Abstract
Childhood cancer survivors are at increased risk for future health problems. As such, physical activity (PA) has been targeted as a health promotion priority in child and adolescent cancer survivors. Research indicates that a large portion of pediatric survivors do not meet PA recommendations. Using Bronfenbrenner’s ecological theory as a framework, this review presents a conceptual model to explain child and adolescent survivors’ PA. The model considers predictors of PA across six domains: (1) demographic; (2) medical; (3) cognitive/emotional; (4) behavioral; (5) social/cultural; and (6) environmental. A structured literature review found 14 empirical articles examining those predictors of PA among child and adolescent cancer survivors. Much existing research is cross-sectional, but suggests multiple factors work together to encourage or discourage PA among survivors of child/adolescent cancer. The conceptual model, which is based in empirical findings to date, can be used to understand the process through which PA is promoted and maintained, to inform the development of empirically-supported clinical interventions, and to guide future research objectives and priorities.
Keywords: cancer, oncology, survivorship, physical activity, childhood
Advances in treatment for children diagnosed with cancer have led to increased survival rates. Current estimates indicate 80% of children diagnosed with cancer are alive five years post-treatment (Greenlee, Murray, Bolden, & Wingo, 2000), and approximately 328,000 childhood cancer survivors1 live in the United States today (Mariotto et al., 2009). The growing number of survivors has prompted increased focus on long-term health and quality of life. Research indicates that two-thirds of survivors experience at least one physical or psychological late effect following treatment for childhood cancer (Hewitt, Weiner, & Simone, 2003). Additionally, long-term childhood cancer survivors are over eight times more likely to die prematurely when compared to age- and gender-matched peers in the general population (Mertens et al., 2008).
Given that childhood cancer survivors are at increased risk for future health problems and premature death, it is important to foster development of health promotion behaviors that may ameliorate some of this risk. Physical activity (PA) has been targeted as a leading health promotion priority in cancer survivorship research (National Cancer Institute, 2009). Regular PA during childhood and adolescence is associated with increased cardiorespiratory fitness, muscle and bone strength, reduced anxiety and depression, and more favorable cardiovascular and metabolic disease risk profiles (Doyle, Kushi, & Byers, 2006; U.S. Department of Health and Human Services, 2008).
In addition to general health benefits for all children, specific physical and psychological benefits of PA are documented in childhood cancer survivors. Higher PA is associated with better health-related quality of life across the domains of physical, social, and cognitive functioning in childhood cancer survivors (Paxton et al., 2010). Two recent exercise intervention trials designed for children and adolescents on or off treatment for cancer found improved fitness, muscular strength, fatigue, and mental health associated with participation in the intervention (Keats & Culos-Reed, 2009; San Juan et al., 2007).
Despite research suggesting PA has protective health benefits, a large proportion of childhood cancer survivors do not meet PA recommendations (San Juan et al., 2011, Stolley et al., 2010, Winter et al., 2010). Survivor PA is generally classified according to Center for Disease Control and Prevention (CDC) guidelines recommending that individuals over age 18 engage in 30 minutes of moderate to vigorous activity at least 5 days a week (Haskell et al., 2007). For children and adolescents under age 18, CDC recommendations are for 60 minutes of moderate to vigorous activity at least 5 days per week (Haskell et al., 2007). Similar to healthy children, childhood cancer survivors appear not to meet these recommendations. Almost all published data indicate that less than 50% of childhood cancer survivors meet PA guidelines (Stolley et al., 2010, Winter et al., 2010). Data also suggest that these survivors are less active than healthy comparison groups (Stolley et al., 2010, Winter et al., 2010). This finding is particularly salient in light of childhood cancer survivors’ increased risk for future health problems.
This review considers the predictors of PA in child and adolescent cancer survivors. We conceptualize various factors that contribute to engagement in PA within this population. Such a review and the accompanying model are important to educate movement toward interventions that increase child and adolescent survivors’ engagement in PA. Previous work in the area has been somewhat atheoretical. Empirical work that does explicitly mention a theoretical basis has approached PA behaviors from multiple theoretical models, including the Theory of Planned Behavior (TPB), Social Cognitive Theory (SCT), the Transtheoretical Model (TTM), and the Health Belief Model (HBM), generally positing that specific intrapersonal, interpersonal, or extrapersonal factors interact to promote or inhibit engagement in PA. The present model conceptualizes behavior similarly.
Previous reviews (San Juan et al., 2011, Stolley et al., 2010; Winter et al., 2010) have addressed PA levels in adolescent and adult survivors, including some of the correlates of PA in those populations. While these reviews provide valuable information on PA in older childhood cancer survivors, they do not focus on the unique factors influencing PA in survivors prior to adolescence. Examining these influences in child and adolescent survivors is critical, given that PA behaviors established early in life are likely to be maintained into adulthood (Aart, Paulussen, & Schaalma, 1997). This review adopts the socio-ecological framework outlining correlates of PA in healthy children (Sallis, Prochaska, & Taylor, 2000) to present a conceptual model integrating demographic, medical, social/cultural, environmental, cognitive/emotional, and behavioral influences on PA in child and adolescent cancer survivors. Below, we present the model and then examine specific predictors within each of the conceptual domains. Because published data on child and adolescent survivors is still limited, we reference relevant data from healthy youth and adult childhood cancer survivors when appropriate to fill gaps in the literature on child cancer survivors. Implications of the model are discussed prior to concluding the paper.
Conceptual Model
Work with healthy children suggests PA is a multidimensional behavior, resulting from a number of multi-level influences (Hinkley et al., 2008; Sallis et al., 2000; Taylor & Sallis, 1997). Child and adolescent cancer survivors’ PA is complicated further with the influences of diagnosis and treatment-related factors, plus cognitive and behavioral reactions by parents and children to those influences. Our structured literature review, as outlined below, yielded six domains of functioning, conceptualized as demographic, medical, social/cultural, environmental, cognitive/emotional, and behavioral that we propose as predictors of child and adolescent survivors’ PA. Within each domain, individual constructs that emerged from the review as unique predictors are listed. Examples of causal mechanisms that might explain how the constructs predict PA are offered below in the review.
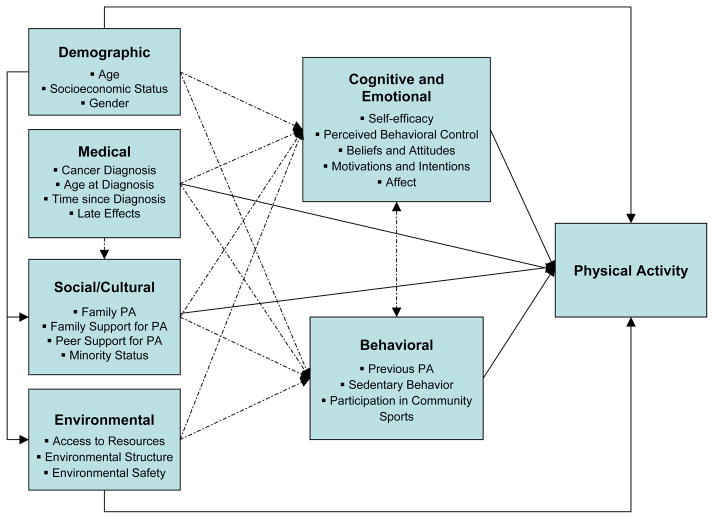
Conceptualizing child and adolescent survivors’ PA through a model (Figure 1) provides a useful framework to demonstrate direct and indirect pathways between the six domains and PA. Solid lines reflect empirically-documented pathways and broken lines reflect hypothesized interactions. The proposed model is unique in that it explains child and adolescent survivors’ PA from a comprehensive vantage point through the inclusion of intrapersonal, interpersonal, and environmental factors. This approach is consistent with ecological systems theory (Bronfenbrenner, 1979, 2001; Kazak, 1986) and emphasizes the role of multiple interactive influences on children and adolescents’ PA. The conceptual model also integrates key components of major health behavior models by considering the roles of intrapersonal factors (e.g., vulnerability), interpersonal factors (e.g., peer relations), and socio-cultural factors (e.g., minority status) on health-related behavior. Uncovering ways in which child and adolescent survivors’ demographic and medical characteristics interact with their social and environmental systems to produce cognitions, emotions, and behaviors will allow for greater accuracy in our ability to explain and promote the development and maintenance of PA in this population.
Figure 1.
Conceptual model of physical activity in child and adolescent cancer survivors. Solid lines reflect empirically-documented pathways and broken lines reflect hypothesized interactions. This figure is conceptual and is not an exhaustive illustration of all possible interactions and pathways.
Methods
To conduct our literature review of influences on child and adolescent cancer survivors’ PA, five databases were searched: PubMed, Medline, PsycINFO, Web of Science, and CINAHL. Search terms were (“physical activity” OR “physical fitness” OR “exercise” OR “exercise intervention”) AND (“childhood cancer survivors” OR “childhood cancer” OR “pediatric cancer survivors” OR “healthy children” OR “healthy adolescents”). Searches were limited to articles published in English between January, 1990 and February, 2011. Studies reporting on predictors or correlates of PA in childhood cancer survivors were selected for inclusion, as were intervention or review articles with a primary outcome of survivor PA. Where applicable, studies reporting on predictors or correlates of PA in healthy youth were also included. References of included articles were searched to identify studies not found in the initial database search, including significant articles published before 1990.
The literature search yielded 78 articles. Following a review of titles and abstracts, the search was narrowed to 26 papers that appeared to meet the inclusion criteria described above. Careful readings of the 26 full manuscripts further reduced the field to 17 papers. Of these, 14 were empirical studies of PA and 3 were review papers. Table 1 summarizes the empirical studies.
Predictors of Physical Activity
Demographic Predictors
Demographic predictors of PA encompass characteristics of the survivor and/or family that directly and indirectly influence PA levels but are not readily modifiable. In pre-adolescent and adolescent survivors, younger age and higher socioeconomic status (SES) predict greater PA (Arroyave et al., 2008; Ness et al., 2009; Tyc et al., 2001). Consistent with PA patterns in healthy children, younger survivors are more active than older survivors, with declines in PA beginning in pre-adolescence and continuing into adulthood (Arroyave et al., 2008; Ness et al., 2009; Sallis et al., 2002; Tyc et al., 2001). While this age-related decline in PA is well-established in both adolescent and adult survivors and healthy youth, the mechanisms of the phenomenon are unknown. Theories range from a decline in parental influence during adolescence (Tyc et al., 2001) to decreases in locomotion-motivating dopamine in adolescent brains (Sallis et al., 2002).
SES also predicts PA, such that adolescent survivors from higher SES levels are more active. This relation is likely due to greater access to financial and environmental resources (Tyc et al., 2001), thus providing more opportunities for youth from wealthier families to engage in PA. For example, child and adolescent survivors from higher SES levels may have the financial resources available to access physical therapy, rehabilitation, or fitness providers outside of the medical setting, thus allowing these survivors to more readily engage in PA following treatment.
Research with healthy children and adult childhood cancer survivors indicates gender may also be a relevant predictor of PA in child and adolescent survivors. In these populations, males tend to be more active than females both in amount and intensity of PA (Finnegan et al., 2007; Florin et al., 2007; Ness et al., 2009; Sallis et al., 2000; Strauss et al., 2001). One study documented gender differences in 19 ALL survivors aged 6–15, reporting that male survivors engaged in significantly more moderate to vigorous PA compared to female survivors (Heath et al., 2010). Future research is needed to confirm this finding in larger, more heterogeneous samples.
As proposed in the conceptual model, it is likely that indirect relations between demographic influences and PA also exist in child and adolescent survivors in addition to the direct influences of age, SES, and gender. Research in healthy children and adolescents suggests that engagement in community sports and activities mediates the relations between SES and PA engagement (Gordon-Larson et al., 2006). Research also suggests that the relation between gender and PA is mediated by activity preferences such that boys prefer more vigorous activities while girls tend to prefer more mild to moderate activities (Trost et al., 2002). These hypothesized interactions should be explored in child and adolescent survivors.
Medical Predictors
Medical predictors of PA are those factors related to the cancer experience, including diagnosis and treatment-related factors as well as late effects. While no published studies directly assessed medical predictors of PA in child survivors, several assessed these influences on PA in adolescent and adult cancer survivors. Adolescent and adult survivors diagnosed with CNS tumors or osteosarcoma evidence lower levels of PA compared to survivors of ALL, lymphoma, and other solid tumors (Arroyave et al., 2008; Ness et al., 2009; Reeves et al., 2007; Winter et al., 2010). Treatment modality also predicts PA, with childhood cancer survivors who received cranial radiation evidencing lower levels of PA compared to non-irradiated survivors, regardless of diagnosis (Florin et al., 2010; Mayer et al., 2000; Nathan et al., 2009; Ness et al., 2009). Given these findings, patients who receive diagnoses of CNS tumors or osteosarcoma along with those receiving cranial radiation may benefit from referrals to physical therapy during or immediately following treatment to help maintain, re-establish, or initiate recommended levels of PA.
The influence of age at diagnosis and time since diagnosis on PA is unclear, as studies assessing these factors have yielded equivocal results. Using structural equation modeling with a sample of 838 adult survivors, one study concluded that age at diagnosis directly explained PA in male but not female survivors, with younger age of diagnosis associated with more PA (Cox et al., 2009). However, a similar study with 9,301 adult survivors contradicted these findings (Ness et al., 2009). Studies assessing time since diagnosis yielded similarly mixed results, with one study supporting a positive association between time since diagnosis and PA (Carpentier et al., 2008) and others not confirming this link (Cox et al., 2009; Ness et al., 2009; Winter et al., 2010). One explanation may be that relations between PA and age at diagnosis or time since diagnosis are moderated by cancer type. For example, lymphoma is more often diagnosed in adolescence whereas ALL is more often diagnosed in childhood, with the sharpest peak in toddlerhood (Ries et al., 1999). Lymphoma survivors may have more time to establish PA patterns pre-diagnosis than ALL survivors. Further, treatment duration differs for the two diagnoses, with many lymphomas (e.g., Hodgkins, Burkitt’s lymphomas) being treated for less than a year whereas treatment for ALL can extend up to 3 years. For these reasons, it is important to assess age at diagnosis and time since diagnosis within subsamples of survivors to reduce variation in diagnosis and treatment-related factors.
Recent research has focused on the influence of specific treatment-related effects on PA. Survivor-reported fatigue is associated with lower levels of PA (Cox et al., 2009) and is the most commonly reported barrier to adolescent survivors’ engagement in PA (Arroyave et al., 2008). Additionally, similar to findings with healthy children (Sallis et al., 2000), obesity predicts lower levels of PA in adult childhood cancer survivors (Florin et al., 2007). Because certain cancers are associated with increased risk of obesity (van Waas et al., 2010; Warner, 2008), this factor must be considered for research and practice.
As with the demographic predictors, medical factors may indirectly influence survivor PA via cognitive, emotional, and behavioral factors. As an example, survivors who have treatment-related effects that limit their ability to engage in PA as they did prior to diagnosis may have lower self-efficacy or perceived behavioral control for PA compared to survivors who do not have activity-limiting late effects. Late effects may also be mediated by social influences such that survivors who experience more debilitating late effects may receive less support for PA if family and peers do not believe the survivor is capable of engaging in PA. Future research should address the influence of medical factors on the cognitive, emotional, and behavioral predictors of PA as well as direct effects of medical factors on PA.
Social/Cultural Predictors
Social influence is the influence of family, friends, or community groups on one’s thoughts, feelings, or behaviors. Social influences are considered strong determinants of PA, especially in youth (Tinsley et al., 1995; Sallis et al., 2000). Children and adolescents are in the process of developing self-identity and internal social values that dictate lifelong behavioral patterns, and as such are heavily influenced by external social influences (Kohl & Hobbs, 1998). Research with child and adolescent cancer survivors, as well as healthy children, sheds light on several factors.
Research with healthy children indicates parental PA influences young children’s PA (Hinkley et al., 2008). Preschool-aged children with one or more active parents are more likely to be active than children without physically active parents (Hinkley et al., 2008). As children grow older, this relation becomes less consistent (Gustafson & Rhodes, 2006; Heitzler et al., 2006; Sallis et al, 2000), with the influence of parental PA diminishing during adolescence and being replaced by sibling and peer PA (Duncan et al., 2007; Sallis et al., 2000). A recent study of 17 childhood cancer survivors aged 10 to 17 evaluated relations between family PA and child/adolescent cancer survivors’ PA (Norris, Moules, Pelletier, Culos-Reed, 2010). Survivors’ PA was related to maternal PA, but not father or sibling PA.
Both direct and indirect support from parents is related to PA in healthy children (Gustafson & Rhodes, 2006; Heitzler et al., 2006; Sallis et al., 1999; Whitt-Glover et al., 2009) and adolescents (Sallis et al., 2000; Whitt-Glover et al., 2009). Direct support involves tasks like transportation and engagement of parents in physical activities, and is associated with higher PA in children and adolescents (Gustafson & Rhodes, 2006; Whitt-Glover et al., 2009). Indirect support through parental encouragement is also associated with increased PA in children (Gustafson & Rhodes, 2006; Heitzler et al., 2006; Sallis et al., 2000). Differences in types of parental support may exist between children and adolescents. It is possible that children may be more influenced by parental encouragement and engagement in PA whereas adolescents may be more influenced by parental support that facilitates PA. More research is needed to evaluate potential developmental differences.
Given the developmental disruption caused by a diagnosis of cancer during childhood, and the increased reliance on family support during treatment, these relations may differ in child and adolescent survivors compared to healthy youth. It is possible that survivors are more strongly influenced by parental support for PA across childhood and adolescence given the major role family support plays during treatment. Alternatively, relations between parental support for PA and survivor PA could be moderated by time since treatment, such that child and adolescent survivors who have completed treatment more recently may be more strongly influenced by parental support whereas survivors who are further from treatment may show relations similar to those in healthy youth. Research is needed to evaluate these hypotheses.
In addition to family support, peer support predicts PA in preadolescent and adolescent youth, but not younger children (Heitzler et al., 2010; Strauss et al., 2001; van der Horst et al., 2007). Youth who report greater support from friends engage in higher PA levels compared to youth who report lower levels of peer support (Heitzler et al., 2010; Strauss et al., 2001). Again, research with child and adolescent cancer survivors is sparse.
Little is known about the cultural influences on PA beyond the relation between minority status and lower PA in healthy children and adult survivors (Florin et al., 2007; Ness et al., 2009; Whitt-Glover et al., 2009). Cross-cultural and international research is needed to assess influences such as societal norms, rules of behavior, and leisure-time traditions.
While published research has not addressed indirect influences of social/cultural predictors on child and adolescent survivor’s PA, it seems likely that they exist. Survivors who receive less support from family and peers are likely to demonstrate lower levels of self-efficacy, less positive beliefs or attitudes towards PA, and lower levels of motivation and intention to exercise that in turn may lead to lower levels of PA. This is especially relevant for survivors, as family members may perceive them as more vulnerable to physical threats and thus provide less encouragement for PA. Similar indirect relations likely exist for cultural influences on PA.
Environmental Predictors
In the past decade, increased interest has developed concerning the relation between the environment and youth PA (Ferreira et al., 2006). Access to PA-promoting resources, the structure and safety of the environment, and rural rather than urban residence are all hypothesized to increase PA (Ferreira et al., 2006; Heitzler et al., 2010).
While the influence of the environment has not been directly assessed in child and adolescent cancer survivors, a leading barrier to PA reported by adolescent survivors is a lack of access to resources (Arroyave et al., 2008). Research with healthy children and adolescents indicates that access to resources significantly predicts PA, such that youth who are enrolled in community sports programs or who have access to PA facilities show higher PA levels (Heitzler et al., 2006; Sallis, Prochaska, & Taylor, 2000; Whitt-Glover et al., 2009). Of note, Gordon-Larson et al. (2006) concluded that disparities in access to community resources partially explained ethnic and socioeconomic disparities in PA in healthy adolescents.
Additional environmental influences are also documented in healthy youth, though the relative importance of these factors differs by developmental level. For children, time spent outdoors is a strong predictor of PA (Sallis, Prochaska, & Taylor, 2000; Whitt-Glover et al., 2009). Low crime rates and perceptions of greater neighborhood safety predict higher levels of adolescent PA (Whitt-Glover et al., 2009). Interestingly, specific neighborhood hazards, including heavy traffic, no signaled light crossings, community disorder, and pollution, are consistently unrelated to youths’ PA (Ferreira et al., 2006).
As the conceptual model proposes, in addition to the direct influences of environment on survivor PA, these influences are also likely to be mediated by cognitive, emotional, and behavioral factors. Survivors who do not have access to PA-promoting resources or those whose environments do not support PA are likely to engage in more sedentary behaviors and show lower levels of self-efficacy and perceived behavioral control for PA. This relation may be particularly strong for survivors who have recently completed treatment and are attempting to return to pre-diagnosis levels of activity.
Cognitive/Emotional Predictors
Cognitive and emotional factors have received substantial attention as predictors of PA in the childhood cancer literature (Cox et al., 2009; Finnegan et al., 2007; Keats, Culos-Reed, Courneya, & McBride, 2007; Keats & Culos-Reed, 2009). Several health behavior theories, including HBM, TPB, SCT, and TTM, emphasize the role of cognitive and emotional influences on health behavior outcomes. While a thorough review of the individual theories is beyond the scope of this paper, they each share a common foundation asserting that attitudes, beliefs, and/or thoughts influence the development and maintenance of health-related behaviors, including engagement in PA. In adolescent and adult childhood cancer survivors, relevant cognitive and emotional influences include self-efficacy and perceived behavioral control, behavioral beliefs and attitudes, behavioral motivations and intentions, and affect (Cox et al., 2009; Finnegan et al., 2007; Keats et al., 2007; Keats & Culos-Reed, 2009; Ness et al., 2009). According to the proposed model, these factors directly influence child and adolescent survivor PA and are also hypothesized to mediate relations between the demographic, medical, social/cultural, and environmental factors and PA.
Self-efficacy and perceived behavioral control positively predict adolescent and adult survivors’ PA (Finnegan et al., 2007; Keats et al., 2007; Keats & Culos-Reed, 2009). Self-efficacy refers to an individual’s belief about his/her ability to perform a given behavior. Perceived behavioral control refers to an individual’s perception that the execution of a given behavior is under his/her personal control (Ajzen, 2002). Research supports the independent, significant contributions of both constructs to health behavior outcomes (Tavousi et al., 2009; Terry & O’Leary, 1995).
Adolescent and young adult survivors who endorse greater self-efficacy for PA are more likely to be active compared to survivors who report lower self-efficacy (Finnegan et al., 2007; Keats et al., 2007, Keats & Culos-Reed, 2009). Perceived behavioral control shows a similar association, such that adolescent and adult survivors who feel like they have greater control over their engagement in PA are more likely to be active (Keats & Culos-Reed, 2009). While both constructs significantly account for variance in models of adolescent survivors’ PA, self-efficacy is the stronger predictor of PA when both constructs are included in the same model (Keats et al., 2007; Keats & Culos-Reed, 2009). Replication is needed among younger survivors.
The influence of beliefs and attitudes on PA is documented in both adolescent and adult childhood cancer survivors (Finnegan et al., 2007; Keats et al., 2007). Adolescent and young adult survivors’ negative beliefs about PA (e.g. self-reported cons or barriers to being active) consistently predict lower PA (Arroyave et al., 2008; Finnegan et al., 2007). Interestingly, positive beliefs about PA or its benefits are not predictive of higher PA. Negative beliefs about PA appear to have a stronger impact on PA disengagement than do positive beliefs on PA engagement (Finnegan et al., 2007).
Mixed results have been found for attitudes toward PA (Keats et al., 2007; Keats & Culos-Reed, 2009). In a study applying the TPB to understand PA motivation and behavior in 95 adolescent cancer survivors, results indicated that survivors’ positive attitudes toward PA were associated with increased intentions to exercise (Keats et al., 2007). Survivor intentions to exercise were, in turn, associated with higher PA. In a follow-up intervention designed to increase PA in 10 adolescent survivors, no relations were found between positive attitude and either intention to exercise or PA (Keats & Culos-Reed, 2009). Intention to exercise, perceived behavioral control, and self-efficacy were strongly related to PA, suggesting that attitude may not be a strong influence on PA after covarying the effect of more salient cognitive factors. This hypothesis should be confirmed with a larger sample size.
Motivation significantly predicts PA in adolescent and adult childhood cancer survivors, such that adolescent and adult survivors who are more motivated to be active tend to report higher PA (Cox et al., 2009; Finnegan et al., 2007; Keats et al., 2007; Keats & Culos-Reed, 2009). Interestingly, when motivation is separated into intrinsic (e.g., internal desire to improve skill level; sense of personal accomplishment) versus extrinsic (e.g., please others; gain rewards) components, gender differences emerge (Cox et al., 2009). Young adult male survivors’ PA is predicted by both intrinsic and extrinsic motivation whereas female survivors’ PA is predicted by intrinsic motivation only.
Finally, affect has been evaluated as a predictor of PA in adolescent and adult childhood cancer survivors (Cox et al., 2009; Finnegan et al., 2007; Ness et al., 2009). Self-report generalized (not disease-specific) depression predicts lower PA in adult cancer survivors (Cox et al., 2009; Ness et al., 2009). Mixed results emerge with anxiety as a predictor, likely due to variability in the underlying constructs measured. One study (Cox et al., 2009) assessed the relation between cancer-related anxiety and PA in a sample of 838 adult childhood cancer survivors (mean age = 30 years; mean time since diagnosis = 23 years), and concluded that current cancer-related anxiety predicted higher PA in both males and females. In contrast, another study with a sample of 117 adult childhood cancer survivors aged 18 to 37 years concluded that general worries, both in the present and for the future, predicted lower levels of PA for both male and female survivors (Finnegan et al; 2007). Given the differences in the underlying constructs, it may be that the focus of anxious thoughts influences survivor PA outcomes. For example, cancer or health-related anxiety may be associated with increased survivor PA while more generalized anxiety may be associated with lower PA. Future research should utilize measures of anxiety with both general and cancer-specific scales in the same sample to explicate this relation.
Behavioral Predictors
Behavioral predictors include any action performed by the survivor that may lead to changes in their PA. Similar to cognitive and emotional factors, behavioral predictors are hypothesized in the conceptual model to influence survivor PA directly and also to mediate the relations between PA and the demographic, medical, social/cultural, and environmental domains. Behavioral predictors of PA have not been examined in child and adolescent cancer survivors, but there are relevant published data from samples of healthy children and from adult childhood cancer survivors. The strongest behavioral predictor of PA in both healthy adolescents and adult childhood cancer survivors is previous PA (Cox et al., 2009; Sallis et al., 2000). Individuals who report engaging in PA behaviors in the past are more likely to report current engagement.
Sedentary behavior also is a strong predictor of PA, such that healthy children who spend more time engaging in sedentary behaviors report lower levels of PA (Sallis et al., 2000). These findings, not surprising given that time spent performing sedentary activities necessarily decreases time available to perform physical activities, are consistent with Arroyave and colleagues’ (2008) finding that a significant barrier to PA in adolescent survivors of cancer is preference for sedentary behaviors such as watching television and using the computer. Given the physical limitations placed on children and adolescents during treatment, it is not surprising that they may be inclined to prefer more sedentary activities. In addition, many survivors may lose confidence in their ability to perform PA during the course of treatment and thus may prefer to engage in more sedentary behaviors.
A final related behavioral predictor of PA in healthy adolescents is participation in community sports. Healthy adolescents who participate in community sports report higher PA (Sallis et al., 2000). This is particularly salient given the age-related decline in PA documented during preadolescence and adolescence. Community sports participation may protect against this PA decline. For child and adolescent survivors, engagement in community sports may be a particularly relevant predictor of PA as many youth have to stop participating in community sports during treatment. Keeping survivors engaged in community athletic teams throughout treatment, regardless of the child or adolescent’s ability to physically participate, may ease the return to community sports following treatment.
As the model implies, cognitive and emotional predictors are hypothesized to interact with behavioral predictors through bi-directional relations. For example, during treatment, survivors tend to become more sedentary. Over time, this may lead to lower self-efficacy and perceived behavioral control for PA that further reinforces the preference for sedentary activities even after treatment. On the other hand, survivors who are educated on the importance of PA following treatment may have higher motivation and intention to exercise that in turn results in engagement in more active behaviors as opposed to sedentary behaviors.
Limitations of Existing Research
The current literature provides a strong foundation from which we can begin to understand the multiple influences on child and adolescent survivors’ PA. Several limitations should be noted, however. Despite the recent attention to childhood cancer survivors’ PA behaviors, the literature on child and adolescent survivors remains sparse and findings with older adolescents and adult survivors should be replicated among younger survivors, who have different developmental influences. Additionally, sample sizes within the studies that have been conducted with child and adolescent survivors are small, typically numbering less than 60. Multi-center studies will be necessary to provide sufficient statistical power to identify small effect sizes and fully understand interactions between the various factors influencing survivor PA. Finally, with the exception of one small (N=10) study of adolescent survivors, all of the studies that have been published to date utilized cross-sectional designs and thus limit our ability to draw causal inferences. Future studies should employ prospective and longitudinal designs to look at relations within and across predictors over time.
Implications
The proposed conceptual model and accompanying review might be used for a wide range of purposes, but we see three as primary: (1) understanding the processes through which PA is developed and maintained in the target population, (2) informing development of empirically-supported intervention programs, and (3) guiding further research objectives.
Development of effective interventions to increase PA among pediatric cancer survivors requires an understanding of the processes that determine survivors’ PA. Examination of specific relations between the current model’s six domains and their corresponding factors will illuminate the process leading to survivors’ PA. The model conceptualizes cognitions, emotions, and behaviors as mediators of the relations between the other domains and PA. As child and adolescent survivors have experienced a significant threat to their health, they often exhibit unique cognitive, emotional, and behavioral reactions to their external world as compared to healthy peers. For example, following completion of treatment, survivors may exhibit decreased self-efficacy and perceived behavioral control for PA, as they have been required to be more sedentary for a considerable amount of time. These cognitions may be exacerbated if a survivor is experiencing significant late effects that require alternate forms of PA (e.g. osteosarcoma survivor with lower limb amputation; CNS tumor survivor experiencing balance issues).
Following documentation of the processes that lead to the development and maintenance of PA, empirically-supported interventions can be designed to target modifiable characteristics related to survivors’ engagement in PA. To date, interventions targeting PA in child and adolescent survivors of cancer have provided mixed results (Winter et al., 2010). Consistent with the conceptual model, interventions targeting both behavioral and social factors have demonstrated greater success than interventions targeting behavioral factors alone (Keats & Culos-Reed, 2009; Takken et al., 2009). Following Bronfenbrenner’s ecological systems theory, the present conceptual model suggests that successful interventions will include multiple components targeting behavioral, cognitive, emotional, environmental, and social factors rather than focusing on one or two single domains. Based on the current model, an ideal PA intervention might include both survivors and their families to address the behavioral and social influences on survivor PA. In addition, cognitive-behavioral techniques should be employed to address any thoughts or feelings that may be impeding survivors’ PA engagement. Finally, problem-solving training would be used to help survivors and families navigate barriers to PA within the home or local community environment.
In addition to guiding development of interventions, the conceptual model informs future research objectives related to PA in survivors. Areas of future research may include validation of the model across subsets of survivors (e.g. child versus adolescent, male versus female) or between survivors and other child populations (e.g. youth with other chronic illnesses, healthy children). Future studies also may evaluate the model’s applicability to the prediction of other health-promoting behaviors in child and adolescent survivors such as diet, sleep, dental, and safety behaviors. It is possible that processes underlying engagement in PA may also underlie similar health-related behaviors.
Conclusions
The review and conceptual model indicate that PA in child and adolescent cancer survivors is a multi-dimensional phenomenon influenced by multiple psychosocial domains. The proposed model can document the processes underlying PA behaviors in young survivors. Understanding these processes is vital to the development of empirically-supported interventions to increase PA levels in this population. Because childhood cancer survivors are at greater risk for future health problems compared to the general population, successful promotion of PA may have significant implications on the future health of these youth.
Table I.
Empirical Studies Assessing Predictors of Physical Activity in Childhood Cancer Survivors
| Publication | Assessment Point | N, age | Cancer Diagnosis | Design/Methods | Theory | Primary Results |
|---|---|---|---|---|---|---|
| Child and Adolescent Participants | ||||||
| Heath et al., 2010 | .5 – 6 y off tx | 19, 6 – 15 y | ALL | Cross-sectional/Self-report, actigraph | None | Female gender associated with lower PA. |
| Keats et al., 2007 | Mean time since dx = 2.6 y | 59, 15 – 20 y | Mixed | Cross-sectional/Self-report | Theory of Planned Behavior | Lower PA associated with lower self-efficacy and intention. Lower intention to exercise associated with negative attitudes toward exercise. |
| Keats et al., 2009 | 0.5 – 13.6 y off tx | 10, 14 – 18 y | Mixed | Longitudinal/Self-report | Theory of Planned Behavior | Lower PA associated with lower self-efficacy, perceived behavioral control, and intention to exercise. |
| Mayer et al., 2000 | 3.4 – 14.6 y off tx | 39, 10 – 20 y | ALL | Cross-sectional/Self-report, physiological measures | None | Cranially-irradiated ALL survivors report lower PA compared to non-irradiated ALL survivors. |
| Norris et al., 2010 | .92 – 10.6 y since dx | 17, 10 – 17 y | Mixed | Cross-sectional/Self-report | None | Survivor PA levels associated with maternal PA levels. Survivor-sibling and survivor-father PA unassociated. |
| Tyc et al., 2001 | 1– 4 y off tx | 46, 10 – 18 y | Mixed | Cross-sectional/Self-report | Cognitive-Motivational Theory | Lower physical activity associated with older age and lower SES. |
| Adolescent and Adult Participants | ||||||
| Arroyave et al., 2008 | ≥ 1 y off tx | 118, 13 – 35 y | Mixed | Cross-sectional/Self-report | General health behavior theory | Common barriers to exercise included being too tired, too busy, and lack of equipment or gym memberships. Additional barriers for adolescents included poor weather, worries about injury, and inexperience with exercise. |
| Reeves et al., 2007 | 50% of sample ≤ 5 y off tx; 50% of sample > 5 y off tx. | 28, 17 – 25 y | Mixed | Cross-sectional/Self-report | None | Diagnosis with Leukemia or CNS tumor associated with lower PA. |
| Adult Participants | ||||||
| Castellino et al., 2005 | 8.767, 18 – > 50 y | Mixed | Cross-sectional/self-report | None | Males more likely to be active. | |
| Cox et al., 2009 | ≥ 5 y off tx | 838, 18 – > 50 y | Mixed | Cross-sectional/Self-report, medical chart abstraction | Interaction Model of Client Health Behavior | Male and female survivors’ PA associated with motivation, anxiety, stamina, fatigue, pain, and baseline exercise frequency. Male PA also associated with age at diagnosis, health fears, education, affect, physician expertise, and discussion of future cancer risk. Female PA also associated with recency of visits to the physician and quality of interactions with the physician. |
| Finnegan et al., 2007 | Mean time off tx = 11 y | 117, 18 – 37 y | Mixed | Cross-sectional/Self-report | Interaction Model of Client Health Behavior | PA associated with male gender, autonomous motivation, PA cons, self-efficacy, and present and future worries. |
| Florin et al., 2007 | 16 – 34 y off tx | 2,648, 18 – 44 y | ALL | Cross-sectional/Self-report | None | Female gender, minority status, and cranial irradiation associated with inactivity. |
| Ness et al., 2009 | ≥ 5 y off tx | 9,301, 18 – > 50 y | Mixed | Cross-sectional/Self-report, medical chart abstraction | None | Inactivity associated with cranial irradiation, amputation, diagnosis, female gender, black race, older age, lower education, weight status, smoking, and depression. |
| Participants at All Ages | ||||||
| Demark-Wahnefried et al., 2005 | 209, 11– 33 y | Mixed | Cross-sectional/Self-report | None | Adolescents were more likely to exercise compared to adults. Leukemia and lymphoma survivors were more likely to meet PA guidelines compared to CNS survivors. |
|
Dx, diagnosis; Tx, treatment; y, years; PA, physical activity; ALL, Acute Lymphoblastic Leukemia; PA, physical activity; CNS, Central Nervous System; SES, socioeconomic status
Acknowledgments
This work has been supported in part by the National Cancer Institute sponsored Cancer Prevention and Control Training Program, Grant No. P30 CA13148-39 and R25 CA047888. The authors thank Wendy Demark-Wahnefried, Ph.D., Avi Madan-Swain, Ph.D., and Kimberly Whelan, M.D. for helpful comments on a draft of this article.
Footnotes
In this paper, the term “childhood cancer survivors” refers to survivors of all ages who were diagnosed with cancer during childhood or adolescence. Specific age groups of participants in research studies are referenced when applicable.
No competing interests, personal or financial, exist for either author.
References
- Arroyave WD, Clipp EC, Miller PE, Jones LW, Ward DS, Bonner MJ, Demark-Wahnefried W. Childhood cancer survivors’ perceived barriers to improving exercise and dietary behaviors. Oncology Nursing Forum. 2008;35:121–130. doi: 10.1188/08.ONF.121-130. [DOI] [PubMed] [Google Scholar]
- Aarts H, Paulussen T, Schaalma H. Physical exercise habit: On the conceptualization and formation of habitual health behaviours. Health Education Research. 1997;12:363–374. doi: 10.1093/her/12.3.363. [DOI] [PubMed] [Google Scholar]
- Ajzen I. Perceived behavioral control, self-efficacy, locus of control, and the theory of planned behavior. Journal of Applied Social Psychology. 2002;32:665–683. [Google Scholar]
- Bronfenbrenner U. The Ecology of Human Development: Experiments by Nature and Design. Cambridge, MA: Harvard University Press; 1979. [Google Scholar]
- Bronfenbrenner U. The bioecological theory of human development. In: Smelser N, Baltes P, editors. International Encyclopedia of the Social and Behavioral Sciences. New York, NY: Elsevier; 2001. pp. 6973–6970. [Google Scholar]
- Carpentier MY, Mullins LL, Elkin TD, Wolfe-Christensen C. Predictors of health-harming and health-protective behaviors in adolescents with cancer. Pediatric Blood & Cancer. 2008;51:525–530. doi: 10.1002/pbc.21605. [DOI] [PubMed] [Google Scholar]
- Castellino SM, Casillas J, Hudson MM, Mertens AC, Whitton J, Brooks SL, Oeffinger KC. Minority adult survivors of childhood cancer: A comparison of long-term outcomes, health care utilization, and health-related behaviors from the childhood cancer survivor study. Journal of Clinical Oncology. 2005;23:6499–6507. doi: 10.1200/JCO.2005.11.098. [DOI] [PubMed] [Google Scholar]
- Cox CL, Montgomery M, Oeffinger KC, Leisenring W, Zeltzer L, Whitton JA, Robison LL. Promoting physical activity in childhood cancer survivors: Targets for intervention. Cancer. 2009;115:642–654. doi: 10.1002/cncr.24043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Werner C, Clipp EC, Guill AB, Bonner M, Jones LW, Rosoff PM. Survivors of childhood cancer and their guardians. Cancer. 2005;103:2171–2180. doi: 10.1002/cncr.21009. [DOI] [PubMed] [Google Scholar]
- Doyle C, Kushi LH, Byers T, Courneya KS, Demark-Wahnefried W, Grant B, Andrews KS. Nutrition and physical activity during and after cancer treatment: An American Cancer Society guide for informed choices. CA: A Cancer Journal for Clinicians. 2006;56:323–353. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- Duncan SC, Duncan TE, Strycker LA, Chaumeton NR. A cohort-sequential latent growth model of physical activity from ages 12 to 17 years. Annals of Behavioral Medicine. 2007;33:81–89. doi: 10.1207/s15324796abm3301_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira I, van der Horst K, Wendel-Vos W, Kremers S, van Lenthe FJ, Brug J. Environmental correlates of physical activity in youth – A review and update. Obesity Reviews. 2006;8:129–154. doi: 10.1111/j.1467-789X.2006.00264.x. [DOI] [PubMed] [Google Scholar]
- Finnegan L, Wilkie DJ, Wilbur J, Campbell RT, Zong S, Katula S. Correlates of physical activity in young adult survivors of childhood cancers. Oncology Nursing Forum. 2007;34:E60–E69. doi: 10.1188/07.ONF.E60-E69. [DOI] [PubMed] [Google Scholar]
- Florin TA, Fryer GE, Miyoshi T, Weitzman M, Mertens AC, Hudson MM, Oeffinger KC. Physical inactivity in adult survivors of childhood acute lymphoblastic leukemia: A report from the childhood cancer survivor study. Cancer Epidemiology, Biomarkers & Prevention. 2007;16:1356–1363. doi: 10.1158/1055-9965.EPI-07-0048. [DOI] [PubMed] [Google Scholar]
- Gordon-Larsen P, Nelson MC, Page P, Popkin BM. Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics. 2006;117:266–278. doi: 10.1542/peds.2005-0058. [DOI] [PubMed] [Google Scholar]
- Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA: A Cancer Journal for Clinicians. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- Gustafson SL, Rhodes RE. Parental correlates of physical activity in children and early adolescents. Sports Medicine. 2006;36:79–97. doi: 10.2165/00007256-200636010-00006. [DOI] [PubMed] [Google Scholar]
- Haskell WL, Lee I, Pate RR, Powell KE, Blair SN, Franklin BA, Bauman A. Physical activity and public health: Recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine and Science in Sports & Exercise. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- Heath JA, Ramzy JM, Donath JM. Physical activity in survivors of childhood acute lymphoblastic leukemia. Journal of Paediatrics and Child Health. 2010;46:149–153. doi: 10.1111/j.1440-1754.2009.01653.x. [DOI] [PubMed] [Google Scholar]
- Heitzler CD, Martin SL, Duke J, Huhman M. Correlates of physical activity in a national sample of children aged 9–13 years. Preventive Medicine. 2006;42:254–260. doi: 10.1016/j.ypmed.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Heitzler CD, Lytle LA, Erickson DJ, Barr-Anderson D, Sirard JR, Story M. Evaluating a model of youth physical activity. American Journal of Health Behavior. 2010;34:593–606. doi: 10.5993/ajhb.34.5.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt M, Weiner SL, Simone JV. Childhood cancer survivorship: Improving care and quality of life. Washington, DC: National Academics Press; 2003. [PubMed] [Google Scholar]
- Hinkley T, Crawford D, Salmon J, Okely AD, Hesketh K. Preschool children and physical activity: A review of correlates. American Journal of Preventative Medicine. 2008;34:435–441. doi: 10.1016/j.amepre.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Kazak A. Families with physically handicapped children: Social ecology and family systems. Family Process. 1986;25:265–281. doi: 10.1111/j.1545-5300.1986.00265.x. [DOI] [PubMed] [Google Scholar]
- Keats MR, Culos-Reed N, Courneya KS, McBride M. Understanding physical activity in adolescent cancer survivors: An application of the theory of planned behavior. Psycho-oncology. 2007;16:448–457. doi: 10.1002/pon.1075. [DOI] [PubMed] [Google Scholar]
- Keats MR, Culos-Reed N. A theory driven approach to encourage physical activity in pediatric cancer survivors: A pilot study. Journal of Sport & Exercise Psychology. 2009;31:267–283. doi: 10.1123/jsep.31.2.267. [DOI] [PubMed] [Google Scholar]
- Kohl HW, Hobbs KE. Development of physical activity behaviors among children and adolescents. Pediatrics. 1998;101:549–554. [PubMed] [Google Scholar]
- Mariotto AB, Rowland JH, Yabroff KR, Scoppa S, Hachey M, Ries L, Feuer EJ. Long-term survivors of childhood cancers in the United States. Cancer Epidemiology, Biomarkers & Prevention. 2009;18:1033–1040. doi: 10.1158/1055-9965.EPI-08-0988. [DOI] [PubMed] [Google Scholar]
- Mayer EI, Reuter M, Dopfer RE, Ranke MB. Energy expenditure, energy intake and prevalence of obesity after therapy for acute lymphoblastic leukemia during childhood. Hormone Research. 2000;53:193–199. doi: 10.1159/000023566. [DOI] [PubMed] [Google Scholar]
- Mertens AC, Liu Q, Neglia JP, Wasilewski K, Leisenring W, Armstrong GT, Yasui Y. Cause-specific late mortality among 5-year survivors of childhood cancer: The childhood cancer survivor study. Journal of the National Cancer Institute. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan PC, Ford JS, Henderson TO, Hudson MM, Emmons KM, Casillas JN, Oeffinger KC. Health behaviors, medical care, and interventions to promote healthy living in the childhood cancer survivor study cohort. Journal of Clinical Oncology. 2009;27:2363–2373. doi: 10.1200/JCO.2008.21.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute. National Cancer Institute Fact Sheet: Physical activity and cancer. 2009 Retrieved from http://www.cancer.gov/cancertopics/factsheet/prevention/physicalactivity.
- Ness KK, Leisenring WM, Huang S, Hudson MM, Gurney JG, Whelan K, Oeffinger KC. Predictors of inactive lifestyle among adult survivors of childhood cancer. Cancer. 2009;115:1984–1994. doi: 10.1002/cncr.24209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris J, Moules NJ, Pelletier G, Culos-Reed SN. Families of young pediatric cancer survivors: A cross-sectional survey examining physical activity behavior and health-related quality of life. Journal of Pediatric Oncology Nursing. 2010;27:196–208. doi: 10.1177/1043454209358411. [DOI] [PubMed] [Google Scholar]
- Paxton RJ, Jones LW, Rosoff PM, Bonner M, Ater JL, Demark-Wahnefried W. Associations between leisure-time physical activity and health-related quality of life among adolescent and adult survivors of childhood cancer. Psycho-Oncology. 2010;19:997–1003. doi: 10.1002/pon.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves M, Eakin E, Lawler S, Demark-Wahnefried W. Health behaviours in survivors of childhood cancer. Australian Family Physician. 2007;36:95–96. [PubMed] [Google Scholar]
- Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, Bunin GR, editors. NIH Publication No. 99-4649. Bethesda, MD: National Institute of Health; 1999. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. [Google Scholar]
- Sallis JF. Age-related decline in physical activity: A synthesis of human and animal studies. Medicine & Science in Sports & Exercise. 2000;32:1598–1600. doi: 10.1097/00005768-200009000-00012. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Prochaska JJ, Taylor WC. A review of correlates of physical activity of children and adolescents. Medicine & Science in Sports & Exercise. 2000;32:963–975. doi: 10.1097/00005768-200005000-00014. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Taylor WC, Dowda M, Freedson PS, Pate RR. Correlates of vigorous physical activity for children in grades 1 through 12. Comparing parent-reported and objectively measured physical activity. Pediatric Exercise Science. 2002;14:30–44. [Google Scholar]
- San Juan AF, Fleck SJ, Chamorro-Vina C, Mate-Munoz JL, Moral S, Perez M, Lucia A. Effects of an intrahospital exercise program intervention for children with leukemia. Medicine & Science in Sports & Exercise. 2007;39:13–21. doi: 10.1249/01.mss.0000240326.54147.fc. [DOI] [PubMed] [Google Scholar]
- San Juan AF, Wolin K, Lucia A. Physical activity and pediatric cancer survivorship. Recent Results in Cancer Research. 2011;186:319–347. doi: 10.1007/978-3-642-04231-7_14. [DOI] [PubMed] [Google Scholar]
- Stolley MR, Restrepo J, Sharp LK. Diet and physical activity in childhood cancer survivors: A review of the literature. Annals of Behavioral Medicine. 2010;39:232–249. doi: 10.1007/s12160-010-9192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss RS, Rodzilsky D, Burack G, Colin M. Psychosocial correlates of physical activity in healthy children. Archives of Pediatrics and Adolescent Medicine. 2001;155:897–902. doi: 10.1001/archpedi.155.8.897. [DOI] [PubMed] [Google Scholar]
- Takken T, van der Torre P, Zwerink M, Hulzebos EH, Bierings M, Helders PJM, van der Net J. Development, feasibility and efficacy of a community-based exercise training program in pediatric cancer survivors. Psycho-Oncology. 2009;18:440–448. doi: 10.1002/pon.1484. [DOI] [PubMed] [Google Scholar]
- Tavousi M, Hidarnia AR, Montazeri A, Hajizadeh E, Taremain F, Ghofranipour F. Are perceived behavioral control and self-efficacy distinct constructs? European Journal of Scientific Research. 2009;30:146–152. [Google Scholar]
- Taylor WC, Sallis JF. Determinants of physical activity in children. In: Simopolous AP, Pavlou KN, editors. Nutrition and Fitness: Metabolic and Behavioral Aspects in Health and Disease. World Review of Food and Nutrition. Vol. 82. Basel, Switzerland: Karger; 1997. pp. 159–167. [DOI] [PubMed] [Google Scholar]
- Terry DJ, O’Leary JE. The theory of planned behaviour: The effects of perceived behavioural control and self-efficacy. British Journal of Social Psychology. 1995;34:199–220. doi: 10.1111/j.2044-8309.1995.tb01058.x. [DOI] [PubMed] [Google Scholar]
- Tinsley BJ, Holtgrave DR, Reise SP, Erdley C, Cupp RG. Developmental status, gender, age, and self-reported decision-making influences on students’ risky and preventive health behaviors. Health Education Quarterly. 1995;22:3244–3259. doi: 10.1177/109019819502200211. [DOI] [PubMed] [Google Scholar]
- Trost SG, Pate RR, Sallis JF, Freedson PS, Taylor WC, Dowda M, Sirard J. Age and gender differences in objectively measured physical activity in youth. Medicine & Science in Sports & Exercise. 2002;34:350–355. doi: 10.1097/00005768-200202000-00025. [DOI] [PubMed] [Google Scholar]
- Tyc VL, Hadley W, Crockett G. Prediction of health behaviors in pediatric cancer survivors. Medical and Pediatric Oncology. 2001;37:42–46. doi: 10.1002/mpo.1161. [DOI] [PubMed] [Google Scholar]
- U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2007 Incidence and Mortality Web-based Report. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2010. Retrieved from http://www.cdc.gov/uscs. [Google Scholar]
- U.S. Department of Health and Human Services. Physical activity guidelines advisory committee report. Washington, DC: U.S. Department of Health and Human Services; 2008. 2008. [Google Scholar]
- van der Horst K, Chin A, Paw MJ, Twisk JWR, van Mechelen W. A brief review on correlates of physical activity and sedentariness in youth. Medicine & Science in Sports & Exercise. 2007;39:1241–1250. doi: 10.1249/mss.0b013e318059bf35. [DOI] [PubMed] [Google Scholar]
- van Waas M, Neggers SJ, van der Lelij AJ, Pieters R, van den Heuvel-Elbrink MM. The metabolic syndrome in adult survivors of childhood cancer, a review. Journal of Pediatric Hematology/Oncology. 2010;32:171–179. doi: 10.1097/MPH.0b013e3181d419c3. [DOI] [PubMed] [Google Scholar]
- Warner JT, Bell W, Webb DK, Gregory JW. Daily energy expenditure and physical activity in survivors of childhood malignancy. Pediatric Research. 1998;19:607–613. doi: 10.1203/00006450-199805000-00008. [DOI] [PubMed] [Google Scholar]
- Whitt-Glover MC, Taylor WC, Floyd MF, Yore MM, Yancey AK, Matthews CE. Disparities in physical activity and sedentary behaviors among U.S. children and adolescents: Prevalence, correlates, and intervention implications. Journal of Public Health Policy. 2009;30:S309–334. doi: 10.1057/jphp.2008.46. [DOI] [PubMed] [Google Scholar]
- Winter C, Muller C, Hoffmann C, Boos J, Rosenbaum D. Physical activity and childhood cancer. Pediatric Blood & Cancer. 2010;54:501–510. doi: 10.1002/pbc.22271. [DOI] [PubMed] [Google Scholar]