Abstract
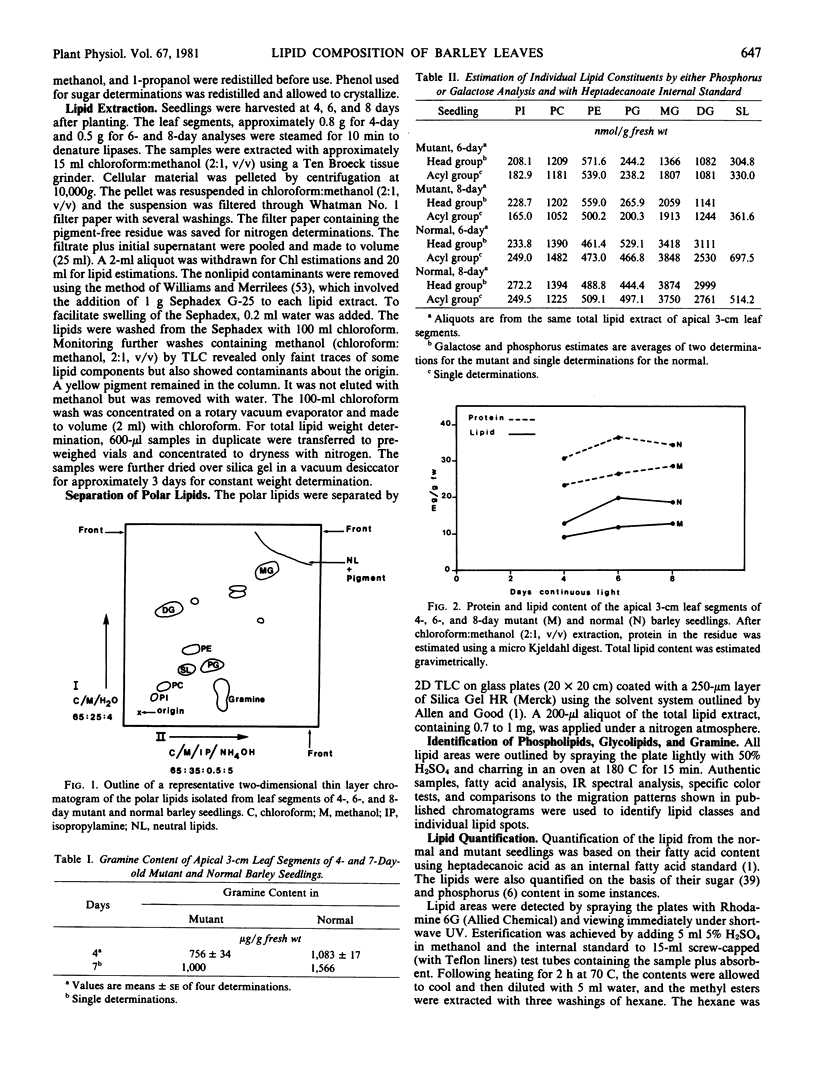
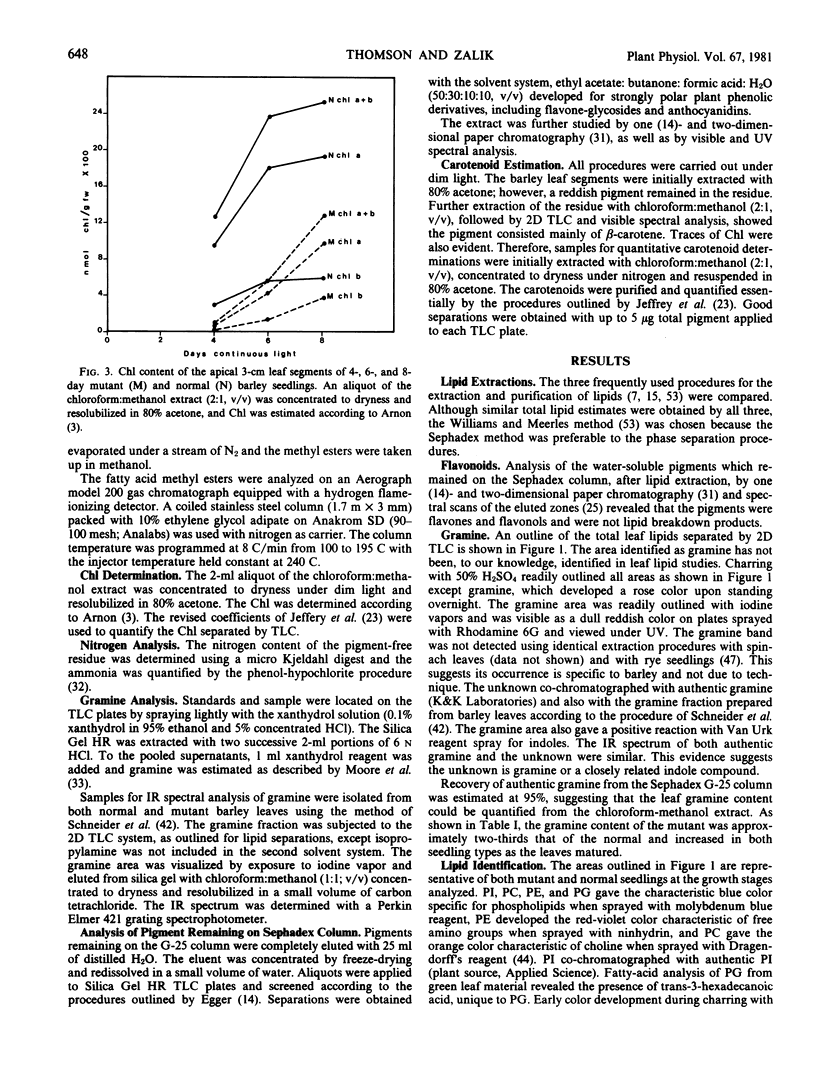
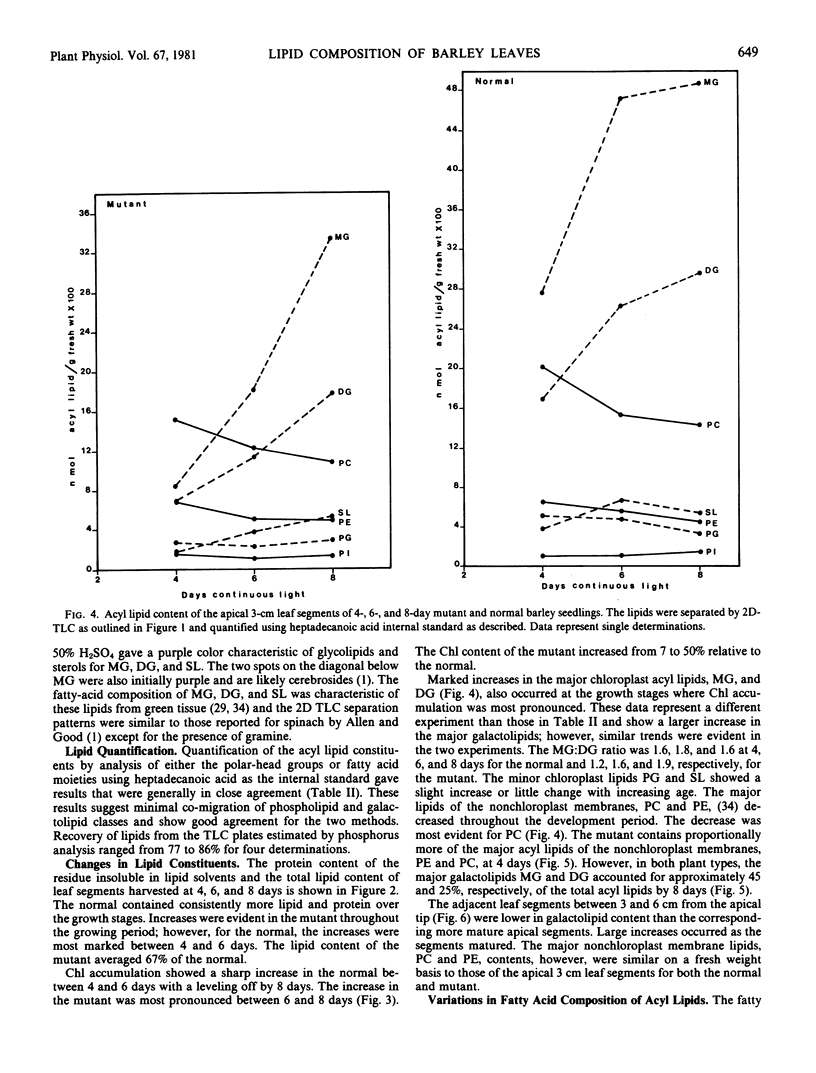
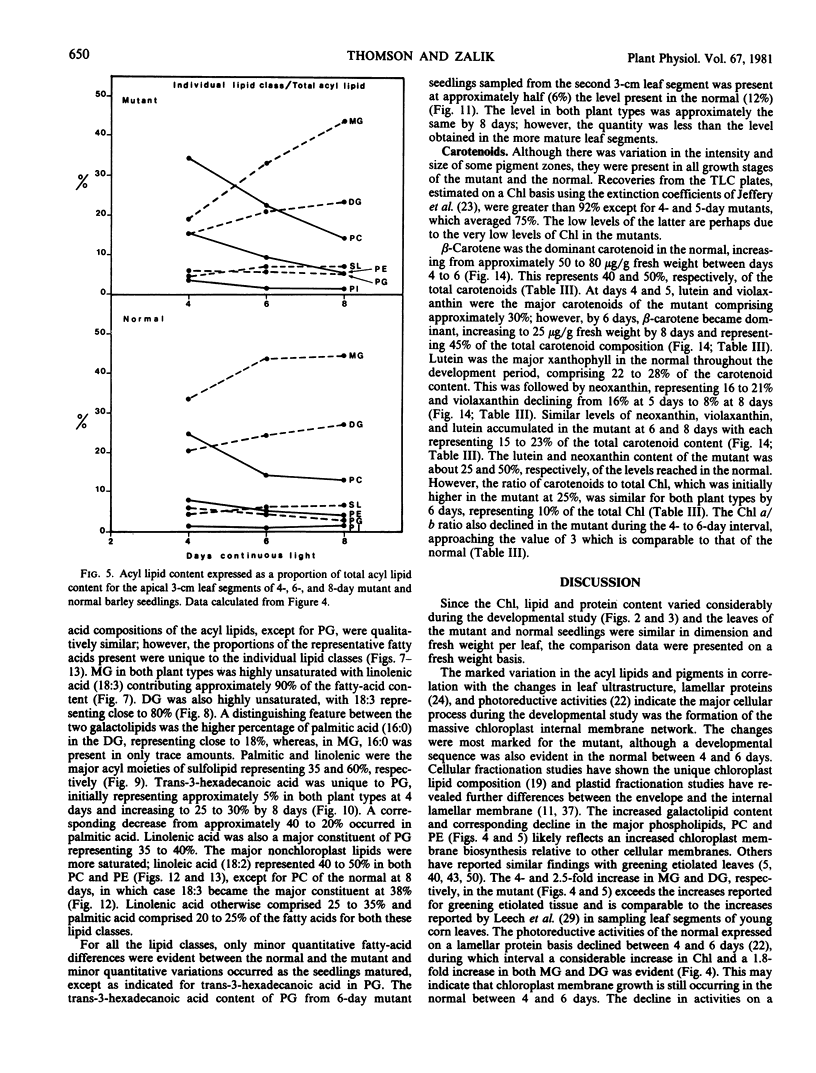
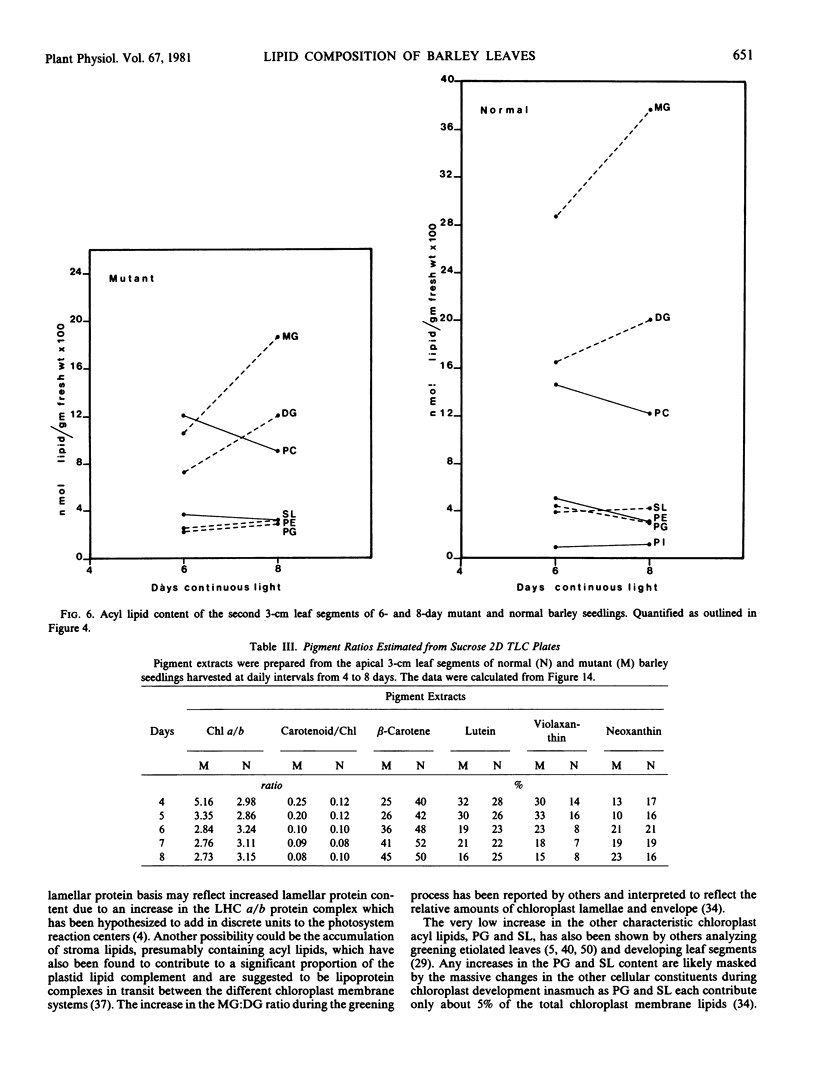
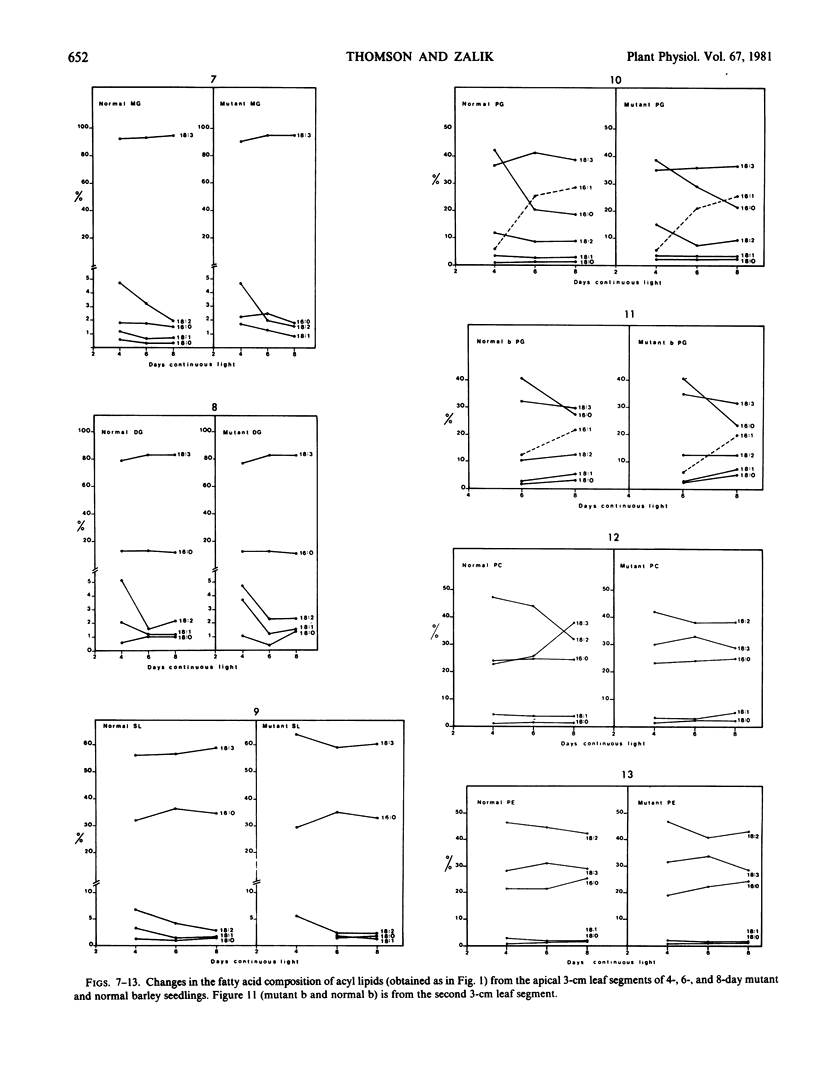
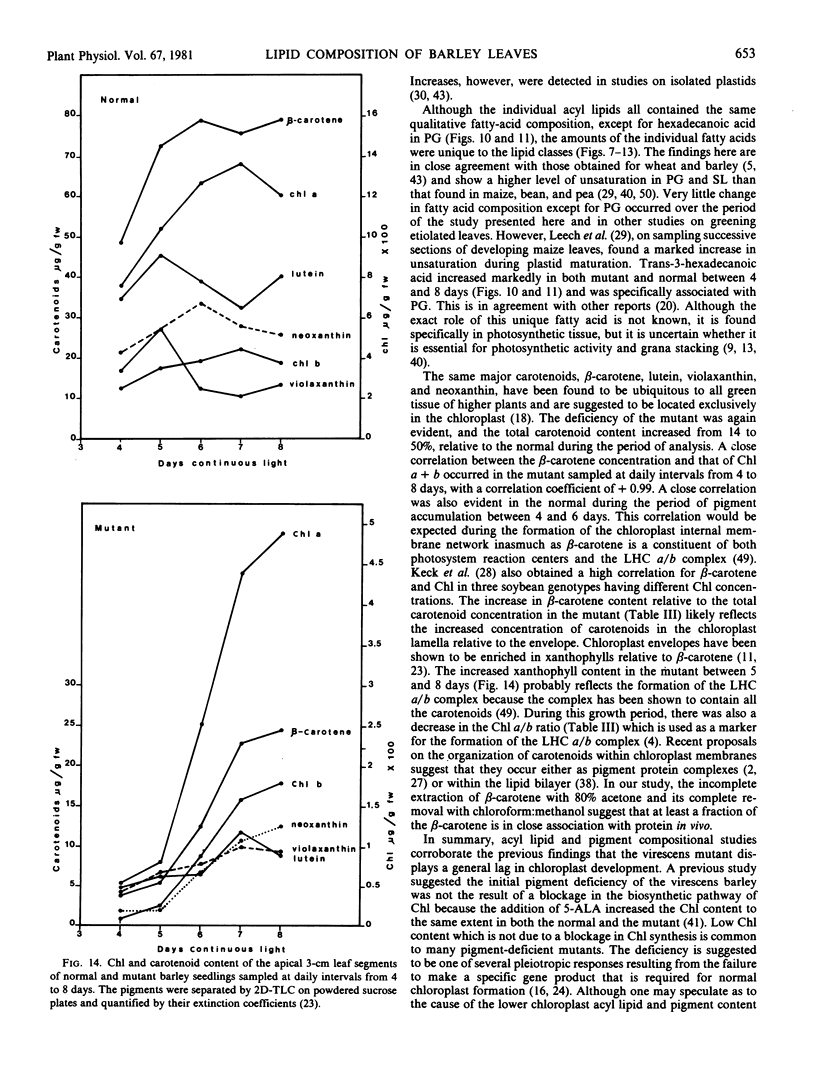
Changes in acyl lipids and pigments during leaf development in a virescens barley mutant (M) and the normal (N) were studied. Apical 3-cm leaf segments were extracted with chloroform-methanol, the extracts were purified on Sephadex G-25 columns, and the polar lipids were separated on two-dimensional-thin layer chromatography silica gel plates. The pigment remaining on the Sephadex column was identified as flavonoids and a zone on the TLC plates which did not correspond to the usual standards was identified as gramine. Quantification of acyl lipids by either polar head group analysis or fatty acid analysis using heptadecanoate as an internal standard gave similar results. The per cent of the total lipid extract quantified for the M between 4 and 8 days ranged from 46 to 65% and that for the N ranged from 60 to 68%. Of these, acyl lipids represented 37 to 48% in the M and 43 to 50% in the N. By 8 days, mono- and digalacto-syldiglyceride (MG and DG) accounted for 45 and 25% of the total acyl lipid of both the M and N. For the period of study here, this represented a 4-fold increase in MG and a 2.5-fold increase in DG in the M but only a 1.8-fold increase for MG and DG in the N. These increases were closely correlated with the increases in chlorophyll. Chlorophyll increased sharply between 4 and 6 days for the N, whereas, in the M, it rose from 7 to 50% relative to the normal by 8 days. The proportions of the various fatty acids were unique for the lipid classes. The only major quantitative change for a fatty acid was for hexadecanoate in phosphatidylglycerol which increased from 5% at 4 days to 25 to 30% by 8 days. Relative to the N, the carotenoid content of the M increased from 14 to 50% between 4 and 8 days. In both the M and N, the increase in β-carotene and chlorophyll were closely correlated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bolton P., Wharfe J., Harwood J. L. The lipid composition of a barley mutant lacking chlorophyll b. Biochem J. 1978 Jul 15;174(1):67–72. doi: 10.1042/bj1740067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Holtz R. B., Benson A. A. Isolation and properties of the envelope of spinach chloroplasts. J Biol Chem. 1973 Oct 25;248(20):7215–7222. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Harwood J. L., James A. T. Metabolism of trans-3-hexadecenoic acid in broad bean. Eur J Biochem. 1975 Jan 2;50(2):325–334. doi: 10.1111/j.1432-1033.1975.tb09807.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lucas C., De Caleya R. F., Carbonero P., Garcia-Olmedo F. Control of galactosyl diglycerides in wheat endosperm by group 5 chromosomes. Genetics. 1977 Mar;85(3):521–527. doi: 10.1093/genetics/85.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey S. W., Douce R., Benson A. A. Carotenoid transformations in the chloroplast envelope. Proc Natl Acad Sci U S A. 1974 Mar;71(3):807–810. doi: 10.1073/pnas.71.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck R. W., Dilley R. A. Chloroplast composition and structure differences in a soybean mutant. Plant Physiol. 1970 Nov;46(5):692–698. doi: 10.1104/pp.46.5.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R. M., Rumsby M. G., Thomson W. W. Plastid differentiation, acyl lipid, and Fatty Acid changes in developing green maize leaves. Plant Physiol. 1973 Sep;52(3):240–245. doi: 10.1104/pp.52.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leese B. M., Leech R. M. Sequential changes in the lipids of developing proplastids isolated from green maize leaves. Plant Physiol. 1976 May;57(5):789–794. doi: 10.1104/pp.57.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. M., Williams J. D., Chia J. Factors affecting concentrations of dimethylated indolealkylamines in Phalaris tuberosa L. Aust J Biol Sci. 1967 Dec;20(6):1131–1140. doi: 10.1071/bi9671131. [DOI] [PubMed] [Google Scholar]
- Murphy D. J., Stumpf P. K. Light-dependent Induction of Polyunsaturated Fatty Acid Biosynthesis in Greening Cucumber Cotyledons. Plant Physiol. 1979 Feb;63(2):328–335. doi: 10.1104/pp.63.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poincelot R. P. Isolation and lipid composition of spinach chloroplast envelope membranes. Arch Biochem Biophys. 1973 Nov;159(1):134–142. doi: 10.1016/0003-9861(73)90437-2. [DOI] [PubMed] [Google Scholar]
- Rohmer M., Bouvier P., Ourisson G. Molecular evolution of biomembranes: structural equivalents and phylogenetic precursors of sterols. Proc Natl Acad Sci U S A. 1979 Feb;76(2):847–851. doi: 10.1073/pnas.76.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Batt R. D. Quantitative analysis of sulfolipid (sulfoquinovosyl diglyceride) and galactolipids (monogalactosyl and digalactosyl diglycerides) in plant tissues. Anal Biochem. 1968 Jan;22(1):74–88. doi: 10.1016/0003-2697(68)90261-3. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Boardman N. K. Lipid Composition of Pea and Bean Leaves during Chloroplast Development. Plant Physiol. 1972 Jul;50(1):31–34. doi: 10.1104/pp.50.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson L. W., Zalik S. Acetyl coenzyme a carboxylase activity in developing seedlings and chloroplasts of barley and its virescens mutant. Plant Physiol. 1981 Apr;67(4):655–661. doi: 10.1104/pp.67.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson L. W., Zalik S. Lipids in rye seedlings in relation to vernalization. Plant Physiol. 1973 Sep;52(3):268–273. doi: 10.1104/pp.52.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trémolières A., Lepage M. Changes in Lipid Composition during Greening of Etiolated Pea Seedlings. Plant Physiol. 1971 Feb;47(2):329–334. doi: 10.1104/pp.47.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein D. V., Kahn A., Nielsen O. F., Gough S. Genetic regulation of chlorophyll synthesis analyzed with mutants in barley. Science. 1974 May 17;184(4138):800–802. doi: 10.1126/science.184.4138.800. [DOI] [PubMed] [Google Scholar]
- Williams J. P., Merrilees P. A. The removal of water and nonlipid contaminants from lipid extracts. Lipids. 1970 Apr;5(4):367–370. doi: 10.1007/BF02532100. [DOI] [PubMed] [Google Scholar]


