Abstract
Background
Mitigating urethral injury remains a great challenge for urologists due to lack of ideal biomaterials for urethroplasty. The application of amniotic membrane (AM) over other synthetic materials makes it a better potential source for urethral reconstruction. We separated the basement layer of AM to obtain denuded human amniotic scaffold (dHAS) and then inoculated primary rabbit urethral epithelial cells on the surface of dHAS to determine whether this strategy minimizes potential rejection and maximizes the biocompatibility of human AM.
Material/Methods
After the successful acquisition of dHAS from AM, cell-seeded dHAS were prepared and characterized. Both cell-seeded dHAS and acellular dHAS were subcutaneously implanted. Immune responses were compared by histological evaluation and CD4+ cell and CD8+ cell infiltrations. Then they were applied as urethroplastic materials in the rabbit models of urethral injury to fully explore the feasibility and efficacy of tissue-engineered dHAS xenografts in urethral substitution application.
Results
Mild inflammatory infiltration was observed in cell-seeded dHAS grafts, as revealed by fewer accumulations of CD4+ cells and CD8+ cells (or neutrophils or other immune cells). Urethral defects of rabbits in the urethroplastic group with dHAS implantation (n=6) were completely resolved in 1 month, while there were 1 infection and 1 fistula in the control group with acellular dHAS patches (n=6). Histopathological analysis revealed mild immune response in the cell-seeded dHAS group (P<0.05).
Conclusions
Tissue-engineered dHAS minimizes potential rejection and maximizes the biocompatibility of AM, which makes it a potential ideal xenograft for urethral reconstruction.
MeSH Keywords: Amnion; Anastomosis, Surgical; Immunity, Active; Transplantation, Heterologous; Urethral Stricture
Background
Urethral injury results from various acquired and congenital abnormalities, including trauma, infection, cancer, and hypospadias. The reconstruction of urethral injuries remains a great challenge for urologists, owing to the difficulties of a successful urethroplasty that achieves desirable outcomes without complications such as fistula and stricture [1,2]. Although diverse remedies such as direct suture, transurethral resection, and urethral substitution with different tissues or xenografts have been applied to maintain urethra continuity during reconstructive surgery, few are highly effective or reliable, especially in the management of a long urethral defect [3–5].
Amniotic membrane (AM), a biocompatible material that has been intensively investigated in ophthalmology and dermatology reconstructive surgery, shows great potential for urethral reconstruction [6,7]. The basic structure of AM consists of 3 layers: epithelial layer, basement layer, and avascular stromal layer. It has been demonstrated that AM exhibits excellent properties of reducing inflammation, scarring, and the risk of rejection [8], as well as facilitating the migration, localization, and proliferation of epithelial cells, which largely depend on the function of the basement layer [9]. Amniotic membranes have been applied successfully in the reconstruction of long ureteral strictures in humans [10,11].
However, the use of human AM is relatively infrequent due to the shortage of tissue sources. AM xenografts prepared from non-human donors can dramatically expand AM sources for clinical use, but few studies have achieved satisfactory results using AM xenotransplantation in urethral reconstruction, probably due to anti-xenoresponses [11,12]. To overcome this limitation, we separated the basement layer of AM to obtain denuded human amniotic scaffold (dHAS), then engrafted primary rabbit urethral epithelial cells on the surface of dHAS to minimize potential xenograft rejection and maximize the biocompatibility of human AM. Both dHAS and cell-seeded dHAS were then applied as urethroplastic materials in rabbit models of urethral injury to fully explore the feasibility and efficacy of tissue-engineered dHAS in urethral substitution application.
Material and Methods
Animals and experimental groups
All animal experiments were approved by the ethics committee and conducted in accordance with guidelines of the Fourth Military Medical University for animal experiments. Twenty male New Zealand white rabbits weighing 2.5–3.5 kilograms each were purchased from the experimental animal center of the Fourth Military Medical University and housed individually in a temperature-controlled cage with 50–55% humidity and a 12-h light–dark cycle, with free access to standard commercial feed and tap water. Eight New Zealand rabbits underwent subcutaneous implantation of dHAS (n=4) and human AM (n=4). Others were randomly segregated into 2 groups for urethral reconstruction surgery: the experimental group receiving tissue-engineered dHAS (n=6) and the control group receiving intact AM patches (n=6).
Preparation of denuded human amniotic scaffold
Human AM was obtained with informed consent from mothers before delivery. The dHAS was prepared and preserved using a method described elsewhere [13]. In brief, the human placenta was obtained immediately after delivery with negative serologic tests for human immunodeficiency virus, human hepatitis type B and C, and syphilis. Under sterile conditions, the AM was washed with 0.01M phosphate buffer solution (PBS, Invitrogen) containing antibiotic-antimycotic liquid solution (penicillin 50 μg/mL, streptomycin 50 μg/mL, neomycin 100 μg/mL, and amphotericin B 2.5 μg/mL, Sigma, St. Louis, USA). Part of the human AM was then deprived of amniotic epithelial cells to obtain dHAS by mixed digestive solution (0.25% trypsin +0.02% ethylene diamine tetraacetic acid; Sigma, St. Louis, USA) at a temperature of 37°C for 2 hours. Finally, the prepared dHAS was rewashed 3 times with PBS.
Characterizations of denuded human amniotic scaffold
Scanning electron microscopy (SEM) and hematoxylin-eosin (HE) and immunofluorescence staining were performed to characterize the surface structure and histology of dHAS. For SEM analysis, the sample was fixed on a stub with double-sided carbon tape. After the sample was spattered with an ultrathin gold layer, the surface structure of dHAS was observed under a SEM S-3400N (Hitachi, Tokyo, Japan) at an accelerating voltage of 5 kV for imaging. For histological analysis, 6-μm polylysine-coated frozen sections were obtained from dHAS embedded in optimal cutting temperature (OCT) compound (Leica, Nussloch, Germany) and subjected to hematoxylin-eosin staining. For immunofluorescence assay, 6-μm polylysine-coated frozen sections were fixed with cold acetone for 15 min and blocked with 10% goat serum for 30 min. Sections were then incubated at room temperature for 1 h with primary antibodies: rabbit anti-human collagen I, collagen III, and collagen IV polyclonal antibody, mouse anti-human fibronectin, vascular endothelial growth factor monoclonal antibody (1:500, Santa Cruz, CA). Appropriate nonspecific mouse or rabbit IgG (Dako, Kyoto, Japan) at the same concentration was used as control. After washing 3 times over a period of 15 min in PBS containing 0.15% Triton X-100 (Sigma, USA), sections were incubated at room temperature for 1 h with a fluorescent secondary antibody (Rhodamine Red-X goat anti-mouse or rabbit IgG; 1:500, Invitrogen). Sections were then rewashed 3 times with PBS and mounted using anti-fade mounting medium containing propidium iodide (Vectashield; Vector, Burlingame, CA). Finally, sections were observed under an Olympus BX51 fluorescence microscope (Olympus, Tokyo, Japan).
Inoculation of primary rabbit urethral epithelium cells to dHAS
After i.p. anesthetization and disinfection of male New Zealand white rabbits, mucous membrane (4×3 mm) was harvested from the posterior wall of the urethra and rinsed repeatedly with D-Hanks solution containing 50 μg/mL penicillin, 50 μg/mL streptomycin, and 2.5 μg/mL amphotericin B (Life Technologies Inc., Gaithersburg, MD) followed by disintegration using 2.24 U/ml Dispase II for 30 min (Gibco-Invitrogen, Carlsbad, CA). Then the epithelium layer was carefully separated from the mucous membrane and cut into pieces. Mixed digestive solution was used to digest the epithelial layer. Cell suspension was collected and cultured in Dulbecco’s Modified Eagle Medium; Nutrient Mix F-12 medium (containing N2, 15% fetal bovine serum, hydrocortisone, L-glutamin, and β-mercaptoethanol; Invitrogen, Grand Island, NY) under a humidified environment containing 5% CO2 at 37°C. Cell culture with epithelium origin was confirmed by immunofluorescence with cytokeratin 18. For the following inoculation, 3–5 passages of rabbit urethral epithelium cells were used. Briefly, 1 ml of cell suspension (105 cells/ml) was dripped on the surface of dHAS placed in a 6-cm petri dish. Then the cell-seeded sheet was cultured for 2 weeks in the previously mentioned medium and environment to obtain tissue-engineered dHAS.
Histocompatibility comparison of dHAS and cell-seeded dHAS
The histocompatibility was investigated by the implantation of dHAS and cell-seeded dHAS subcutaneously into 2 groups of 4 male New Zealand white rabbits each weighing 2.5–3.5 kg. The rabbit that underwent intraperitoneal anesthesia with ketamine (dose: 5 mg/kg) and Xylazine (dose: 8 mg/kg) was placed on the operating table in prone position. After shaving, disinfection with povidone-iodine scrub and covering the rest of its body with sterile drapes, 1-cm vertical incisions were made at both dorsal parts spaced 3 cm away from the middle line of the vertebrae, with a deep reaching aponeurotic fascia. Two lacunas were made by blunt dissection of skin and subcutaneous tissue. Prepared materials (1×1 cm) were then implanted into both lacunas, followed by the suture of incisions and sterilization. Finally, the rabbits were caged alone until regaining consciousness.
Xenografts were obtained 1, 2, 4, and 8 weeks after implantation surgery in rabbits. The grafts were fixed in 4% paraformaldehyde and then underwent HE staining for histological analyses. Immunohistochemical analysis of CD4+ and CD8+ cells was performed to assess the intensity of immune response using goat anti-rabbit CD4, CD8 monoclonal antibodies (1:500) and HRP conjugated mouse anti-goat IgG (1:500, Biolegend, San Diego, CA). The sections were visualized with DAB staining (Dako, Kyoto, Japan). Positive cells were marked by brown color. Semi-quantification of the percentage of CD4+ and CD8+ cells were assessed by Image Pro Plus 6.0 software [14].
Urethral reconstruction using cell-seeded dHAS in a rabbit urethral injury model
The surgical procedures of urethral injury model and urethroplasty have been described in detail elsewhere [15]. Briefly, after i.p. anesthetization and local preparation of the lower abdomen, a transurethral Fr6 catheter was inserted into the bladder through the urethral orifice. Under an operating microscope with 2.5× magnification, a 5×10 mm defect that exposed the catheter was made on the ventral part of the urethra 1 cm away from the distal urethral meatus with the penis on stretch. Human AM graft or tissue-engineered human amniotic scaffold was covered onto the defect, which was closed by interrupted suturing with absorbable sterile Vicryl 6-0 suture.
The urethral catheter was fixed to the glans of the penis by silk 4-0 with a short 1-cm protrusion to avoid dislocation caused by movement of the rabbit. The rabbit was caged alone until it awoke. Daily antibiotic injections (penicillin 50 000 unit/kg) were started at the time of surgery and continued for 1 week. One week after urethroplasty, the catheter was removed. Rabbits were closely observed for abnormal mental status, growth, diet, defecation, and the development of infection and fistula. At intervals of 2 weeks and again at 3 months after surgery, implants in the rabbits were collected for histological examinations.
Statistical analysis
Numerical data are described as Mean ±SEM. The t test was used for the comparison of statistical significance between 2 groups. The value of statistical significance was set at P less than 0.05.
Results
Preparation of dHAS
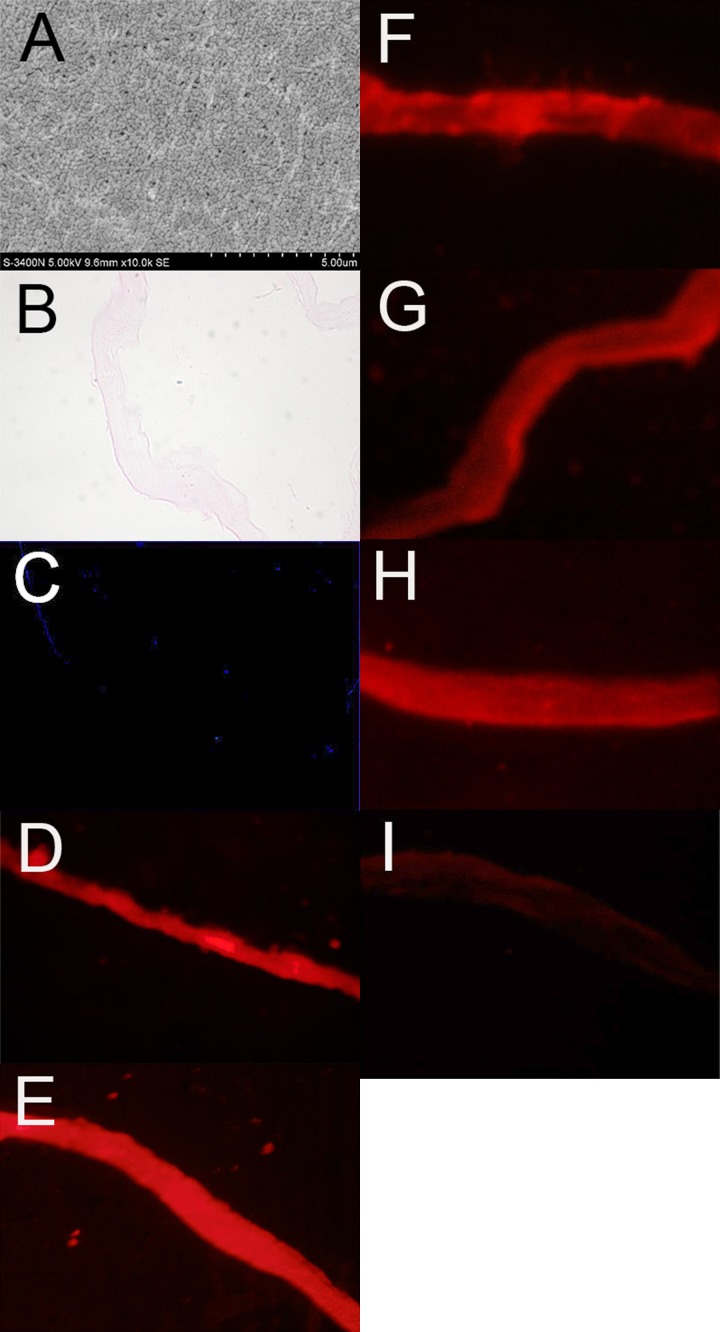
Trypsinization with mixed digestive solution successfully removed the epithelial layer of the amniotic membrane, as revealed by SEM and HE staining (Figure 1A, 1B). Nuclei staining using DAPI revealed the absence of epithelial cells (Figure 1C). The expression patterns of extracellular matrix molecules and growth factor in dHAS were characterized by immunofluorescence imaging (Figure 1C–1I). Ubiquitous expressions of collagen (types I, III, and IV), fibronectin, and vascular endothelial growth factor (VEGF) were demonstrated, although fibronectin and VEGF expressions were lower compared to collagen expression. No positive staining was found under the microscopic field in negative controls.
Figure 1.
(A–F) Characterizations of denuded human amniotic scaffold (dHAS). (A) Scanning electron micrograph of dHAS showed a continuous flat layer of smooth basement membrane; (B) Hematoxylin-eosin (HE) staining of dHAS confirmed a complete removal of the epithelial layer of amniotic membrane (AM); (C) DAPI staining revealed the absence of epithelial cells; (D) immunofluorescence of Collagen I expression in dHAS; (E) Collagen III; (F) Collagen IV. (G–I) Characterizations of denuded human amniotic scaffold (dHAS). (G) Fibronectin; (H) VEGF; (I) negative control group incubated with nonspecific IgG (magnification at ×400).
Inoculation of rabbit urethral epithelial cells to dHAS
Rabbit urethral epithelial cells were successfully collected after trypsinization. The immunostaining using cytokeratin 18 confirmed the epithelial origin of harvested cells (Figure 2A). In the first few days of co-culture with rabbit urethral epithelial cells and dHAS, slow growth of urethral epithelial cells was observed. By the seventh day, colonies of urethral epithelial cells began to form on the surface of dHAS. After 2 weeks of co-culture, a confluent layer of urethral epithelial cells was found on the entire surface of the dHAS sheet (Figure 2B).
Figure 2.
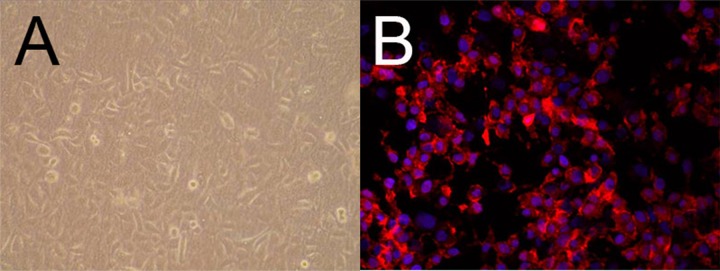
Co-culture and characterization of rabbit urethral epithelial cells on dHAS. (A) dHAS seeded with rabbit urethral epithelial cells were observed under an inverted microscope 2 weeks after co-culture; (B) Cell culture with epithelium origin was confirmed by red fluorescence of cytokeratin 18 (blue: DAPI, magnification at ×400).
Histocompatibility studies of dHAS
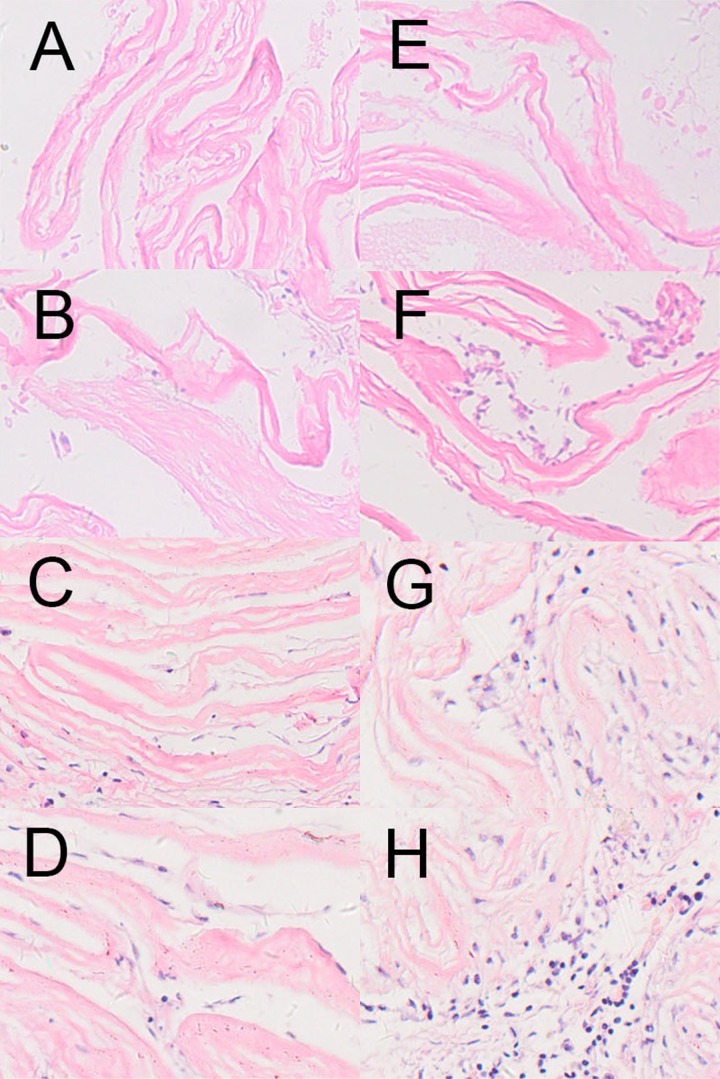
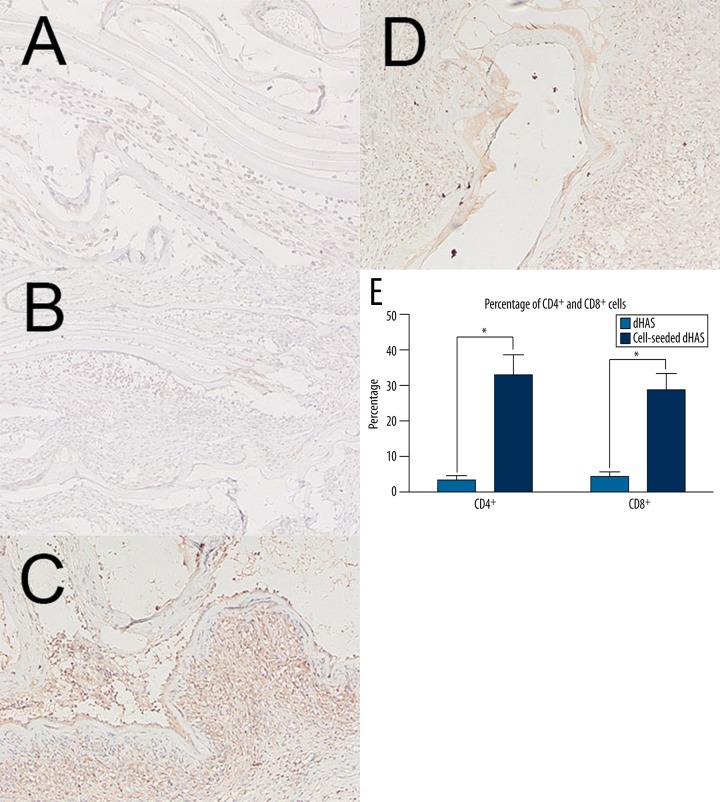
To investigate the histocompatibility of cell-seeded dHAS, the prepared dHAS and cell-seeded dHAS were implanted subcutaneously to posterior portions of rabbits. In the follow-up studies with duration up to 8 weeks, no serious inflammation or rejection was observed in the cell-seeded dHAS implantation group, as indicated by HE staining (Figure 3), as well as the scant infiltration of CD4+ and CD8+ cells revealed through immunohistochemical analysis (Figure 4). Apparent accumulation of CD4+ and CD8+ cells was found in the group that underwent AM implantation. There was a significant statistical difference (p<0.05) in CD4+ and CD8+ infiltrations between these 2 groups (Figure 4E).
Figure 3.
HE staining of the cell-seeded dHAS and dHAS after subcutaneous implantation. (A–D): HE stainings of cell-seeded dHAS engrafts at week 1, 2, 4, and 8 after subcutaneous implantation; (E–H): HE stainings of dHAS engrafts at week 1, 2, 4, and 8. Fewer inflammatory infiltrations in dHAS xenografts were found, in comparison to AM grafts (magnification at ×200).
Figure 4.
Immunohistochemical analyses of CD4+ cell and CD8+ cell infiltrations. (A) CD4+ staining in cell-seeded dHAS engrafts; (B) CD8+ staining in cell-seeded dHAS engrafts; (C) CD4+ staining in dHAS engrafts; (D) CD8+ staining in dHAS engrafts (magnification at ×200); (E) Semi-quantitative results revealed fewer CD4+ cell and CD8+ cell infiltrations in cell-seeded dHAS engrafts after subcutaneous implantation at week 2, indicating a lower immune response. In contrast, there was significant accumulation of CD4+ cells and CD8+ cells in dHAS group (* P<0.05).
Application of cell-seeded dHAS for urethroplasty
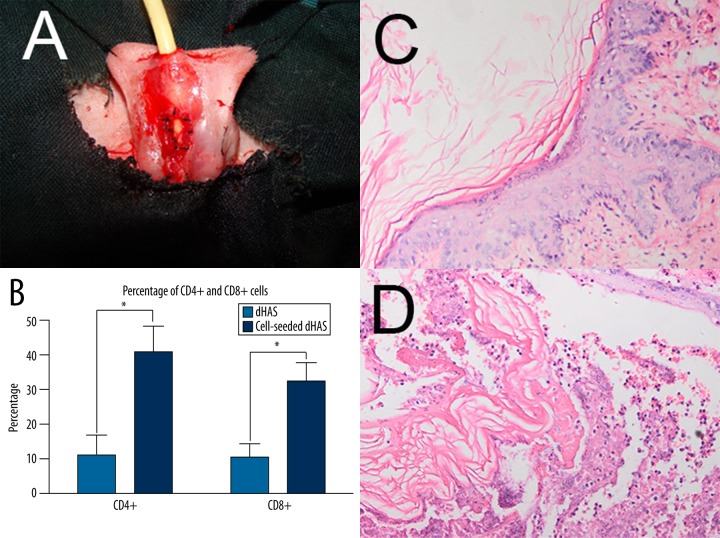
Urethral injury was induced and then urethroplasty was performed successfully in all rabbits (Figure 5A). They exhibited normal behaviors during the 3-month follow-up examination. One rabbit developed serious infection and another was found with fistula in the group that received dHAS patches. Neither infection nor fistula was observed in the group with cell-seeded dHAS implantation. Two weeks after surgery, cell-seeded dHAS was intact without obvious inflammatory cell infiltration, as confirmed by immunohistochemical evaluation of CD4+ cell and CD8+ cell infiltrations (Figure 5B). The anastomosis repair between the defective and normal urethral tissue was revealed by HE staining in the cell-seeded dHAS group (Figure 5C), in contrast to few repairs in the control group using dHAS (Figure 5D). Three months after surgery, the urethral defect was completely repaired in the cell-seeded dHAS group. The formation of a smooth muscular layer and rich blood vessels was apparent.
Figure 5.
Urethral reconstruction using cell-seeded dHAS in a rabbit urethral injury model. (A) Urethral reconstruction surgery utilizing a cell-seeded dHAS patch. (B) immunohistochemical evaluation of CD4+ and CD8+ infiltrations 2 weeks after urethroplasty. Significant accumulation of CD4+ cells and CD8+ cells in the dHAS group were found (* P<0.05); (C) HE staining of cell-seeded dHAS graft 2 weeks after surgery. The graft was intact without obvious infiltration of inflammatory cells. The continuity of epithelial cells was recovered (magnification at ×200); (D) HE staining of dHAS patch 2 weeks after surgery revealed serious inflammatory infiltration (magnification at ×200).
Discussion
Urethral injury that originates from either congenital abnormality or acquired disease negatively affects the quality of life for patients. To best retain urethral function, reconstructive surgery is required [16]. Autologous tissues, such as buccal mucosa, intestine and small intestine submucosa, and biodegradable materials are widely accepted as patches for urethroplasty to close the long defects of the urethra [3,17]. Nonetheless, additional surgeries to obtain these substitutes add complexity to the operation and put patients at risk of various complications such as serious infection, intestinal obstruction, urethral stricture, metabolic disturbance, and chronic renal failure [5,18,19]. Moreover, the presence of bowel in the urethra elevates the risk of fistula formation, lithiasis, and cancers [20]. Therefore, it remains a critical challenge to develop novel biomaterials for effective urethra reconstruction.
Human amniotic membrane has been accepted as a reliable, safe, and available material for tissue repair with relatively low immunogenicity. The first documented application of AM in urethroplasty involved female urethral reconstruction with AM grafts in year 2000, which was considered easy, quick, and effective for reconstructive surgery [21]. Koziak et al. successfully used cryopreserved human amniotic membrane for the reconstruction of extensive ureteral wall defects in 11 patients with rare complications [10]. Excretory urography showed the unobstructed state and normal width of the postoperative urethra. Applications of amniotic membrane as xenograft for urethroplasty were also described in a rabbit model of urethral injury. One in 20 rabbits experienced infection, followed by fistula formation, and 2 urethral strictures [15]. In Chen’s study, the role of intact human amniotic membrane in repairing ureteric defect was explored in a rabbit urethral injury model. Fresh human amnions were clipped into pieces and sutured around the wound, using epidural tubes as a stent. Twenty-two out of 36 rabbits successfully developed a functional urethra without significant contraction 3 months after surgery [22]. However, the potential xenorejection remains a critical issue in using AM xenografts as patches for the management of urethral injury [23].
The basement membrane of the amniotic membrane contains collagens, laminin, fibronectin, multiple growth factors, and high molecular weight hyaluronan with immunosuppressive properties that can facilitate the adhesion, migration, and proliferation of epithelial cells, promote epithelial differentiation, and prevent epithelial apoptosis [24]. Natural inhibitors of metalloproteinases in the matrix of AM stabilize the expression of matrix metalloproteinases in the inflammatory environment, playing a crucial role in regulation of the healing process [25]. Therefore, tissue-engineered dHAS has been developed and used as an optimal xenograft for rabbit urethral reconstruction. The modified dHAS presents favorable biocompatibility, likely due to the absence of human epithelial cells that exhibit pro-inflammatory capability. Moreover, dHAS retains the advantages of AM for cell cultivation and regrowth. These findings corroborate with previous reports revealing that denuded AM is a more amenable substrate for the cultivation of corneal epithelial cells than intact AM, human peritoneum, or omentum [26,27]. In comparison with other allografts and xenografts such as buccal mucosa, bladder mucosa, and intestine, tissue-engineered dHAS is readily available and safe for urethroplasty, with the advantages of unsophisticated surgical techniques required for its application, thereby reducing the operation time, mitigating postoperative patient care, and, most importantly, lowering the risk of serious postoperative complications.
Conclusions
Cell-seeded dHAS displays good biocompatibility and may be an ideal implant for urethral reconstruction with low immunogenicity. The use of dHAS seeded with epithelial cells from the recipients may effectively mitigate potential immune response. The major drawback in this experimental study is that the dHAS was seeded with rabbit urethral epithelium cells and tested in rabbits, so we are not able to initiate a human-based investigation. Moreover, a longer period of postoperative observation might be required to uncover latent adverse effects and complications. Therefore, these preliminary results are still far from the stage of clinical application. Confirmation on a larger scale with longer follow-up is necessary to facilitate future investigations of tissue-engineered dHAS as a satisfactory implant for human urethral reconstruction.
Acknowledgments
We acknowledge support from the Shanxi Province Technological Pooling Fund 2012KTCL03-03 and the NSFC (Natural Science Foundation of China) 81220108011, NSFC 81370039, and the linguistic modification of Raph de White.
Footnotes
Source of support: Shanxi Province technological pooling fund 2012KTCL03-03; NSFC (Natural Science Foundation of China) 81220108011; NSFC 81370039
References
- 1.Atala A. Tissue engineering in urologic surgery. Urol Clin North Am. 1998;25:39–50. doi: 10.1016/s0094-0143(05)70431-6. [DOI] [PubMed] [Google Scholar]
- 2.Schoeneich G, Winter P, Albers P, et al. Management of complete ureteral replacement. Experiences and review of the literature. Scand J Urol Nephrol. 1997;31:383–88. doi: 10.3109/00365599709030625. [DOI] [PubMed] [Google Scholar]
- 3.Palminteri E, Berdondini E, Colombo F, Austoni E. Small Intestinal Submucosa (SIS) Graft Urethroplasty: Short-term Results. Eur Urol. 2007;51:1695–701. doi: 10.1016/j.eururo.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Mehrsai A, Djaladat H, Salem S, et al. Outcome of buccal mucosal graft urethroplasty for long and repeatedstricture repair. Urology. 2007;69:17–21. doi: 10.1016/j.urology.2006.09.069. [DOI] [PubMed] [Google Scholar]
- 5.Chung BI, Hamawy KJ, Zinman LN, Libertino JA. The use of bowel for ureteral replacement for complex ureteral reconstruction: long-term results. J Urol. 2006;175:179–83. doi: 10.1016/S0022-5347(05)00061-3. [DOI] [PubMed] [Google Scholar]
- 6.Tseng SC, Prabhasawat P, Barton K, et al. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998;116:431–41. doi: 10.1001/archopht.116.4.431. [DOI] [PubMed] [Google Scholar]
- 7.Huang G, Ji S, Luo P, et al. Accelerated expansion of epidermal keratinocyte and improved dermal reconstruction achieved by engineered amniotic membrane. Cell Transplant. 2013;22:1831–44. doi: 10.3727/096368912X657945. [DOI] [PubMed] [Google Scholar]
- 8.Koizumi NJ, Inatomi TJ, Sotozono CJ, et al. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20:173–77. [PubMed] [Google Scholar]
- 9.Mohammadi AA, Seyed Jafari SM, Kiasat M, et al. Effect of fresh human amniotic membrane dressing on graft take in patients with chronic burn wounds compared with conventional methods. Burns. 2013;39:349–53. doi: 10.1016/j.burns.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Koziak A, Salagierski M, Marcheluk A, et al. Early experience in reconstruction of long ureteral strictures with allogenic amniotic membrane. Int J Urol. 2007;14:607–10. doi: 10.1111/j.1442-2042.2007.01781.x. [DOI] [PubMed] [Google Scholar]
- 11.Salehipour M, Mohammadian R, Jahanbini S, et al. Is amniotic membrane a suitable biomaterial for reconstruction of long ureteral defects? Saudi J Kidney Dis Transpl. 2013;24:135–38. doi: 10.4103/1319-2442.106311. [DOI] [PubMed] [Google Scholar]
- 12.Scalea J, Hanecamp I, Robson SC, Yamada K. T-cell-mediated immunological barriers to xenotransplantation. Xenotransplantation. 2012;19:23–30. doi: 10.1111/j.1399-3089.2011.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura T, Yoshitani M, Rigby H, et al. Sterilized, freeze-dried amniotic membrane: a useful substrate for ocular surface reconstruction. Invest Ophthalmol Vis Sci. 2004;45:93–99. doi: 10.1167/iovs.03-0752. [DOI] [PubMed] [Google Scholar]
- 14.Yi X, Zhang G, Yuan J. Renoprotective role of fenoldopam pretreatment through hypoxia-inducible factor-1alpha and heme oxygenase-1 expressions in rat kidney transplantation. Transplant Proc. 2013;45:517–22. doi: 10.1016/j.transproceed.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 15.Shakeri S, Haghpanah A, Khezri A, et al. Application of amniotic membrane as xenograft for urethroplasty in rabbit. Int Urol Nephrol. 2009;41:895–901. doi: 10.1007/s11255-009-9532-2. [DOI] [PubMed] [Google Scholar]
- 16.Li ZL, Fu DL, Chong T, Li HC. Reconstruction for the distal urethral end incorrectly anastomosed to the proximal false passage in the treatment of urethral stricture. Am J Case Rep. 2014;15:239–42. doi: 10.12659/AJCR.890378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L, Li N, Lei T, et al. The in vitro biological properties of Mg-Zn-Sr alloy and superiority for preparation of biodegradable intestinal anastomosis rings. Med Sci Monit. 2014;20:1056–66. doi: 10.12659/MSM.890638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiala R, Vidlar A, Vrtal R, et al. Porcine small intestinal submucosa graft for repair of anterior urethral strictures. Eur Urol. 2007;51:1702–8. doi: 10.1016/j.eururo.2007.01.099. [DOI] [PubMed] [Google Scholar]
- 19.Hauser S, Bastian PJ, Fechner G, Müller SC. Small intestine submucosa in urethral stricture repair in a consecutive series. Urology. 2006;68:263–66. doi: 10.1016/j.urology.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 20.Tanagho EA. A case against incorporation of bowel segments into the closed urinary system. J Urol. 1975;113:796–802. doi: 10.1016/s0022-5347(17)59582-8. [DOI] [PubMed] [Google Scholar]
- 21.Brandt FT, Albuquerque CD, Lorenzato FR. Female urethral reconstruction with amnion grafts. Int J Surg Investig. 2000;1:409–14. [PubMed] [Google Scholar]
- 22.Chen WG, Hou JQ, Pu JX, et al. Reconstruction of rabbit ureter using human amnion. Journal of Clinical Rehabilitative Tissue Engineering Research. 2009;13:217–20. [Google Scholar]
- 23.Sartoneva R, Haimi S, Miettinen S, et al. Comparison of a poly-L-lactide-co-epsilon-caprolactone and human amniotic membrane for urothelium tissue engineering applications. J R Soc Interface. 2011;8:671–77. doi: 10.1098/rsif.2010.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Litwiniuk M, Grzela T, Brawura-Biskupski-Samaha R. Clinical immunology: Chronic inflammation in venous leg ulcer – problems and perspectives. Centr Eur J Immunol. 2009;34:247–51. [Google Scholar]
- 25.Litwiniuk M, Bikowska B, Niderla-Bielinska J, et al. Potential role of metalloproteinase inhibitors from radiationsterilized amnion dressings in the healing of venous leg ulcers. Mol Med Rep. 2012;6:723–78. doi: 10.3892/mmr.2012.983. [DOI] [PubMed] [Google Scholar]
- 26.Koizumi N, Fullwood NJ, Bairaktaris G, et al. Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Invest Ophthalmol Vis Sci. 2000;41:2506–13. [PubMed] [Google Scholar]
- 27.Sharifiaghdas F, Hamzehiesfahani N, Moghadasali R, et al. Human amniotic membrane as a suitable matrix for growth of mouse urothelial cells in comparison with human peritoneal and omentum membranes. Urol J. 2007;4:71–78. [PubMed] [Google Scholar]