Summary
Background
The aim of this study was to investigate the utility of diffusion weighted imaging (DWI) using Apparent Diffusion Coefficient (ADC) values in discriminating between patients with tumors and normal prostate tissue before the initial systematic core biopsy. The relationship between histological grade of prostate cancer and ADC values in the peripheral zone was also investigated.
Material/Methods
Our study included 62 patients who underwent magnetic resonance imaging (MRI) of the pelvis. The examinations were performed in T1-, T2-weighted, DWI and T1 after dynamic contrast administration sequences. In all patients there were abnormal foci within the peripheral zone determined in DWI/ADC. ADC values were compared with the Gleason score (GS) after core needle biopsy (CNB) in patients with low, medium and high stage tumors.
Results
Within the examined group of patients, ADC was statistically higher for normal tissue than for cancerous tissue (p=0.00). Mean ADC values for patients with low, intermediate and high GS were 0.85±0.03, 0.72±0.03, and 0.61±0.04, respectively.
Conclusions
DWI/ADC is useful in differentiating high-risk patients from those at low and intermediate risk, since there is a significant correlation between ADC values determined in patients included in three different groups according to their Gleason score. This information may be helpful in the assessment of prostate cancer aggressiveness.
MeSH Keywords: Magnetic Resonance Imaging, Neoplasm Grading, Prostatic Neoplasms
Background
Prostate cancer is the second, considering frequency, malignant cancer both in Poland and in the USA. Specific difficulties associated with detecting this type of cancer are encountered due to its growth pattern, different from other cancers.
The Gleason score (GS), which represents the sum of dominant and subdominant histological patterns is used to grade pathological appearance of prostate tumors. Aggressiveness and high potential of spreading (local and distant) of the tumor are reflected by high Gleason score. What is more, high GS is also associated with increased risk for the patients [1]).
Except highly aggressive tumors with high GS (>7, high grade), there are also low-grade tumors (GS ≤6), and intermediate-grade tumors (GS=7). The likelihood of prostate cancer recurrence after primary treatment increases with tumor aggressiveness [2].
Prostate cancer is most frequently located in the peripheral zone and has a tendency to grow along the anatomical capsule and to have a rectangular shape. About 85% of tumors are multifocal [3,4].
Surprisingly, prostate cancer can have different growth patterns: nodular – well seen in TransRectal UltraSound (TRUS) examination, infiltrating nodular – in that case only the nodule without infiltration is visible in TRUS; and infiltrating tumor, which remains very difficult to detect.
It is worth to remember that prostate cancer can develop also in the transitional or central zone, as well as in the anterior septum and in such cases the tumor is very difficult to detect in TRUS. That is the reason why new methods for prostate cancer detection are still being searched for.
Up to now, the best method used for prostate gland evaluation and diagnostics has been magnetic resonance imaging (MRI), since it allows to differentiate soft tissues with high contrast, examine them in multiplanar views and provide biological information, which makes this method superior to other methods [5].
Diagnostic accuracy, sensitivity (76%) and specificity (82%) in the detection of prostate cancer have been improved and this method combines data from T2-weighted imaging and diffusion weighted imaging (DWI) [6,7].
DWI provides important information about the movement and functional environment of water in tissue and reflects cellular status of normal and pathological tissue. In conventional MR imaging, diffusion of water molecules in the tissues has an extremely small contribution to the MR signal. In DWI, powerful magnetic gradients with echo planar sequence are used providing images that are dependent on water diffusion. Reduced diffusion of water in prostate cancer has been attributed to increased cellularity of malignant lesions that restricts water motion in a reduced extracellular space. Moreover, DWI also provides an important quantitative biophysical parameter, called the apparent diffusion coefficient of water (ADC). Apparent diffusion coefficient (ADC) value is a quantitative parameter of DWI representing water diffusion in extracellular and extravascular space and capillary perfusion. ADC values have been shown to be decreased in various malignancies of different organs due to hypercellularity. The main advantages of DWI as a functional imaging technique over other modalities are ability to assess molecular diffusion in vivo and to provide information about biophysical features of the examined tissue, mainly about cell organization, density and microstructure [8].
Prostate cancer has a low signal intensity on diffusion-weighted images, which means restricted diffusion. Such a diffusion pattern often correlates with fibrous tissue or reflects the degree of cellularity and macromolecular density. Diffusion imaging allows for differentiation between hemorrhage and tumor located in the peripheral zone [9].
The aim of this study was to investigate the utility of DWI using ADC values in discriminating between patients with tumors and normal prostate tissue before the initial systematic core biopsy. The relationship between histological grade of prostate cancer and ADC values in the peripheral zone was also investigated.
Material and Methods
Patients
Our material included data of 62 patients aged 54 to 84 years (mean 65±0.89), who underwent MRI of the pelvis between May 2011 and August 2012 at the Centre of Oncology of Maria Sklodowska-Curie Memorial Institute in Cracow, Poland. The mean serum PSA value equaled 14.83 ng/mL (min 2.25 ng/mL; max 56.12 ng/mL) (Table 1).
Table 1.
Characteristics of patients who underwent prostate MRI.
| Characteristics | N=62 | p |
|---|---|---|
| Age (years) | 0.00 | |
| ≤65 yrs. | 35 | |
| 65–70 yrs. | 13 | |
| 71–75 yrs. | 10 | |
| >75 | 4 | |
| Gleason score | 0.00 | |
| ≤5 | 6 | |
| 6 | 22 | |
| 7 | 26 | |
| 8–10 | 8 | |
| Tumor stage related to Gleason score | 0.00 | |
| Low | 28 | |
| Medium | 26 | |
| High | 8 | |
| Initial PSA (ng/mL) | 0.00 | |
| ≤4 | 4 | |
| 4–10 | 25 | |
| 10–20 | 20 | |
| >20 | 13 |
Imaging technique
All MRI examinations were performed with a 1.5-T magnet (Avanto; Siemens, Erlangen, Germany) by using the multichannel body matrix phased-array coil. The examinations were performed in T1-WI, T2-WI, DWI and T1-weighted sequences after dynamic contrast administration. ADCs were calculated from transverse DWI obtained by using a single-shot echo-planar imaging sequence with the following parameters: TR/TE: 3400/75 ms and b values of 0, 100, 300, 800 and 1000 sec/mm2. Full echo information was obtained with a bandwidth of 1220 Hz/pixel and a matrix size of 256×256. The field of view was 220 mm, with 4-mm section thickness and no intersection gap, covering the entire prostate and seminal vesicles. T1-WI images were assessed to exclude bleeding signs among patients. Prostate cancer was diagnosed in T2-weighted images, dynamic contrast-enhanced MRI and DWI according to standard criteria, and lesion location was determined in accordance with the biopsy result as a golden standard. In MRI, lesions considered as prostate cancer complied with the following criteria: in T2-weighted images, round, oval, or triangular lesions with a low signal intensity in the peripheral zone, in DWI examinations – areas with a higher signal intensity than that surrounding the peripheral zone and a low-signal-intensity area in ADC examination (Figures 1 and 2).
Figure 1.
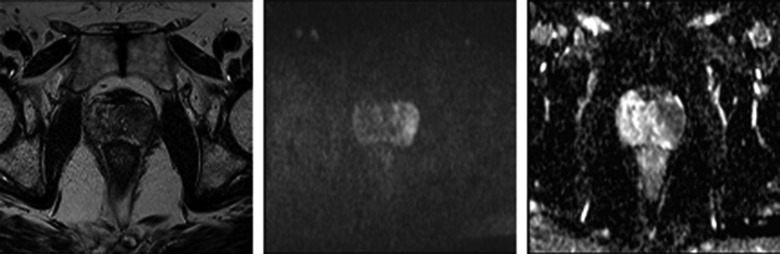
A 68-year-old man with PSA of 16.3 ng/mL; (A) T2-weighted transversal image shows a hypo-intense nodule in the left lateral peripheral zone, indicative of malignancy; (B) DWI with b=800; (C) ADC map: in the peripheral zone, on the left side, a dark area is visible (value of 0.7 mm2/s).
Figure 2.
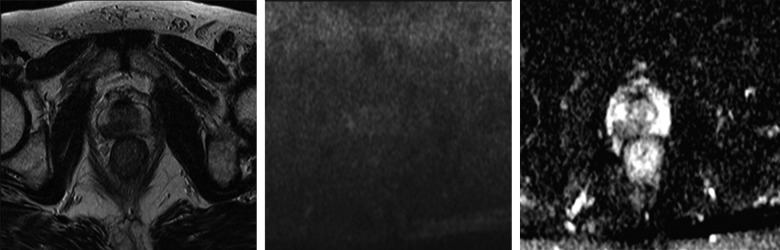
A 66-year-old man with PSA of 12.1 ng/mL; (A) T2-weighted transversal image shows a hypo-intense nodule in the right lateral peripheral zone, indicative of malignancy; (B) DWI with b=800; (C) ADC map: in the peripheral zone, on the right side, a dark area is visible (value of 0.82 mm2/s).
In all patients, an abnormal focus in DWI/ADC within the peripheral zone was determined. Its borders were outlined by hand. Similarly, an area of healthy, normal tissue of the peripheral zone was outlined in order to compare numerical values. A radiologist with a 10-year experience in prostate imaging with TRUS and MRI techniques and a urologist with a 15-year experience in TRUS and CNB under TRUS guidance established unanimously all regions of interests (ROIs) relevant to the areas.
After CNB a prostate cancer was diagnosed in all patients. ADCs values were compared with the Gleason score after CNB in patients with low, intermediate and high stage tumors. ADC values were also compared between patients who were treated with radiotherapy and surgery, taking into consideration whether ADC values influence treatment management.
Histopathology
Following radical prostatectomy, haematoxylin-eosin stained specimens were examined microscopically in every case. Those slides were produced by step section of the gland from the apex to the base every 5 mm. An oversized slide containing the entire short axis of the gland was produced from each 5-mm section. Specimens were examined by one of the two experienced pathologists dedicated to this study and blinded to ADC results. The histological assessment of the cancer was based on the Gleason system [10].
Statistics
Statistical analysis was performed using Statistica version 10 (StatSoft, Poland). In all statistical procedures, the p value of <0.05 was considered statistically significant. A chi-square test was used to verify an equal number of patients within the groups based on age, Gleason score and PSA. A t-test was used to estimate differences between mean values of ADC and surface area for normal and cancerous tissue and also their relation to treatment option. A one-way ANOVA was used to analyze whether mean ADC values depend on clinical and histological features of the cancer. The results of the correlation between ADC value and surface area were analysed using the Student’s t-test. A ROC (Receiver Operating Characteristic) analysis was performed in order to examine whether the ADC value can be used to differentiate between less and more aggressive cancers. Patients were divided into two groups of less and more aggressive tumors according to the Gleason score. A ROC curve was drawn and the size of the area under ROC curve was examined using the Z-test.
Results
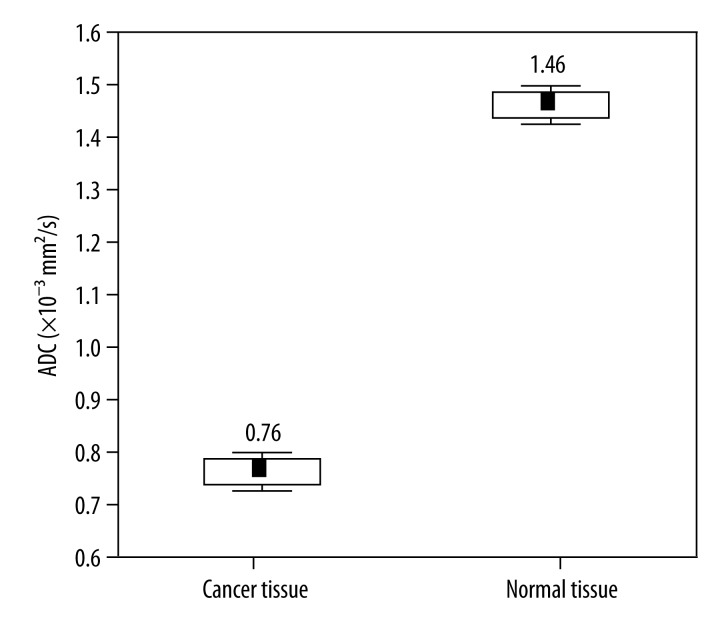
Mean ADC values for normal and cancerous tissue and mean values of the surface area are presented in Table 2
Table 2.
Mean values of ADC and surface area of imaging for normal and tumor tissue.
| Type of tissue | ADC | Surface area of imaging |
|---|---|---|
| Normal | 1.46±0.02 | 0.60±0.03 |
| Tumor | 0.76±0.02 | 0.56±0.03 |
| p | 0.000 | 0.087 |
Within the examined group of patients ADC was statistically higher for normal tissue than for tumor tissue (p=0.00) (Figure 3).
Figure 3.
Box plots of ADC values for cancer and normal tissue. Horizontal lines indicate mean ±SE and mean ±1.96*SE (standard error – SE) which is the border of the 95% confidence interval.
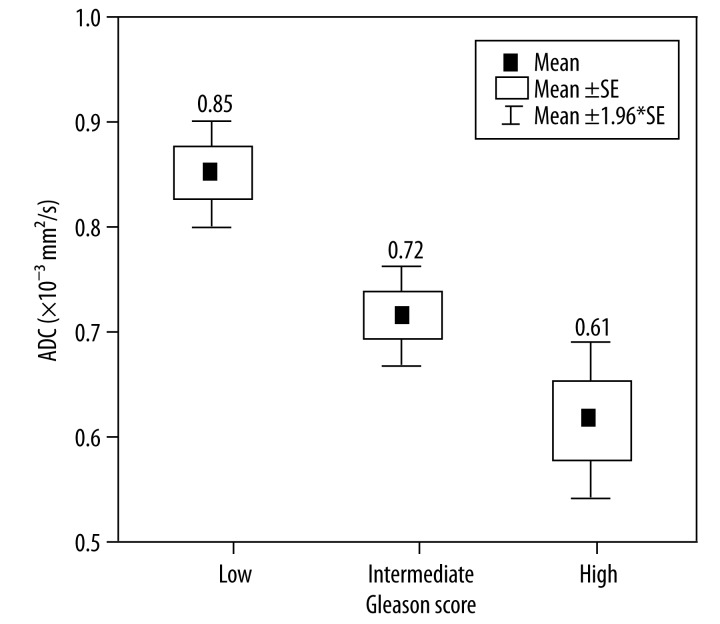
Mean values of ADC for patients with low-grade GS were 0.85±0.03, for patients with medium GS 0.72 ± 0.03, and 0.61±0.04 for patients with high GS.
The mean value of ADC depends on prostate cancer stage in accordance with the Gleason score (p=0.00) (Figure 4).
Figure 4.
Comparison of ADC values for low, intermediate and high Gleason score. Horizontal lines indicate mean ±SE and mean ±1.96*SE which is a border of the 95% confidence interval.
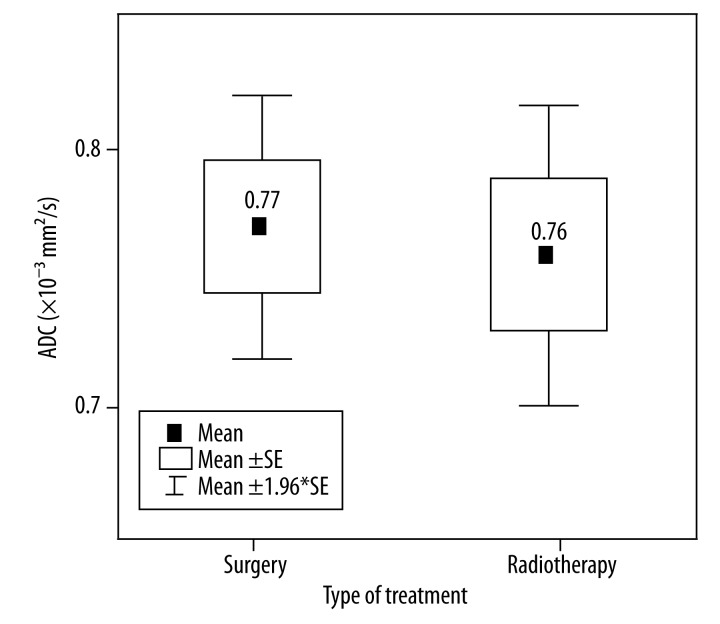
Twenty-nine patients underwent surgical treatment and the mean value of ADC for those patients was 0.77±0.03, while radiotherapy was administered in 33 patients, with the mean value of ADC equal to 0.76±0.03 (Figure 5).
Figure 5.
Box plots of ADC values for surgery and radiotheraphy. Horizontal lines indicate mean ±SE and mean ±1.96*SE which is a border of the 95% confidence interval.
There was no significant difference between mean ADC values in both types of treatment, surgery or radiotherapy (p=0.89 and p=0.75, respectively). However, patients with higher ADC and lower stage of cancer are more often treated with surgical procedures. Consequently, patients who undergo radiotherapy have lower ADC values but higher stage in GS.
ROC curve for ADC values
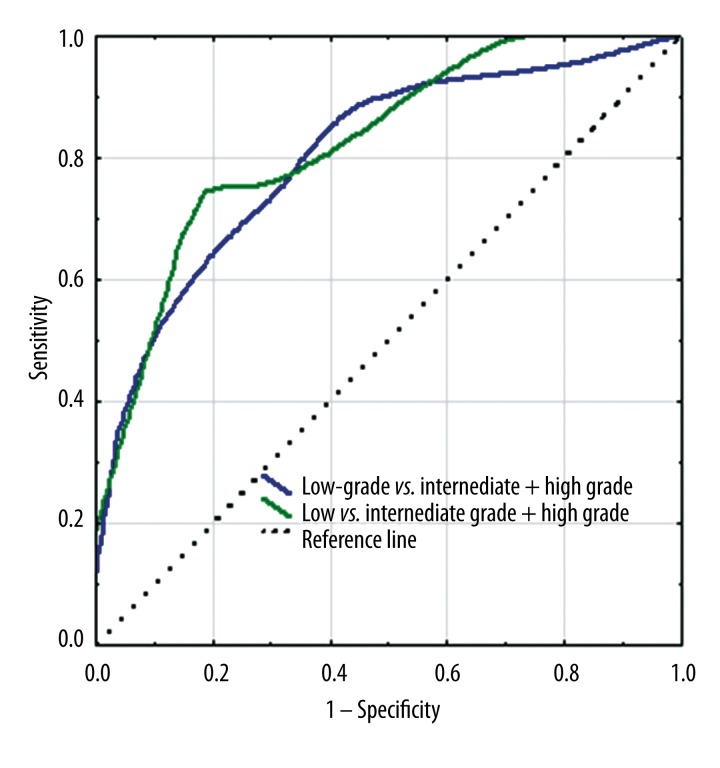
We evaluated whether ADC results allow to determine cancer stage. The patients were divided into two groups using two different ways, depending on cancer stage in GS. The first way: patients with low Gleason score (Gleason, ≤6) were compared with patients with more advanced disease (GS=7 together with GS >7). The second way: patients with less advanced disease (a combined group of patients with low GS ≤6 and medium GS=7) were compared with patients with high GS >7. The ROC curve of the analysed parameter located closer to the left upper corner indicates higher precision of examination considering the parameter given (100% of specificity and sensitivity), which results in a bigger area under ROC curve (AUC – area under curve) (Figure 6).
Figure 6.
ROC curves of ADC value. The blue line shows the ROC curve calculated to differentiate low, intermediate, and high grade. The green line shows the ROC curve calculated to differentiate low, intermediate – and high grade. The dashed line shows the control line (A control line represents the ROC curve for random distribution of cases in more and less aggressive tumors).
Discussion
The study included 62 patients with elevated serum PSA level, who underwent MRI examination before TRUS/CNB. Prostate cancer was determined in all patients. Except T1-, T2-weighted sequences, and T1 after contrast medium administration, DWI/ADC was also performed in MRI examination. The area of abnormality visible in the peripheral zone in ADC examination was drawn by hand, as well as normal prostate gland tissue. The examination revealed that mean ADC values are significantly lower in healthy and abnormal tissue (p=0.00). The obtained data are consistent with the literature, since in other publications, higher average ADC was determined in healthy tissue of the peripheral zone (less overlap with cancerous tissue) in comparison to the transition zone and prostate base. Differentiation of prostate cancer from noncancerous tissue is limited by overlap [11].
Differences in ADC values between patients with low, intermediate and highly malignant disease (p=0.000) are consistent with the literature data, which state that higher Gleason score is associated with low ADC, probably due to specific infiltrative growth of prostate tumors, assumed as dedifferentiated. Such an organization is opposite to the glandular organization of more well-differentiated prostate cancer, which is more similar to healthy prostatic tissue [12,13].
The statistical significance of the correlation between ADC value and treatment option was not confirmed in our study. However, we noticed a tendency of qualifying patients with higher ADC value (which means lower stage) to radical prostatectomy and patients with lower ADC values (higher stage) to radiotherapy.
Examinations were performed with a 1.5 T MRI Avanto Siemens. Although the 3.0 T device is known to be more accurate and to have higher resolution, the results of our studies show that examining the patient with the 1.5 T apparatus and performing not only T1 and T2 images but also DWI/ADC allows for differentiating between cancerous and noncancerous tissue, similarly to the 3.0 apparatus. Literature data confirm that using the transrectal coil for examination is more accurate than using the Flex Body coil. However, this examination is much more expensive and due to financial limitations is not widely available. The other reason is patient inconvenience.
There are many publications related to the significance of DWI and ADC in prostate cancer diagnostics. The literature reports on importance of DWI/ADC in prostate cancer diagnostics, especially in case of difficulties encountered with classical imaging aimed at tumor localization in the peripheral zone.
In some publications ADC values are divided into benign and malignant. It is also proved, that DWI and ADC mapping can increase the sensitivity (54–98%) and specificity (58–100%) of MR imaging in detection of prostate cancer when diffusion-weighted imaging is used in conjunction with T2-weighted imaging [14]. Based on the initial results, it is possible that diffusion-weighted imaging has a potential to increase the specificity of prostate cancer detection and to support prediction of tumor aggressiveness [15,16].
Similarly to other publications, we found a correlation between cancer stage in the Gleason score and ADC value in our material. There is a statistically significant negative correlation between the mean ADC value and the Gleason score of tumors. Increased tumor cellularity, structural change of gland stroma (which is found to be more fibrous), and disorganization of texture causing a relatively more restricted motion of water molecules within high Gleason score tumors could explain such a correlation. What is more, there is a significant difference between mean ADC values of low, intermediate, and high clinical risk tumors [17,18]. It was confirmed by the results of Tamada et al. 2 who also reported a negative correlation between ADC values and Gleason score. Mazaheri et al. [19] and deSouza et al. also demonstrated significant differences between the two groups at 1.5 T after comparing ADC values for low- and high-risk prostate cancers. Our material analysis showed that although we examined the patients with 1.5 T MRI we are able to diagnose them with prostate cancer [21,22]. If there is no diffusion restriction in the peripheral zone, and serum PSA level is elevated but not increasing, it is possible to choose MRI examination as a follow-up treatment option instead of performing CNB under MRI guidance.
We are aware that our study has several limitations. Among them there was the number of patients, which was not satisfactory, and so was the radiologist experience in MRI assessment. Secondly, the study was retrospective. Another one was the lack of possibility to perform CNB under MRI guidance. Not all patients underwent surgical procedure, which is also considered as a limitation. Due to the last two limitations it is not entirely certain whether the material obtained during TRUS CNB was taken from the area of restricted diffusion in MRI examination.
Conclusions
DWI may be helpful in differentiating high-risk patients from those at low and intermediate risk, since there is a significant correlation between ADC values determined in patients included in three different groups according to their Gleason scores. This information may be useful in the assessment of prostate cancer aggressiveness.
References
- 1.Epstein JI, Allsbrook WC, Amin MB, Egevad LL. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29(9):1228–42. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 2.Bianco FJ, Wood DP, Cher ML, et al. Ten-year survival after radical prostatectomy: specimen Gleason score is the predictor in organ-confined prostate cancer. Clin Prostate Cancer. 2003;1(4):242–47. doi: 10.3816/cgc.2003.n.006. [DOI] [PubMed] [Google Scholar]
- 3.Byar DP, Mostofi FK. Carcinoma of the prostate: prognostic evaluation of certain pathologic features in 208 radical prostatectomies. Examined by the step-section technique. Cancer. 1972;30(1):5–13. doi: 10.1002/1097-0142(197207)30:1<5::aid-cncr2820300103>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.McNeal JE, Redwine EA, Freiha FS, Stamey TA. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am J Surg Pathol. 1988;12(12):897–906. doi: 10.1097/00000478-198812000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Wang S, Peng Y, Medved M, et al. Hybrid multidimensional T2 and diffusion-weighted MRI for prostate cancer detection. J Magn Reson Imaging. 2014;39(4):781–88. doi: 10.1002/jmri.24212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibbs P, Liney GP, Pickles MD, et al. Correlation of ADC and T2 measurements with cell density in prostate cancer at 3.0 Tesla. Invest Radiol. 2009;44(9):572–76. doi: 10.1097/RLI.0b013e3181b4c10e. [DOI] [PubMed] [Google Scholar]
- 7.Wu L-M, Xu J-R, Ye Y-Q, et al. The clinical value of diffusion-weighted imaging in combination with T2-weighted imaging in diagnosing prostate carcinoma: a systematic review and meta-analysis. Am J Roentgenol. 2012;199(1):103–10. doi: 10.2214/AJR.11.7634. [DOI] [PubMed] [Google Scholar]
- 8.Le Bihan D, Breton E, Lallemand D, et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168(2):497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 9.Miller FH, Hammond N, Siddiqi AJ, et al. Utility of diffusion-weighted MRI in distinguishing benign and malignant hepatic lesions. J Magn Reson Imaging. 2010;32(1):138–47. doi: 10.1002/jmri.22235. [DOI] [PubMed] [Google Scholar]
- 10.Erbay G, Koc Z, Karadeli E, et al. Evaluation of malignant and benign renal lesions using diffusion-weighted MRI with multiple b values. Acta Radiologica. 2012;53(3):359–65. doi: 10.1258/ar.2011.110601. [DOI] [PubMed] [Google Scholar]
- 11.Gibbs P, Tozer DJ, Liney GP, Turnbull LW. Comparison of quantitative T2 mapping and diffusion-weighted imaging in the normal and pathologic prostate. Magn Reson Med. 2001;46(6):1054–58. doi: 10.1002/mrm.1298. [DOI] [PubMed] [Google Scholar]
- 12.Outwater EK, Montilla-Soler JL. Imaging of prostate carcinoma. Cancer Control J Moffitt Cancer Cent. 2013;20(3):161–76. doi: 10.1177/107327481302000304. [DOI] [PubMed] [Google Scholar]
- 13.Spencer JA, Chng WJ, Hudson E, et al. Prostate specific antigen level and Gleason score in predicting the stage of newly diagnosed prostate cancer. Br J Radiol. 1998;71(851):1130–35. doi: 10.1259/bjr.71.851.10434906. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Kim JK, Park B-W, et al. Apparent diffusion coefficient: prostate cancer versus noncancerous tissue according to anatomical region. J Magn Reson Imaging. 2008;28(5):1173–79. doi: 10.1002/jmri.21513. [DOI] [PubMed] [Google Scholar]
- 15.Tamada T, Sone T, Jo Y, et al. Apparent diffusion coefficient values in peripheral and transition zones of the prostate: comparison between normal and malignant prostatic tissues and correlation with histologic grade. J Magn Reson Imaging. 2008;28(3):720–26. doi: 10.1002/jmri.21503. [DOI] [PubMed] [Google Scholar]
- 16.Rosenkrantz AB, Kopec M, Kong X, et al. Prostate cancer vs. post-biopsy hemorrhage: diagnosis with T2- and diffusion-weighted imaging. J Magn Reson Imaging. 2010;31(6):1387–94. doi: 10.1002/jmri.22172. [DOI] [PubMed] [Google Scholar]
- 17.Haider MA, van der Kwast TH, Tanguay J, et al. Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. Am J Roentgenol. 2007;189(2):323–28. doi: 10.2214/AJR.07.2211. [DOI] [PubMed] [Google Scholar]
- 18.DEsouza NM, Reinsberg SA, Scurr ED, et al. Magnetic resonance imaging in prostate cancer: the value of apparent diffusion coefficients for identifying malignant nodules. Br J Radiol. 2007;80(950):90–95. doi: 10.1259/bjr/24232319. [DOI] [PubMed] [Google Scholar]
- 19.Nagarajan R, Margolis D, Raman S, et al. Correlation of Gleason scores with diffusion-weighted imagAssessing the aggressiveness of tumour: particularly when biopsies may have under-sampled tumour with important surgical implicationsing findings of prostate cancer. Adv Urol. 2012;2012:374805. doi: 10.1155/2012/374805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Amico AV, Moul J, Carroll PR, et al. Cancer-specific mortality after surgery or radiation for patients with clinically localized prostate cancer managed during the prostate-specific antigen era. J Clin Oncol. 2003;21(11):2163–72. doi: 10.1200/JCO.2003.01.075. [DOI] [PubMed] [Google Scholar]
- 21.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 22.Mazaheri Y, Hricak H, Fine SW, et al. Prostate tumor volume measurement with combined T2-weighted imaging and diffusion-weighted MR: correlation with pathologic tumor volume. Radiology. 2009;252(2):449–57. doi: 10.1148/radiol.2523081423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.deSouza NM, Riches SF, Vanas NJ, et al. Diffusion-weighted magnetic resonance imaging: a potential non-invasive marker of tumour aggressiveness in localized prostate cancer. Clin Radiol. 2008;63(7):774–82. doi: 10.1016/j.crad.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 24.D’Onofrio M, Gallotti A, Mantovani W, et al. Perfusion CT can predict tumoral grading of pancreatic adenocarcinoma. Eur J Radiol. 2013;82(2):227–33. doi: 10.1016/j.ejrad.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 25.Turkbey B, Shah VP, Pang Y, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology. 2011;258(2):488–95. doi: 10.1148/radiol.10100667. [DOI] [PMC free article] [PubMed] [Google Scholar]