Abstract
Recent whole-genome analyses in Drosophila suggest that critical developmental control genes sometimes contain “shadow” enhancers [1]. These can be located in remote positions, including the introns of neighboring genes. They nonetheless produce patterns of gene expression that are the same or similar as those produced by more proximal primary enhancers. It was suggested that shadow enhancers help foster robustness in gene expression in response to environmental or genetic perturbations [2,3]. Here, we critically test this hypothesis by employing a combination of BAC recombineering and quantitative confocal imaging methods [2,4]. Evidence is presented that the snail gene is regulated by a distal shadow enhancer located within the neighboring Tim17b2 locus. snail encodes a zinc finger transcription factor that has been implicated in epithelial/mesenchyme transitions (EMT) in a broad spectrum of developmental processes and cancers [5-7]. Removal of the proximal primary enhancer does not significantly perturb snail function, including the repression of neurogenic genes and formation of the ventral furrow during gastrulation at normal temperatures of development. However, at elevated temperatures there is sporadic loss of snail expression and coincident disruptions in gastrulation. Similar defects are observed at normal temperatures upon reductions in the levels of Dorsal, a key activator of snail expression [reviewed in 8]. Altogether, these results suggest that shadow enhancers represent a novel mechanism of canalization, whereby complex developmental processes “bring about one definite end-result regardless of minor variations in conditions…” [9].
Despite both intrinsic and environmental sources of noise, which introduce variability in complex developmental processes, the patterning of the Drosophila embryo unfolds with high fidelity (e.g., [10]). It has been postulated that gene interactions in developmental regulatory networks can channel these variable inputs into faithful outcomes, as a ball bouncing inside of a funnel is channeled to the center, a process termed “canalization” [9]. Here we present evidence that shadow enhancers [1] are important mediators of canalization, ensuring reliable and robust expression of critical patterning genes.
snail is a key determinant of dorsal-ventral patterning [5,6,11,12]. It encodes a zinc finger repressor that establishes a sharp boundary between the presumptive mesoderm and neurogenic ectoderm, and is essential for the formation of the ventral furrow and invagination of the mesoderm. Whole genome ChIP-chip assays identified a cluster of Dorsal and Twist (key activators of snail expression) binding sites in the immediate 5′ flanking region of the snail transcription unit that coincide with the known enhancer [12,13]. Unexpectedly, these studies also identified a second cluster of binding sites within the neighboring Tim17b2 locus, located ∼7 kb upstream of snail. A small genomic DNA fragment (∼1 kb) encompassing this second cluster of binding sites was attached to a lacZ reporter gene and expressed in transgenic embryos (Fig. 1). The fusion gene exhibits localized expression in the presumptive mesoderm, similar to that seen for the endogenous gene (e.g., Fig. 1) or obtained with the proximal enhancer (the first 2.8 kb of the 5′ flanking region; see ref. [12]). We arbitrarily refer to the newly identified distal enhancer as the shadow enhancer and the original, proximal enhancer as the primary enhancer [1].
Figure 1. Identification of a snail shadow enhancer.
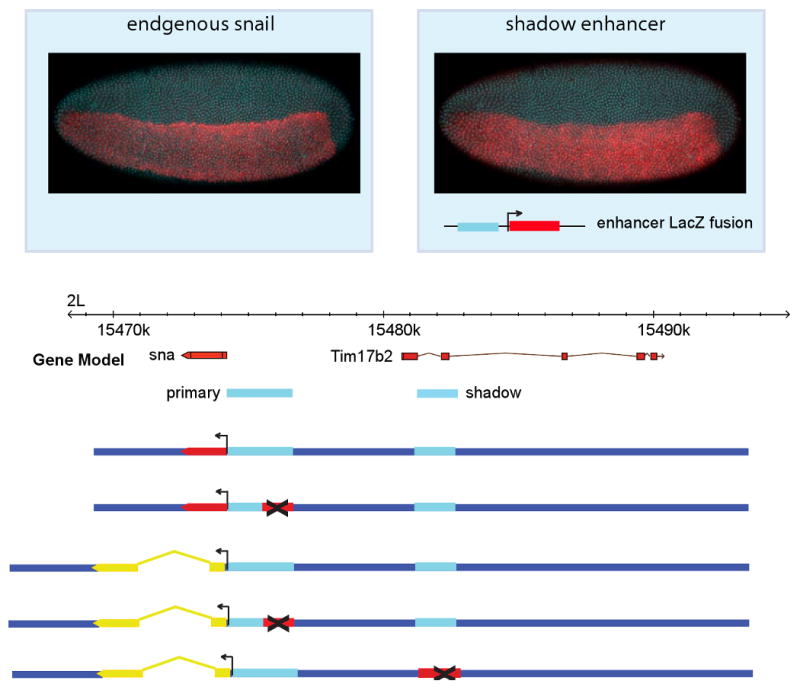
The snail gene is expressed in the presumptive mesoderm, (top left in red). An intronic region in neighboring Tim17b2 was shown to be bound by transcription factors that regulate snail [13] and here is shown to drive expression of a lacZ fusion gene in the mesoderm in a pattern qualitatively similar to the endogenous gene (upper right panel). Below, schematic representations of the BAC constructs used in subsequent experiments are aligned to the gene model. In all figures, anterior is to left, dorsal is at top, unless indicated.
A snail fusion gene containing only the primary enhancer rescues the gastrulation of at least some snail mutants in a population of mutant embryos [14]. Since snail is essential for the coordinated invagination of the mesoderm during early gastrulation, variability in expression could lead to occasional disruptions in morphogenesis. Perhaps the additional enhancer provides a mechanism for suppressing such variability, thereby ensuring robust expression in large populations of embryos. This hypothesis was motivated in part by previous preliminary evidence that neurogenic genes with shadow enhancers show less sensitivity to changes in activator concentration than similar genes lacking shadows [2].
An alternative view is that the proximal and shadow enhancers are primarily responsible for controlling distinct dynamic aspects of the snail expression pattern, rather than functioning in an overlapping manner during mesoderm invagination. An expectation of the former “robustness hypothesis” is that transgenes containing either enhancer alone should be sufficient to induce gastrulation in snail mutant embryos. We tested this possibility by creating a series of recombineered BACs [4,15] containing a ∼25 kb genomic interval encompassing the snail and Tim17b2 loci (Fig. 1). Comparable BACs were prepared that either contain or lack the proximal enhancer. This enhancer was not simply deleted, but a ∼1 kb segment containing critical Dorsal activator elements was replaced with a “random” DNA sequence (see Experimental Procedures) in order to retain normal spacing of the regulatory region.
To measure the effect that different enhancers have on transcriptional activity we developed a reporter system for detecting nascent transcripts. The endogenous yellow gene is not transcribed until late in development and contains a large intron (e.g., [16,17]), making it an ideal reporter for the detection of de novo transcripts by in situ hybridization. In contrast, the snail transcription unit lacks introns and is therefore not amenable to quantitative in situ hybridization methods that rely on intronic probes. Consequently, a series of BACs were created that contain yellow in place of snail. These BACs contain both enhancers or have either the primary or shadow enhancer replaced with random DNA (Fig 1). All of the aforementioned BACs were inserted in the same chromosomal location on 2L using phiC31 targeted integration [4,18-19].
BACs containing the snail gene were crossed into a mutant background with a deletion spanning the entire snail transcription unit (Df (2L)osp29) along with a marked balancer to identify homozygous snail null mutants. As noted earlier, the reciprocal situation, proximal enhancer without shadow, can sometimes rescue gastrulation [14]. Mutant embryos homozygous for the snail deficiency chromosome (osp29) are easily recognized by the absence of snail expression and ectopic single-minded (sim) expression, a key regulator of midline formation within the central nervous system that is normally excluded from the mesoderm by the Snail repressor [20,21] (Fig. 2E,F).
Figure 2. The snail shadow enhancer rescues gastrulation.
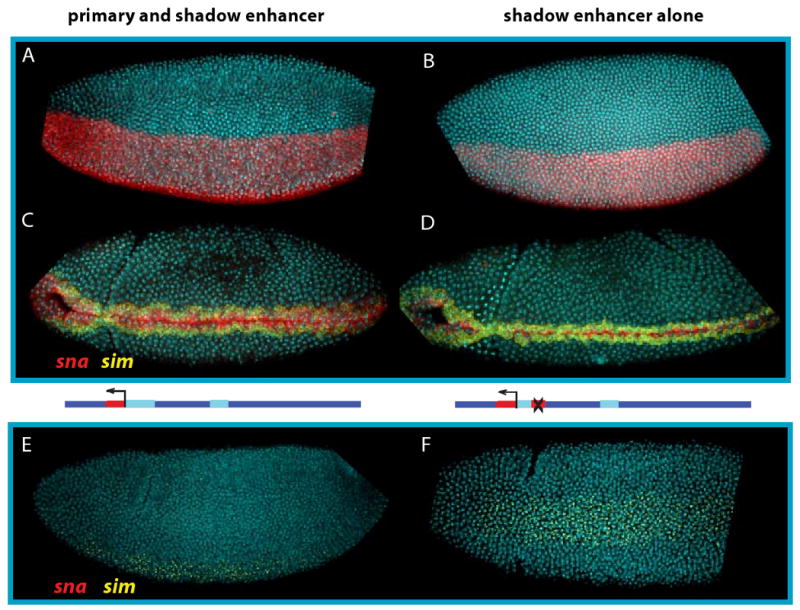
A. The rescue BAC construct in a sna mutant background drives sna expression (red) uniformly throughout the mesoderm in cycle 14 embryos. B. The pattern driven only by the BAC with the primary enhancer deleted is qualitatively similar. C. During gastrulation, all sna expressing cells migrate into the interior of the embryo. A single row of cells flanking the sna domain express sim, shown in yellow. D. sna driven without the primary enhancer is sufficient to induce normal gastrulation and normal sim expression when these embryos are raised at 22C. E. In embryos lacking the snail BAC rescue construct, no sna is expressed. Instead, sim is expressed throughout the ventral region. Without sna there is no mesodermal invagination. Lateral view. F. Embryo as in (E), mesodermal view.
There is neither a ventral furrow nor subsequent ingression of the mesoderm in these mutants (e.g., [5,6]). BAC transgenes containing both enhancers (Fig. 2A) or just the shadow enhancer alone (Fig. 2B) rescue gastrulation of mutant embryos (Fig. 2C,D; compare with E,F). In both cases, a complete ventral furrow is formed, followed by invagination of the mesoderm indistinguishable from that seen in wild-type embryos. Both BACs restore snail expression in the presumptive mesoderm, and sim transcripts are restricted to lateral regions that form the ventral midline of the CNS after gastrulation. These observations, along with previous studies (e.g., [14]), indicate that neither the primary nor shadow enhancer is necessary for the gastrulation of embryos raised at optimal, permissive conditions.
Although the shadow enhancer is sufficient for generating a qualitatively normal pattern of snail expression, additional assays were done to determine whether there might be subtle changes in expression. Quantitative confocal imaging methods were used to investigate this possibility (see [2]). As mentioned earlier, BAC transgenes were prepared that contain the yellow reporter gene in place of the snail transcription unit. In situ hybridization assays with intronic probes permit direct detection of yellow de novo transcripts, and hence, precise measurements of snail transcription with single cell (nucleus) resolution. At normal culturing temperatures, 22C, there is no discernible difference in the initial de novo transcription patterns of BAC transgenes containing both enhancers (Fig. 3A) or just a single enhancer, either the primary enhancer or shadow enhancer (Fig. 3B). In the majority of cases more than 90% of the nuclei in the presumptive mesoderm express yellow transcripts.
Figure 3. Multiple enhancers ensure robust gene expression under different thermal conditions.
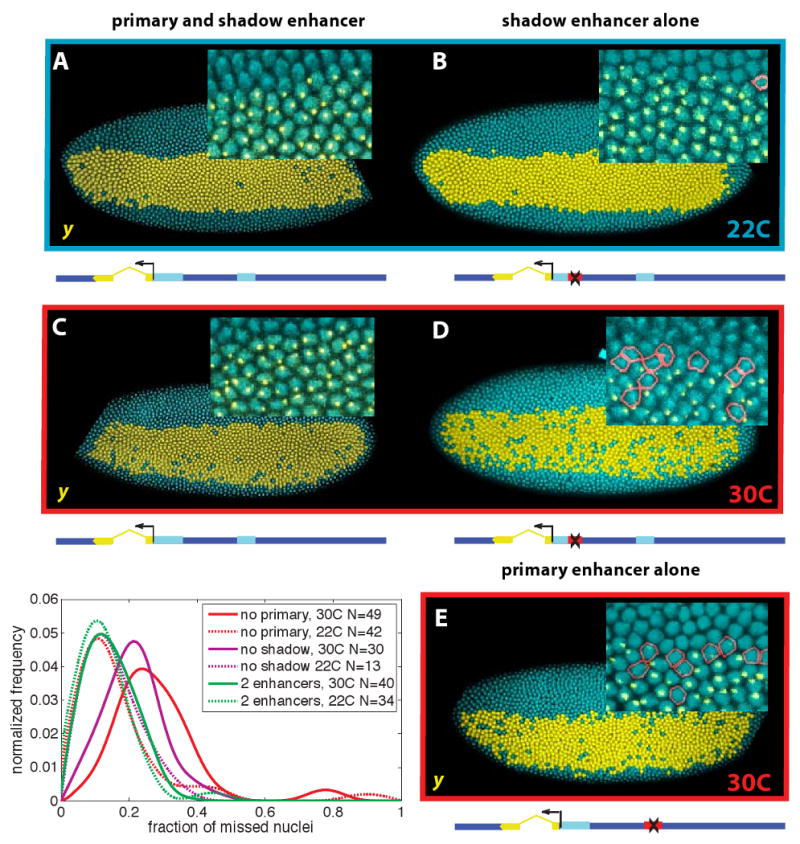
A.Visualization of expression of the yellow reporter gene from the BAC containing the sna locus, stained for the yellow intron. Cells actively transcribing the reporter are shown in yellow. Intronic probes show a single bright point of transcription inside actively transcribing nuclei (inset); all embryos are heterozygous and have one copy of the reporter. Nuclei that express the endogenous gene but not the reporter are outlined in red. A schematic representation of the BAC is shown below the embryo. B. At 22C a similar degree of uniform expression is exhibited by embryos carrying a yellow BAC lacking the primary enhancer. C. At 30C embryos with both enhancers still show straight boundaries and a small percent of inactive nuclei. D. Embryos lacking the primary enhancer at 30C show substantially more ragged boundaries of expression and a greater percent of inactive cells in the mesoderm. E. Embryos lacking the shadow enhancer are similar to those lacking the primary at both temperatures. Lower left: Frequency distributions of the fraction of cells in the sna expressing region that lack yellow expression are plotted for each of the 6 different embryo populations. N indicates the number of embryos in each population sample.
Less reliable expression is observed for BAC transgenes containing a single enhancer at elevated temperatures, 30C (Fig. 3C,D). More than 20% of the nuclei in the presumptive mesoderm lack yellow transcripts in over half of the embryos expressing the BAC transgene without the shadow enhancer. This effect is even more pronounced upon removal of the primary enhancer. The same cut-off value, absence of yellow transcripts in at least 20% of all mesodermal nuclei, occurs in over three-fourths of these embryos (Fig 3). In contrast, the BAC transgene containing both the primary and shadow enhancers continues to display nearly complete patterns of de novo transcription at the elevated temperature.
Similar results were obtained in response to genetic perturbations (Fig. 4A,B). For example, the yellow transgene BAC containing both enhancers exhibits a normal pattern of expression in embryos derived from dl/+ mothers containing half the normal dose of the Dorsal gradient (Fig. 4A). The distribution of nuclei failing to maintain active expression is similar to that seen for wild-type embryos (Fig 4C). However, the comparable BAC transgene containing only the shadow enhancer exhibits erratic patterns of activation in these embryos, particularly in lateral regions (Fig. 4B, quantification in C). These results, along with the preceding analysis of embryos grown at elevated temperatures, suggest that the snail shadow enhancer helps ensure accurate and reproducible patterns of gene expression in large populations of embryos subject to genetic and environmental perturbations.
Figure 4. The effect of intrinsic and extrinsic variability.
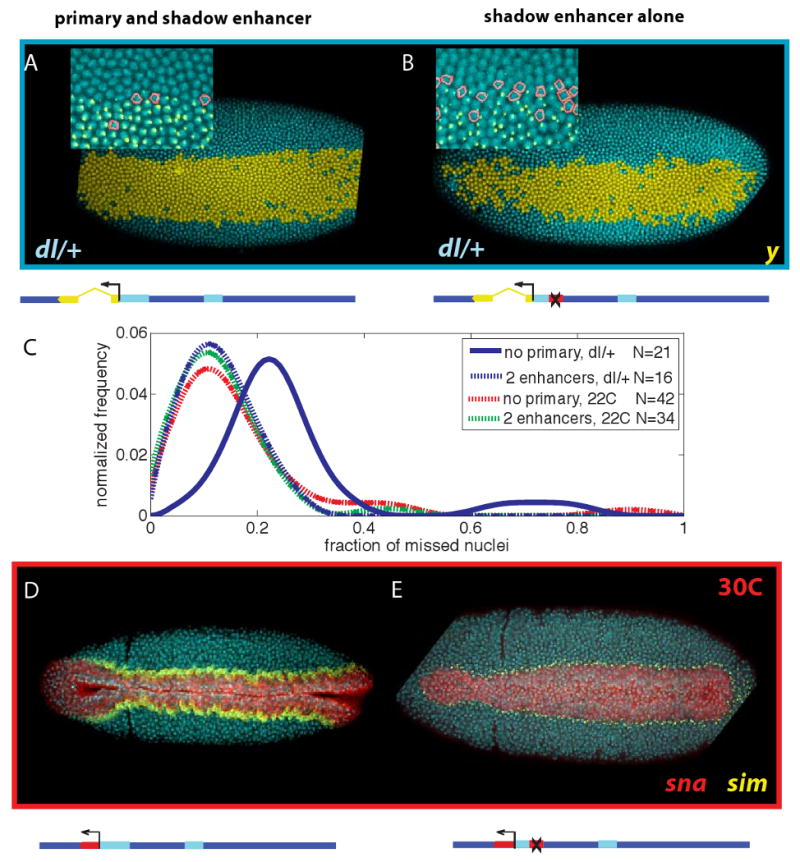
A. Embryos from dorsal heterozygote mothers raised at 25C show uniform yellow expression when driven with both enhancers. Only a few cells are lacking active expression (inset). B. Embryos with a single enhancer in this background show substantially greater loss of expression and ragged boundaries. C. The distribution nuclei which fail to maintain active transcription shifts to the right in the dorsal heterozygote background only for embryos lacking one of the enhancers. D. All observed embryos raised at 30C (N=28) from a population heterozygous for the BAC-constructs containing both enhancers gastrulate normally, forming a straight ventral furrow; note stage of development by presence of cephalic furrow. E. Some embryos from a similar population, but with only the single enhancer and raised at 30C show various defects in gastrulation (N=10 of 14). Note embryo stage by presence of cephalic furrow, yet lack of significant mesodermal invagination. The number of snail expressing cells anterior to the cephalic furrow is also reduced. Supplemental Figure 2 shows a range of defects observed in these embryos; a narrower pattern of anterior expression may result in delays in involution of anterior regions, and some exhibit a more erratic midline.
The preceding results document quantitative changes in the variability and reliability of snail expression upon removal of the primary or shadow enhancer. We next asked whether such variation causes changes in cellular morphogenesis, particularly the formation of the ventral furrow and subsequent invagination of the mesoderm (Fig. 4D,E). snail mutant embryos carrying BACs with both enhancers (Fig. 4D) or just the shadow enhancer (Fig. 4E) were grown at elevated temperatures, 30C. Embryos carrying the transgene with both enhancers exhibit normal patterns of gastrulation (Fig. 4D). In contrast, comparable embryos lacking the primary enhancer display erratic patterns of gastrulation, including the formation of incomplete ventral furrows that do not extend along the entire germband (Fig. 4E) and disruptions in the symmetry of the involuted mesodermal tube (see Suppl Fig. 2). As shown earlier, such defects are not observed at normal temperatures, 22C (Fig. 2 and Supplement).
We have presented evidence that the snail shadow enhancer located within the Tim17b2 locus helps ensure reliable and reproducible patterns of snail expression in the presumptive mesoderm during gastrulation. BAC transgenes lacking either the primary enhancer or the shadow enhancer display erratic patterns of de novo transcription at elevated temperatures. We propose that shadow enhancers come to be fixed in populations by ensuring robustness in the activities of key patterning genes such as snail. Increases in temperature should cause less stable occupancy of critical binding sites, but an additional enhancer could suppress this “noise” by increasing the probability of gene activation. This increased time of active transcription per cell might augment the overall levels of expression, which could be an important function of shadow enhancers.
Other critical dorsal-ventral determinants also contain shadow enhancers, including brinker, vnd, and sog [1]. The recent analysis of shavenbaby suggests that shadow enhancers are essential for the reliable morphogenesis of embryonic bristles in older embryos [22]. There is also evidence that shadow enhancers might be a common feature of vertebrate systems, such as zebrafish [23].
Shadow enhancers appear to represent a novel mechanism of canalization [9], whereby complex developmental processes lead to a fixed outcome despite genetic and environmental perturbations. Other mechanisms of canalization have been suggested, including recursive wiring of gene regulatory networks and “capacitors” such as hsp90 that suppress both altered folding of mutant proteins and transpositioning of mobile elements [24-27].
It is conceivable that primary and shadow enhancers mediate overlapping patterns of activity only during early embryogenesis. They might come to possess distinctive regulatory activities at later stages of development. Nonetheless, during the time when their activities coincide during gastrulation, they maintain reliable patterns of snail expression in response to environmental and genetic variability. Although either enhancer might be sufficient, both enhancers are required for accurate and reliable patterns of expression in response to variability. This precise patterning enables rapid development, without delays arising from corrective feedback mechanisms. It is easy to imagine that delays in embryogenesis would result in selective disadvantages to the resulting larvae, which must compete for limiting sources of food. Regardless of the specific mechanisms that select for shadow enhancers, the occurrence of such enhancers provides an opportunity for the evolution of novel patterns of gene expression. As long as the two enhancers maintain overlapping activities during developmental “hotspots” such as gastrulation, they can drift or be selected to produce divergent patterns of gene expression.
Experimental Procedures
Fly genetics
Positive BAC line males (labeled with w+) were crossed to yw; wg[Sp]/CyO; Pr,Dr/TM3,Sb,Ser virgins. Homozygous BAC lines were created by selfing the red-eyed, Sb,Ser flies from the F1 generation. Males from the BAC lines carrying the yellow reporter constructs were crossed to yw, dl[6]/CyOvirgins.
To test for rescue of the BAC constructs, we generated a white eyed, double balancer strain carrying a CyO linked hunchback-LacZ reporter by crossing and back crossing wnt4/CyO, hb-lacZ (BSC 6650) to yw; wg[Sp]/CyO; Pr,Dr/TM3,Sb,Ser virgins. Positive BAC males were crossed into this line to create w; +/CyO, hb-lacZ; BAC[snail,w+]/TM3,Sb,Ser virgins. Simultaneously, w; Df (2L)osp29/CyO, (BSC 3078) flies carrying a deletion spanning the snail gene were crossed to yw; wg[Sp]/CyO; Pr,Dr/TM3,Sb,Ser virgins. The Df (2L)osp29/wg[Sp], +/TM3,Ser males were crossed to the virgins containing the labeled balancer and the BAC. The progeny were selfed to create homozygous stable lines for the BAC carrying the snail deletion over the hb-lacZ marked CyO balancer. Populations still containing the Ser balancer or a wildtype chr III were analyzed also analyzed to test the effect of single copy rescue. The labeled balancer allowed for the reliable identification of embryos lacking a functional copy of endogenous sna.
Recombineering and transgenesis
Recombineering was performed as described previously [4,15,28-30] with modifications described in Supplemental Experimental Procedures. These supplemental sections also describe construction of the yellow intronic reporter, the use of plasmids with a conditional origin of replication to reduce recombineering colony background [31], and preparation of modified BAC constructs for microinjection. Supplemental Table 1 lists primers used to make BAC modifications; the sna primary enhancer sequence was replaced using an ampicillin resistance cassette as a non-regulatory spacer. BAC CH321-18I14 [15] was used as the basis for all other modifications.
Fluorescent in situ hybridization and quantitative imaging methods
Fluorescent in situ hybridization was performed as described in [32]. Embryos were imaged on a Leica Scanning Confocal SL microscope as a 14-20 section z-stack through the nuclear layer at 1/2 micron intervals, with scanning resolution of approximately 250 nm/pixel. Images were maximum projected and computationally segmented to localize and count nuclei, mRNA expression domains, and nascent transcripts. More details on the automation of image analysis are included in the Supplement.
Supplementary Material
Acknowledgments
The authors thank Chiahao Tsui and Madhurima Pramod for technical support, Jessica Cande, Benjamin Haley, Vivek Chopra, and other members of the Levine lab as well as Nipam Patel for discussion and helpful suggestions, and Russell Vance for recombineering advice and reagents. M.W.P. and A.N.B. are the recipients of NSF predoctoral fellowships; J.B. is the recipient of a Berkeley Fellowship. This work was funded by a grant from the NIH (GM46638) to M.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hong J, Hendrix DA, Levine MS. Shadow enhancers as a source of evolutionary novelty. Science. 2008;321:1314. doi: 10.1126/science.1160631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boettiger AN, Levine M. Synchronous and stochastic patterns of gene activation in the Drosophila embryo. Science. 2009;325:471–473. doi: 10.1126/science.1173976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perry MW, Cande JD, Boettiger AN, Levine M. Evolution of insect dorsoventral patterning mechanisms. Cold Spring Harbor symposium. 2009;74:275–279. doi: 10.1101/sqb.2009.74.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venken KJ, He Y, Hoskins RA, Bellen HJ. P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science. 2006;14:1747–51. doi: 10.1126/science.1134426. [DOI] [PubMed] [Google Scholar]
- 5.Leptin M. twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev. 1991;5:1568–76. doi: 10.1101/gad.5.9.1568. [DOI] [PubMed] [Google Scholar]
- 6.Kosman D, Ip YT, Levine M, Arora K. Establishment of the mesoderm-neuroectoderm boundary in the Drosophila embryo. Science. 1991;254:118–22. doi: 10.1126/science.1925551. [DOI] [PubMed] [Google Scholar]
- 7.Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- 8.Hong JW, Hendrix DA, Papatsenko D, Levine MS. How the Dorsal gradient works: insights from postgenome technologies. Proc Natl Acad Sci. 2008;105:20072–20076. doi: 10.1073/pnas.0806476105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waddington CH. Canalization of Development and the Inheritance of Acquired Characters. Nature. 1942;150:563–565. doi: 10.1038/1831654a0. [DOI] [PubMed] [Google Scholar]
- 10.Gregor T, Tank DW, Wieschaus EF, Bialek W. Probing the limits to positional information. Cell. 2007;130:153–164. doi: 10.1016/j.cell.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boulay JL, Dennefeld C, Alberga A. The Drosophila developmental gene snail encodes a protein with nucleic acid binding fingers. Nature. 1987;330:395–398. doi: 10.1038/330395a0. [DOI] [PubMed] [Google Scholar]
- 12.Ip YT, Park RE, Kosman D, Yazdanbakhsh K, Levine M. dorsal-twist interactions establish snail expression in the presumptive mesoderm of the Drosophila embryo. Genes Dev. 1992;6:1518–30. doi: 10.1101/gad.6.8.1518. [DOI] [PubMed] [Google Scholar]
- 13.Zeitlinger J, Zinzen RP, Stark A, Kellis M, Zhang H, Young RA, Levine M. Whole-genome ChIP-chip analysis of Dorsal, Twist, and Snail suggests integration of diverse patterning processes in the Drosophila embryo. Genes Dev. 2007;21:385–90. doi: 10.1101/gad.1509607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemavathy K, Hu X, Ashraf SI, Small SJ, Ip YT. The repressor function of snail is required for Drosophila gastrulation and is not replaceable by Escargot or Worniu. Dev Biol. 2004;269:411–20. doi: 10.1016/j.ydbio.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 15.Venken KJ, Carlson JW, Schulze KL, Pan H, He Y, Spokony R, Wan KH, Koriabine M, de Jong PJ, White KP, et al. Versatile P[acman] BAC libraries for transgenesis studies in Drosophila melanogaster. Nature methods. 2009;6:431–4. doi: 10.1038/nmeth.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong S, Rebeiz M, Andolfatto P, Werner T, True J, Carroll SB. The evolution of gene regulation underlies a morphological difference between two Drosophila sister species. Cell. 2008;132:783–93. doi: 10.1016/j.cell.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Parkhurst SM, Corces VG. Interactions among the gypsy transposable element and the yellow and the suppressor of hairy-wing loci in Drosophila melanogaster. Mol Cell Biol. 1986;6:47–53. doi: 10.1128/mcb.6.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groth AC, Fish M, Nusse R, Calos MP. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics. 2004;166:1775–1782. doi: 10.1534/genetics.166.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci. 2007;104:3312–7. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasai Y, Nambu JR, Lieberman PM, Crews ST. Dorsal-ventral patterning in Drosophila: DNA binding of snail protein to the single-minded gene. Proc Natl Acad Sci. 1992;89:3414–3418. doi: 10.1073/pnas.89.8.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nibu Y, Zhang H, Levine M. Interaction of short-range repressors with Drosophila CtBP in the embryo. Science. 1998;280:101–104. doi: 10.1126/science.280.5360.101. [DOI] [PubMed] [Google Scholar]
- 22.Frankel N, Davis GK, Vargas D, Wang S, Payre F, Stern DL. Phenotypic robustness conferred by apparently redundant transcriptional enhancers. Nature. 2010 doi: 10.1038/nature09158. advance online pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kikuta H, Laplante M, Navratilova P, Komisarczuk AZ, Engström PG, Fredman D, Akalin A, Caccamo M, Sealy I, Howe K, et al. Genomic regulatory blocks encompass multiple neighboring genes and maintain conserved synteny in vertebrates. Genome Research. 2007;17:545–55. doi: 10.1101/gr.6086307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lott SE, Kreitman M, Palsson A, Alekseeva E, Ludwig MZ. Canalization of segmentation and its evolution in Drosophila. Proc Natl Acad Sci. 2007;104:10926–10931. doi: 10.1073/pnas.0701359104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manu Surkova S, Spirov AV, Gursky VV, Janssens H, Kim A, Radulescu O, Vanario-Alonso CE, Sharp DH, Samsonova M, Reinitz J. Canalization of gene expression in the Drosophila blastoderm by gap gene cross regulation. PLoS biology. 2009;7:e1000049. doi: 10.1371/journal.pbio.1000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rutherford SL, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature. 1998;396:336–42. doi: 10.1038/24550. [DOI] [PubMed] [Google Scholar]
- 27.Specchia V, Piacentini L, Tritto P, Fanti L, D'Alessandro R, Palumbo G, Pimpinelli S, Bozzetti MP. Hsp90 prevents phenotypic variation by suppressing the mutagenic activity of transposons. Nature. 2010;463:662–665. doi: 10.1038/nature08739. [DOI] [PubMed] [Google Scholar]
- 28.Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- 29.Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome research. 2003;13:476–84. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. Simple and highly efficient BAC recombineering using galK selection. Nucleic acids research. 2005;33:e36. doi: 10.1093/nar/gni035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci. 2000;97:6640–5. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosman D, Mizutani CM, Lemons D, Cox WG, McGinnis W, Bier E. Multiplex detection of RNA expression in Drosophila embryos. Science. 2004;305:846. doi: 10.1126/science.1099247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


