Abstract
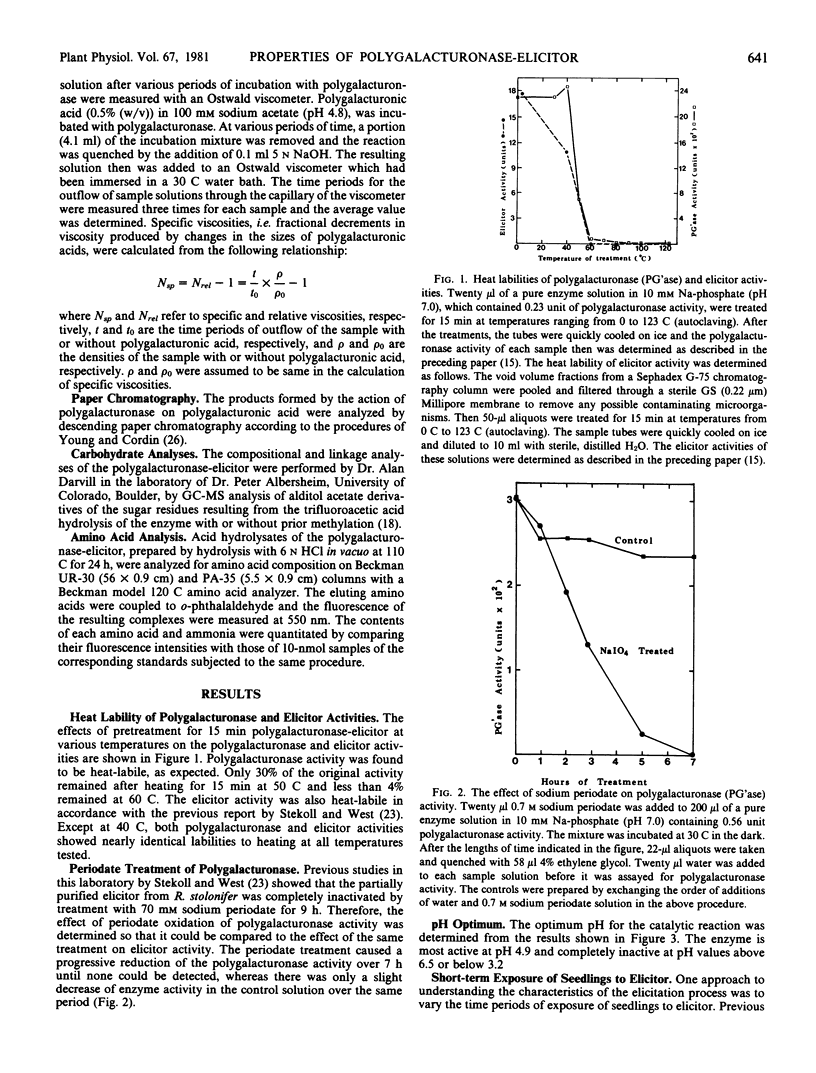
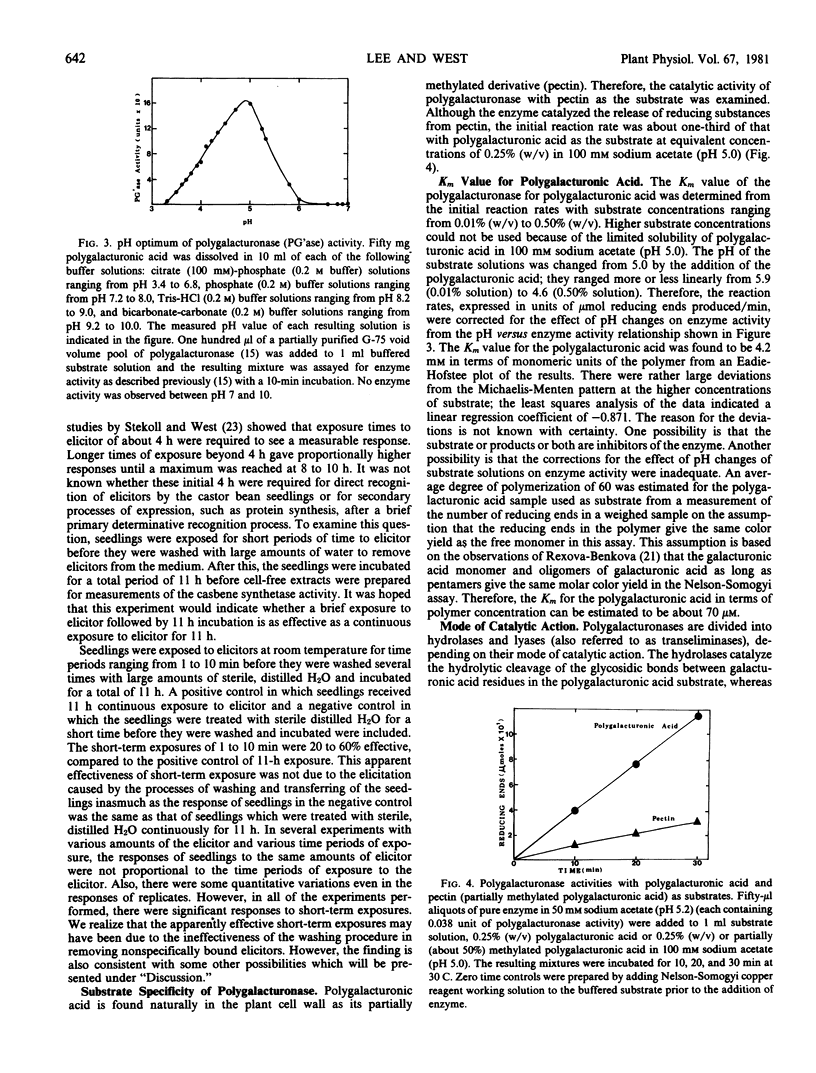
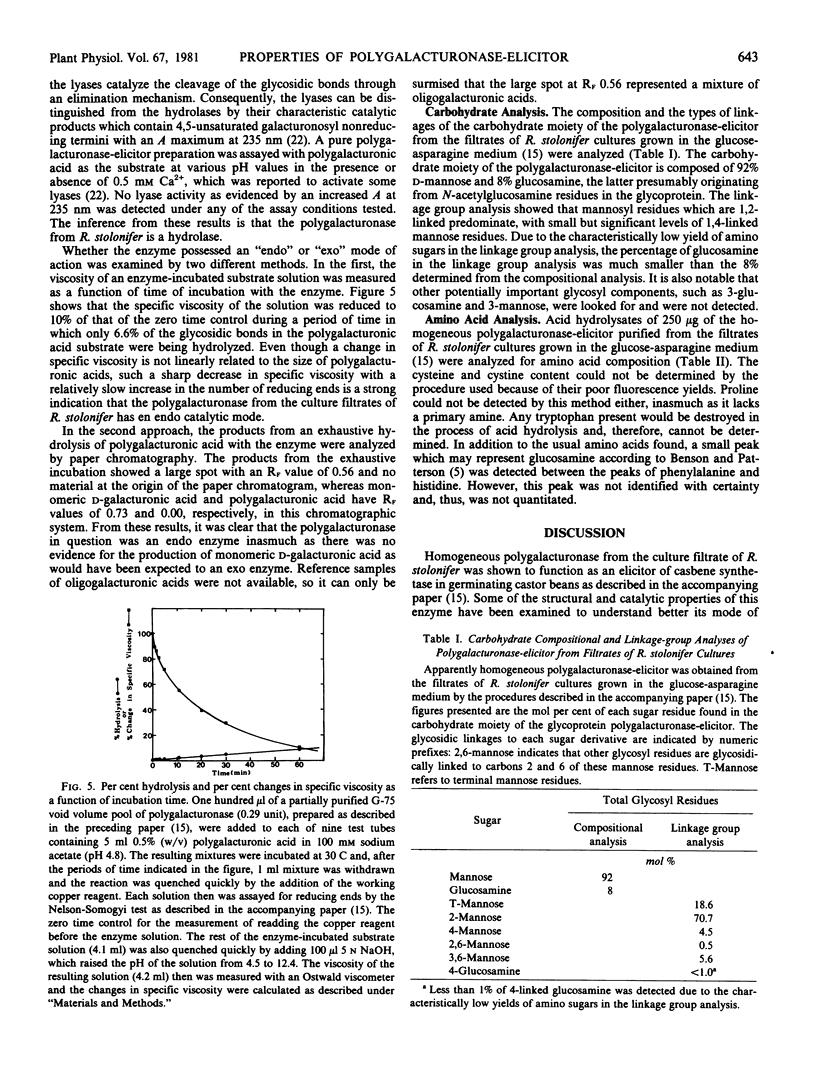
Some properties of the polygalacturonase-elicitor from the filtrates of Rhizopus stolonifer cultures have been examined in an attempt to understand its mode of action as an elicitor of casbene synthetase activity in castor bean seedlings. Both the polygalacturonase activity and the elicitor activity are heat-labile with similar heat-sensitivity profiles. Also, the catalytic activity of the enzyme is lost on treatment with sodium periodate, as had been shown previously for the elicitor activity. The pH optimum of the enzyme activity with polygalacturonic acid as the substrate is 4.9. Exposures of germinating castor bean seedlings to the elicitor for short-term periods of 1 to 10 minutes followed by washing and incubation in sterile, distilled water are partially effective in elicitation in comparison with the continuous exposure of the seedlings over 11 hours to the same amount of the elicitor. The initial rate of reaction catalyzed by the enzyme is about 3 times faster with polygalacturonic acid as a substrate than with partially (50%) methylated polygalacturonic acid (pectin). The Km value of the enzyme for polygalacturonic acid is about 4.2 millimolar in terms of monomeric units and about 0.07 millimolar in terms of polymer concentration. Examination of the types of products formed by the action of the enzyme suggests that it is an endo-hydrolase. The amino acid composition of this enzyme is similar to those of other extracellular fungal proteins reported. The carbohydrate moiety of the glycoprotein polygalacturonase-elicitor is composed of 92% mannose and 8% glucosamine by gas chromatography-mass spectrometry analysis. The linkage group analysis of the carbohydrate moiety showed that mannosyl residues which are 1,2-linked comprise about 70% of the total glycosyl residues and demonstrated the presence of some 1,3,6- and 1,2,6-linked branching mannosyl residues.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayers A. R., Valent B., Ebel J., Albersheim P. Host-Pathogen Interactions: XI. Composition and Structure of Wall-released Elicitor Fractions. Plant Physiol. 1976 May;57(5):766–774. doi: 10.1104/pp.57.5.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. D., Ferber C. E., Kanagasabapathy L. Purification and characterisation of polygalacturonases from a commercial Aspergillus niger preparation. Biochim Biophys Acta. 1976 Dec 8;452(2):440–451. doi: 10.1016/0005-2744(76)90194-7. [DOI] [PubMed] [Google Scholar]
- English P. D., Maglothin A., Keegstra K., Albersheim P. A Cell Wall-degrading Endopolygalacturonase Secreted by Colletotrichum lindemuthianum. Plant Physiol. 1972 Mar;49(3):293–298. doi: 10.1104/pp.49.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M. G., Albersheim P. Host-Pathogen Interactions: XIV. Isolation and Partial Characterization of an Elicitor from Yeast Extract. Plant Physiol. 1978 Jul;62(1):107–111. doi: 10.1104/pp.62.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- Lee S. C., West C. A. Polygalacturonase from Rhizopus stolonifer, an Elicitor of Casbene Synthetase Activity in Castor Bean (Ricinus communis L.) Seedlings. Plant Physiol. 1981 Apr;67(4):633–639. doi: 10.1104/pp.67.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisker N., Katan J., Henis Y. Sequential production of polygalacturonase, cellulase, and pectin lyase by Rhizoctonia solani. Can J Microbiol. 1975 Sep;21(9):1298–1298. doi: 10.1139/m75-196. [DOI] [PubMed] [Google Scholar]
- Rexová-Benková L., Markovic O. Pectic enzymes. Adv Carbohydr Chem Biochem. 1976;33:323–385. doi: 10.1016/s0065-2318(08)60285-1. [DOI] [PubMed] [Google Scholar]
- Rexová-Benková L. The size of the substrate-binding site of an Aspergillus niger extracellular endopolygalacturonase. Eur J Biochem. 1973 Nov 1;39(1):109–115. doi: 10.1111/j.1432-1033.1973.tb03109.x. [DOI] [PubMed] [Google Scholar]
- Stekoll M., West C. A. Purification and Properties of an Elicitor of Castor Bean Phytoalexin from Culture Filtrates of the Fungus Rhizopus stolonifer. Plant Physiol. 1978 Jan;61(1):38–45. doi: 10.1104/pp.61.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlam J. B., Crooks M. J., Brown K. F., Pedersen P. V. Binding of nonsteroidal anti-inflammatory agents to proteins--I. Ibuprofen-serum albumin interaction. Biochem Pharmacol. 1979 Mar 1;28(5):675–678. doi: 10.1016/0006-2952(79)90154-0. [DOI] [PubMed] [Google Scholar]
- YOUNG R. J., CORDEN M. E. PAPER CHROMATOGRAPHY OF GALACTURONIC ACIDS TO DETERMINE POLYGALACTURONASE ACTIVITY. Biochim Biophys Acta. 1964 Mar 2;83:124–126. doi: 10.1016/0926-6526(64)90060-6. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M., Yamauchi K., Masago H. De Novo Messenger RNA and Protein Synthesis Are Required for Phytoalexin-mediated Disease Resistance in Soybean Hypocotyls. Plant Physiol. 1978 Mar;61(3):314–317. doi: 10.1104/pp.61.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler E., Albersheim P. Host-Pathogen Interactions: XIII. Extracellular Invertases Secreted by Three Races of a Plant Pathogen Are Glycoproteins Which Possess Different Carbohydrate Structures. Plant Physiol. 1977 Jun;59(6):1104–1110. doi: 10.1104/pp.59.6.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]


