Abstract
This work investigated the link between genetic and developmental controls of fruit size and composition. On two isogenic lines (CF12-C and CF14-L), differing by fruit weight and sugar content quantitative trait loci (QTLs) identified previously, basal and tip fruits were characterized at anthesis and at maturity through their growth, dry matter and sugar content, number and size of cells and nuclei DNA content. The influence of competition was assessed by removing either basal or tip ovaries at anthesis. On an intact inflorescence, CF12-C fruits grew less than CF14-L fruits, with 1·67 fewer cell layers and similar cell size, suggesting that genes controlling cell division may be responsible for this fruit size variation. Truss thinning masked the QTL effect on fruit size, mainly by reducing the difference in cell number between the two lines and by promoting cell expansion in tip fruits, so that fruit growth was similar at both positions and for both lines. Thus, in these lines, cell number exerts a control on final fruit size only when there is competition among fruits. Different responses of basal and tip fruits after flower removal suggested that this treatment induced changes in hormonal relationships within the truss. No fixed relationship between DNA endoreduplication and cell size was found, as while cell size and dry matter and sugar contents differed with tomato lines, fruit position and truss size, endoreduplication patterns were the same. CF12-C fruits had a higher dry matter (+0·3 % of fresh weight) and carbohydrates (+8 % of dry matter) content than CF14-L fruits. The percentage dry matter was independent of truss size but decreased slightly from basal to tip fruits.
Key words: Carbohydrate, cell division, cell size, endoreduplication, fruit size, Lycopersicon esculentum, quality, tomato
INTRODUCTION
Fruit size and composition are major criteria of quality for fresh tomatoes. They are genetically and environmentally controlled through the successive phases of fruit development. The number of cells, the size of individual cells and the nuclei DNA content are pertinent variables in the analysis of the genetic and phenotypic variations in fruit size and composition. Indeed, both cell number and size contribute to the control of fruit size (Bertin et al., 2002), and in many species small fruit size can be related to low cell number (Cowan et al., 1997; Higashi et al., 1999; Jullien et al., 2001). In tomato, differences in size between basal and tip fruits of the same inflorescence were attributed to differences in the number of cells (Bünger-Kibler and Bangerth, 1983; Bohner and Bangerth, 1988a; Ho, 1996a). Endoreduplication corresponds to an incomplete cell cycle, very common in plants, that leads to the duplication of DNA without division of the cell, increasing the nuclei DNA content (D’Amato, 1964; Galbraith et al., 1991). Although there is no strict relationship between final cell size and endoreduplication, the notion remains that a high nuclear DNA content is present in large and active cells (D’Amato, 1984; Traas et al., 1998; Joubès and Chevalier, 2000). In tomato, high DNA content has been suggested to facilitate the sink function of fruit (Bergervoet et al., 1996), and in these terms it could be expected to play a role in the control of carbohydrate accumulation, though this has never been demonstrated.
In tomato, the period of cell division is relatively short and ends about 2 weeks after anthesis (Mapelli et al., 1978). In the ovary, fertilization takes place during this period a few hours after anthesis (Ho and Hewitt, 1986). After fruit fertilization, the early development of the fruit is commonly divided into two phases: a first phase of very active cell division during which the pericarp develops into multiple cell layers, and a second phase of cell expansion (Gyllaspy et al., 1993). In the pericarp of cherry tomatoes, the mitotic index increases three-fold from anthesis to 10 d after anthesis (10 DAA), decreases during the following 15 d when cell expansion starts, and remains quite low during ripening (Joubès et al., 1999). While the mitotic activity decreases in the pericarp from 10 DAA until ripening, the nuclear ploidy increases (Joubès et al., 1999). These authors reported levels of endoreduplication up to 256C in cherry tomatoes, similar to those observed by Bergervoet et al. (1996) on a large tomato cultivar.
This study was conceived to analyse the implication of cell number, cell size and DNA endoreduplication in the control of final size and composition of tomato during fruit development, in relation to genetic variation. Two near-isogenic lines of tomato differing by four linked quantitative trait loci (QTLs), two of fruit size, one of dry matter and sugar content and one of locule number, were cultivated under controlled climatic conditions. The natural gradient of fruit traits, within the same tomato inflorescence, was considered since fruit size decreases in parallel with the number of pericarp cells from basal to tip fruits (Bohner and Bangerth, 1988a). Inflorescence size was manipulated by removing either basal or tip fruits to assess the influence of competition. Fruits were characterized at anthesis during the cell division period and at maturity, through their growth, dry matter and sugar content, number and size of cells, and nuclear DNA content. Cytological analysis focused on the pericarp tissue, though the epidermis properties are also likely determinants in the control of fruit growth (Thompson, 2001). Nuclei DNA content was measured during early fruit development from initiation to about 20 DAA, and was considered to be an indicator of the progressive shift of the tissue from the phase of active cell division to the phase of cell expansion since cell division and endoreduplication are mutually exclusive in the same cell (Traas et al., 1998).
MATERIALS AND METHODS
Plant material
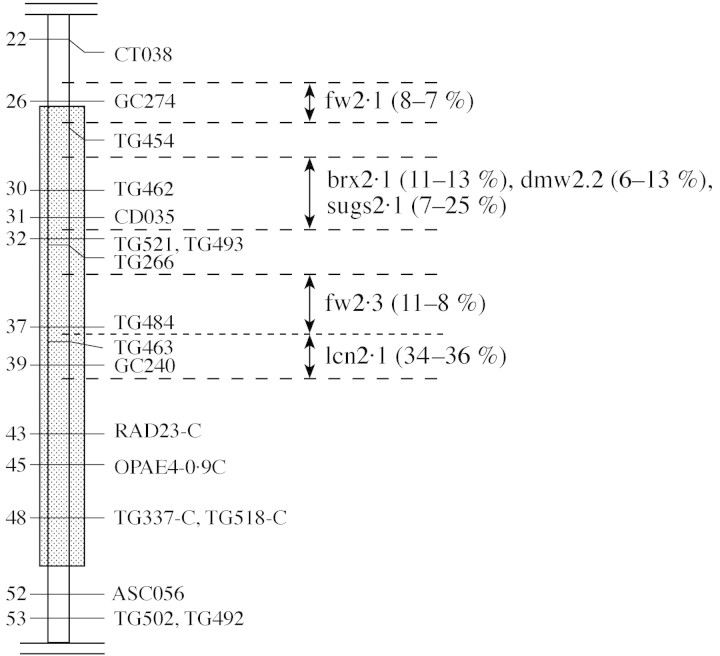
Near isogenic lines were constructed following a programme of QTL detection, performed on a population derived from a cross between a cherry tomato line (Cervil) and a large-fruited line (Levovil). A genetic map was constructed with molecular markers (Saliba-Colombani et al., 2000) and at least four different QTLs were identified in less than 26 cM of the distal region of chromosome 2, one controlling locule number (lcn), one controlling sugar, dry matter and soluble solids contents (sugs, dmw and brx) and two controlling fruit weight (fw) (Saliba-Colombani et al., 2001; Lecomte et al., 2003). The lines CF12-C and CF14-L studied here differ only in this 26 cM segment, with CF12-C carrying the genes originated in the cherry tomato, and CF14-L the large fruited (but low sugar content) genes (Fig. 1).
Fig. 1. Map of the region of chromosome 2 where the CF12-C and CF14-L near-isogenic lines differ (hatched). Distances on the left of the chromosome are centimorgans from the top of the chromosome. The four intervals where QTLs were finely located are indicated on the right, with their codes (fw, fruit weight; brx, soluble solid content; sugs and dmw, reducing sugars and dry matter content; lcn, locular number). The effects of the QTLs, expressed as percentage of average difference, detected in two glasshouse trials, are in parentheses (adapted from Lecomte et al., 2003).
Culture conditions and experimental protocol
Experiments were carried out in two growth chambers under similar culture and climate conditions. Tomato seeds were sown in sand, and six plants of each tomato line were pricked out at a developmental stage of about six visible leaves. Plants were grown in 10 l pots filled with a balanced oxygenated nutrient solution (detailed in Le Bot et al., 1998), whose composition was checked every week and readjusted when necessary. Sowing was done in the controlled-environment growth chamber itself, under climatic conditions similar to those monitored after planting. From sowing to the end of taking measurements, a day/night air temperature of 23–19 ± 0·5 °C was continuously maintained. A 12-h photoperiod was applied with a photon flux of about 500 µmol m–2 s–1 PAR (photosynthetically active radiation) above the canopy. The relative humidity of the air was around 75 %. Air was enriched during the period of illumination to 500 µl CO2 l–1. Flowers were pollinated as they opened using an ‘electrical bee’, and all side shoots were removed as they appeared.
The effects of competition among fruits in relation to fruit position within the inflorescence were studied in two experiments. In the first one, referred to as the ‘competition experiment’, trusses were not pruned and both basal (first four) and tip (seventh to tenth) fruits of the first inflorescence were analysed at the mature green/orange stage (MG, about 40 DAA). Plants were topped two leaves above the fourth truss. In two other experiments, termed ‘pruned experiments’ the first inflorescence was pruned to four flowers. On three plants from each line, only the four basal flowers (nearest to the stem) were left, the tip flower buds being removed at their own anthesis (full flower opening). On three other plants of each line, only four tip flowers (seventh to tenth) were left, the previous and following flowers of the inflorescence being removed at anthesis of the basal ones. On all plants, the second truss was pruned to six flowers and plants were topped two leaves above this truss.
Measurements
In all experiments and on all plants, anthesis of individual flower buds was recorded on the first inflorescence. After fruit setting, fruit diameter was measured twice and then once a week. At the end of the experiment, whole plants were collected and the dry mass of stem, leaves and fruits of other trusses was measured after 4 d in a ventilated oven at 80 °C. Plant leaf area was estimated from total leaf dry mass and LMA (leaf mass per area) measured on a sample of four leaves per plant. The projected area of the leaf laminae in this sample was measured using a LICOR area meter (Lincoln, NE, USA).
In the competition experiment, all fruits of the first inflorescence were picked at the MG stage, and the following variables were measured on a pool of four basal and four tip fruits per plant and six plants per line: fruit fresh weight and diameter, fruit dry matter content, locule number, pericarp cell number and mean size, cell DNA content and number of cell layers in the pericarp.
In the first pruned experiment, a first set of data was obtained at respective anthesis of basal and tip flowers. For this purpose organs removed for basal and tip treatments were used. The second set of data was measured at the beginning of fruit ripening (MG stage). Thus, at each stage a pool of four fruits per plant and three plants per treatment and per line were available for the different measurements: fruit fresh weight and diameter, locule number, fruit dry matter and sugar content (only for ripened fruits), pericarp cell number and mean size, number of cell layers in the pericarp and cell DNA content.
The second ‘pruned’ experiment aimed at describing the dynamic of DNA-endoreduplication during the early stage of fruit development. Flower buds were picked on the first truss from appearance to about 20 DAA for flow cytometric analysis.
Analytical methods
Sucrose, fructose and glucose were measured by high pressure liquid chromatography analysis and starch content was determined by an enzymatic method (Gomez et al., 2002, 2003).
The number of pericarp cells was measured after tissue dissociation according to a method adapted from that of Bünger-Kibler and Bangerth (1983). Details of the applied method are given in Bertin et al. (2002). Complementary measurements of cell number and size were performed in situ by cytological methods in a few fruits of each treatment. A slice (approx. 0·3–0·6 mm thick) was excised from the equatorial region of the fruit, and pericarp fragments (approx. 3 mm long) were cut (for ovaries whole equatorial slices were used) and immersed in Javel water diluted 1 : 10 for 60–90 min at room temperature under partial vacuum. The Javel treatment was used to clear cellular contents and facilitate automatic cell measurements. After rinsing with water, the samples were dehydrated by an ethanol series and embedded in Technovit 7100 (Kulzer, Wehrheim, Germany). Sections (3 µm thick) were obtained with a Reichert 2040 microtome, stained with toluidine blue and photographed on a Zeiss Axiophot microscope with a Spot digital colour camera (Diagnostic instruments, Sterling Heights, MI, USA). Using Image Pro-Plus software (Media Cybernetics, Silver Spring, MD, USA), the following measurements were made: pericarp thickness measured on pericarp portions devoid of vascular bundle, number of cell layers, mean cell area and distribution of cell areas over 40 µm2 classes in an ROI (region of interest) of 350 µm and 2·5 cm length of pericarp for ovaries and mature fruits, respectively (Fig. 2).
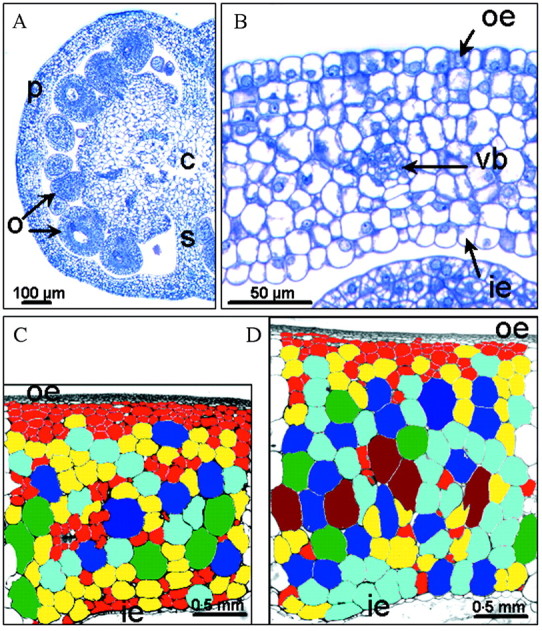
Fig. 2. Microscopic characterization of CF12-C and CF14-L fruits. A, Half equatorial section of a CF12-C ovary sampled at anthesis; B, pericarp portion of a CF14-L ovary at anthesis; C, pericarp portion of a CF12-C basal MG fruit; D, a CF14-L tip MG fruit sampled in the pruned experiment. The rectangle represents the 2·5 mm long region of interest (ROI), in which cells have been coloured according to their class of section area, except for the outer and inner epidermis, for the first two to four layers of very small cells below the outer epidermis and for the vascular bundles (eight 0·02 mm2 wide classes, increasing order: red, yellow, bright blue, cyan, green, brown, dark blue). c, Columella; ie, inner epidermis; o, ovule; p, pericarp; oe, outer epidermis; s, septum; vb, vascular bundle.
The ploidy level of cells from the pericarp and jelly tissues was measured by flow cytometry. A core of fresh tissue was chopped with a razor blade and stained in 2 ml of DAPI solution (4′,6-diamidino-2-phenylindol; PARTEC GmbH, Munich, Germany). Nuclei were filtered through a 30 µm CellTrics filter, and a sample of 15 000–20 000 nuclei was analysed using a PARTEC flow cytometer (PARTEC Ploidy Analyser PA), equipped with an HBO lamp for UV excitation. The fluorescent signals are presented as frequency distribution histograms. Diploid nuclei of tomato leaf were used as standard to adjust and check the peak positions within the scale of the histogram. Histograms were analysed with the WinMDI software (version 2.8) to determine the relative number of nuclei containing different amounts of DNA expressed as C values (from 2C to 256C). Three replicate measurements were made in mature fruit, but only one measurement in flower buds and young ovaries because of the small quantity of material.
Statistical analysis
The effects of treatment (basal or tip fruit) and tomato line (CF12-C and CF14-L) on the measured variables were analysed by two-way ANOVA (SPSS, Chicago, IL, USA) and F-tests were used to determine the statistical significance. When significant effects were detected, a Tukey test was applied for all pairwise comparisons of mean responses. Other data are presented as means ± 95% confidence intervals. To appraise statistically the sensitivity of fruits to the competition exerted by other developing fruits of the same inflorescence, basal or tip fruits of each tomato line were compared in competition and pruned experiments by Student’s t-test or the Mann–Whitney sum rank test in cases of unequal variances.
RESULTS
Differences in the determinant parameters of fruit size between CF12-C and CF14-L lines under natural growth conditions (competition experiment)
Observations of plant development and plant growth analysis performed at the end of each experiment did not show any significant differences between CF12-C and CF14-L in terms of vegetative dry mass, leaf area, LMA or leaf and truss appearance rates. In the absence of truss pruning, similar numbers of fruits (10–15) set on the successive inflorescences of both tomato lines. The locule number differed significantly (P < 0·05) between CF12-C and CF14-L lines (on average 2·1–2·4 and 3·0–3·9, respectively), whereas it was independent of the fruit position.
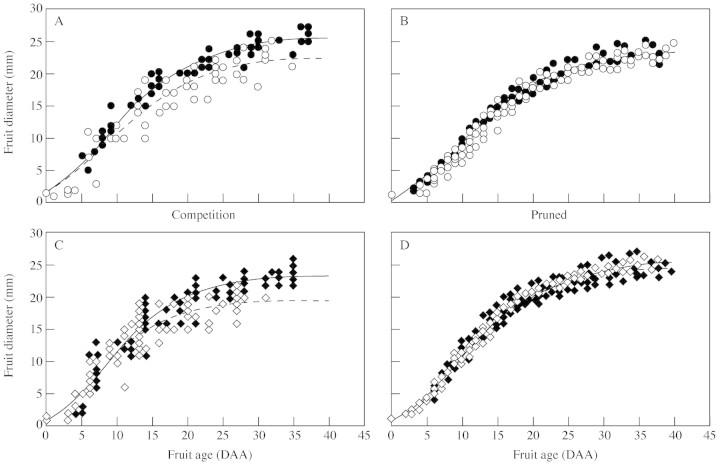
Growth curves of individual fruits for both tomato lines showed significant differences among fruits of the same inflorescence since tip fruits grew significantly less than basal fruits (Fig. 3A and C), and reached a final size 2–3 mm smaller in diameter (approx. 10 % reduction). Despite data scattering, divergence between basal and tip fruits clearly occurred in the second phase of fruit development (after 15 DAA) as the cell division period ended. Adjustment of three parameters by Gompertz function showed significant difference (P < 0·01) in the growth curves of CF12-C and CF14-L fruits both at the basal and tip positions (Fig. 3A and C), fruits of the CF12-C line growing less than fruits of the CF14-L line (about 10 % reduction of the final size).
Fig. 3. Growth curves of basal (filled symbols) and tip (open symbols) fruits measured in the competition (A and C) and pruned (B and D) experiments for the CF14-L (A and B) and CF12-C line (C and D). Individual points are experimental data measured on 12 different fruits for each treatment. Full and broken lines represent the non-linear adjustment of a three-parameter Gompertz function for basal and tip fruits, respectively (R2 > 0·98 in all cases; standard error of estimate = 0·6–1·12).
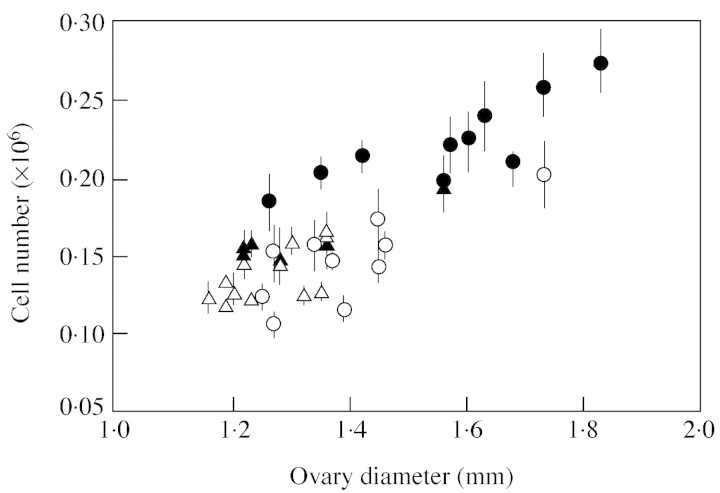
Cytological characteristics of these fruits were recorded at anthesis and at the mature green stage (Table 1). At anthesis, the CF14-L ovaries were significantly bigger (P < 0·05, not shown) than the CF12-C ones at both positions, and contained more cells, though significantly only at the basal position (P < 0·05). The pericarp of CF14-L basal ovaries was thicker (though not significantly) with one more cell layer (P = 0·013) than that of CF12-C ovaries, while the mean cell area was similar for both lines. Cell area ranged from 20 to 300 µm2 and the distribution over 40 µm2 classes was similar for all ovaries (not shown). Basal ovaries generally contained more cells than tip ones, though significantly only for the CF14-L line (P < 0·05). At this stage a good linear relationship could be observed between the number of cells and the ovary diameter (Fig. 4).
Table 1.
Characterization of ovaries and fruits sampled at anthesis and MG stage
| Anthesis | MG stage | ||||||
| Pruned experiment | Competition experiment | Pruned experiment | |||||
| Basal ovaries | Tip ovaries | Basal fruits | Tip fruits | Basal fruits | Tip fruits | ||
| Cell number (106) | CF14-L | 0·22 ± 0·02 | 0·16 ± 0·01 | 0·66 ± 0·04 | 0·63 ± 0·10 | 0·50 ± 0·01 | 0·48 ± 0·11 |
| CF12-C | 0·15 ± 0·02 | 0·14 ± 0·01 | 0·62 ± 0·04 | 0·61 ± 0·05 | 0·63 ± 0·22 | 0·57 ± 0·12 | |
| Pericarp thickness (mm) | CF14-L | 0·09 ± 0·02 | 0·105 ± 0·01 | 2·02 ± 0·16 | 1·90 ± 0·01 | 2·07 ± 0·29 | 2·28 ± 0·32 |
| CF12-C | 0·07 ± 0·00 | – | 1·94 ± 0·56 | 1·64 ± 0·15 | 2·01 ± 0·14 | 2·27 ± 0·25 | |
| No. of cell layers | CF14-L | 9·5 ± 1·5 | 9·0 ± 1·0 | 18·8 ± 1·5 | 17·8 ± 1·5 | 15·5 ± 1·0 | 16·7 ± 0·7 |
| CF12-C | 8·5 ± 0·5 | – | 16·8 ± 1·6 | 16·3 ± 0·3 | 17·3 ± 1·8 | 16·8 ± 1·2 | |
| Cell area | CF14-L | 99 ± 4 | 107 ± 25 | 0·019 ± 0·002 | 0·019 ± 0·001 | 0·021 ± 0·002 | 0·025 ± 0·007 |
| Anthesis (µm2) | CF12-C | 98 ± 1 | – | 0·020 ± 0·006 | 0·014 ± 0·002 | 0·020 ± 0·001 | 0·025 ± 0·005 |
| MG (mm2) | |||||||
The number of pericarp cells (106) was measured after tissue break down. The pericarp thickness, number of cell layers and mean cell area were defined by in situ observations of an ROI delimited by the pericarp thickness and a length of 350 µm and 2·5 mm at anthesis and MG stage, respectively. Each measurement of cell number is the mean of six cell counting per fruit, and data are means of 12 ovaries for the anthesis stage, and four and three MG fruit replicates in competition and pruned experiments, respectively, ± 95 % confidence intervals. In situ observations are means of two or three replicates ± 95 % confidence intervals.
Fig. 4. Relationship between ovary diameter and cell number measured at anthesis in basal (circles) and tip (triangles) ovaries of CF12-C (open symbols) and CF14-L (filled symbols) tomato lines. Data are means of six replicates and vertical bars represent 95 % confidence intervals of the measurements.
From anthesis to MG stage, the number of cell layers was doubled; pericarp thickness and mean cell area presented, respectively, a 20-fold and 200-fold increase (Table 1). At MG stage the biggest cells reached section areas of 0·14 mm2. The two tomato lines and the two types of fruits hardly differed, since a single significant difference was found in the number of cell layers, with 1·67 more layers in the CF14-L tomatoes than in the CF12-C ones (P = 0·049). At this stage, no significant difference in the total number of pericarp cells could be detected, neither between basal and tip fruits, nor between the two tomato lines (Table 1), and the relationship between cell number and fruit size no longer existed (not shown).
Effects of truss pruning on the genetic and developmental control of fruit size within the inflorescence
Reduction of truss size (pruned experiment) induced clear changes in the pattern of fruit growth. For both tomato lines, the growth of tip fruits increased in the latter phase of fruit development, in contrast to the competition experiment, so that the growth of basal and tip fruits were very similar (Fig. 3B and D). Comparison of fitted curves of fruit growth indicated that CF12-C tomatoes grew significantly more (P < 0·01) than CF14-L in the pruned experiment. Under these conditions, no significant differences in the pericarp thickness, final number of pericarp cells and mean cell area could be detected between basal and tip fruits and between the two tomato lines (Table 1).
Truss pruning did not affect the number of cells in CF12-C fruits either at the basal or at the tip position, while independently of the position, it decreased the cell number in the CF14-L fruits (P = 0·004). Pericarp thickness of CF14-L basal fruits did not change after pruning, due to the compensation between the reduced number of cell layers and the increase in mean cell size. However, the pericarp thickness of CF14-L tip fruits increased after pruning because of bigger cells and a similar number of cell layers. None of these differences was statistically significant. In the CF12-C line, only tip fruits were sensitive to pruning and their pericarp thickness significantly increased after pruning (P = 0·013) due to a two-fold increase of the mean cell area (P = 0·016).
DNA endoreduplication is independent of the tomato line, truss size and fruit position
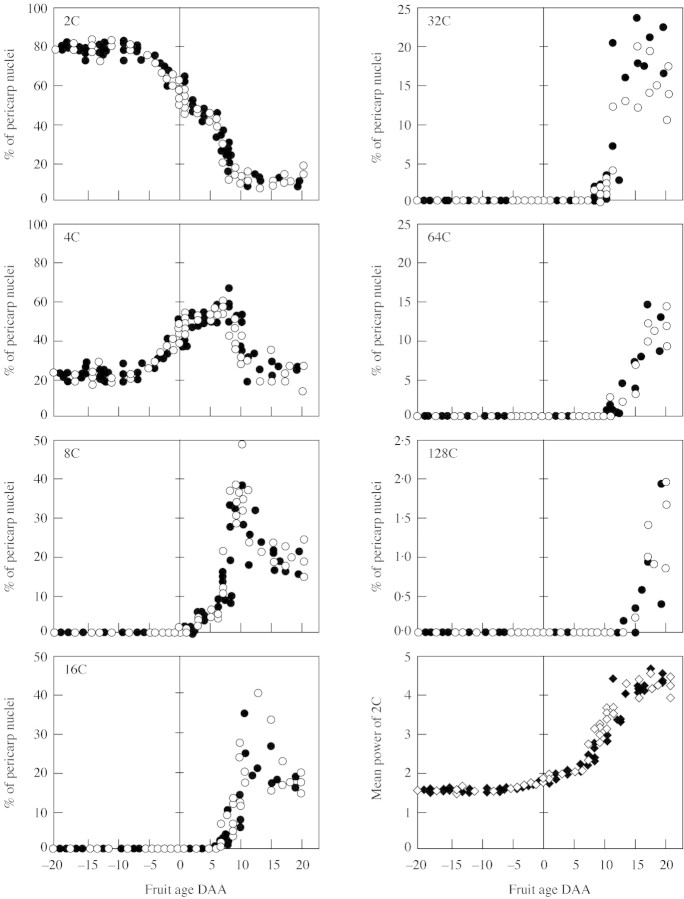
DNA endoreduplication was measured from 20 d before anthesis to 20 DAA in basal and tip organs of each tomato line (pruned experiment). Neither the tomato line, nor the fruit position influenced the pattern of endoreduplication in fruit pericarp. Figure 5 depicts this pattern for the CF14-L and CF12-C lines, pooling basal and tip fruits. Until 10 d before anthesis, about 80 % of nuclei contained 2C amount of DNA and 20 % of nuclei contained 4C (probably corresponding to dividing cells in S phase). After this stage, the percentage of 4C nuclei started to increase at the expense of the 2C nuclei. At 2 DAA, 8C nuclei arose at the expense of the 4C nuclei, and every 3–4 d a new round of DNA replication occurred, so that about 20 DAA pericarp nuclei reached a maximum level of 128C. At this time the mean power n of 2Cn DNA amount was around 3·5.
Fig. 5. Change during ovary and fruit development of the percentages of pericarp nuclei distributed according to their DNA content from 2C to 128C. The last figure represents the change of n, the mean power of 2Cn DNA amount. Filled and open symbols represent, respectively, the CF14-L and CF12-C tomato lines. Each point is an individual fruit of the pruned experiment.
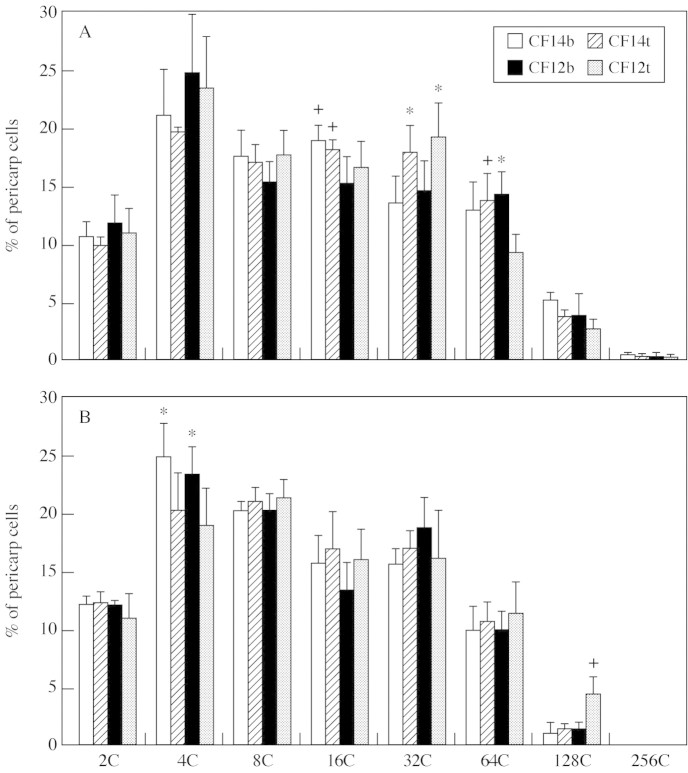
At the MG stage, DNA endoreduplication hardly differed between the two tomato lines, whatever the truss size. In the competition experiment (Fig. 6A), few significant differences were found in the C-level peaks. The CF14-L fruits had more 16C nuclei (P = 0·018) at each fruit position and more 64C nuclei in tip fruits (P = 0·029) than the CF12-C fruits. Basal and tip fruits significantly differed in the percentage of 64C nuclei (P = 0·01) in the CF12-C line and in the percentage of 32C nuclei in both lines (P = 0·006). In the pruned experiment (Fig. 6B), the only significant genetic difference was the percentage of 128C nuclei, higher in the CF12-C than in the CF14-L line. The fruit position also hardly affected the endoreduplication pattern after pruning, since only the percentage of 4C nuclei was higher in basal than in tip fruits.
Fig. 6. Distribution of pericarp nuclei according to their DNA content from 2C to 256C in competition (A) and pruned (B) experiments at basal (CF14b, CF12b) and tip fruit positions (CF14t, CF12t). Data are means of three MG fruits and each measurement is the mean of three replicates per fruit. Vertical bars represent 95 % confidence intervals. + and * indicate significant differences (P < 0·05) between, respectively, the two tomato lines for a given fruit position and the two fruit positions for a given tomato line.
Pruning significantly affected the three higher C-values except for tip fruits of the CF12-C line, which had a similar endoreduplication pattern in both experiments. In basal fruits of the CF12-C line, the 64C and 128C peaks were lower after truss pruning (P = 0·02 and 0·003). In the CF14-L line, percentages of 256C nuclei in basal fruits (P = 0·002), and 128C nuclei in tip fruits (P = 0·001) were reduced by pruning. Averaging basal and tip fruits, the mean power of 2C DNA decreased after pruning from 4·7 and 4·3 to 3·7 and 4·0 for the CF14-L and CF12-C lines, respectively. Differences between the two experiments could not be attributed to developmental differences since fruits were 37 d old in both experiments except tip fruits of the CF12-C line which were 4–5 d younger in the competition experiment.
Endoreduplication of jelly tissue was much more limited and almost all nuclei contained 16C or 32C DNA (not shown). These cells had two rounds endoreduplication less than pericarp cells. No effects of tomato line or fruit position on endoreduplication could be detected.
Final fruit composition differed between tomato lines and fruit positions
In the competition experiment, the percentage of fruit dry matter measured at the MG stage on the first four trusses was on average significantly (P < 0·05) higher in the CF12-C (8·94 %) line than in the CF14-L (8·67 %) one. Fruit dry matter content slightly increased from the first to the fourth truss and, on the first one, a trend could be seen that indicated higher (n.s.) dry matter content in basal than in tip fruits (8·05 vs. 7·54 % for the CF14-L tomatoes and 8·24 vs. 7·99 % for the CF12-C tomatoes). In the pruned experiment, the percentage dry matter also significantly (P = 0·003) differed between the tomato lines with an average content of 8·65 % in the CF12-C fruits and 8·1 % in the CF14-L fruits, independent of the fruit position. For a given truss, pruning did not affect the fruit dry matter content.
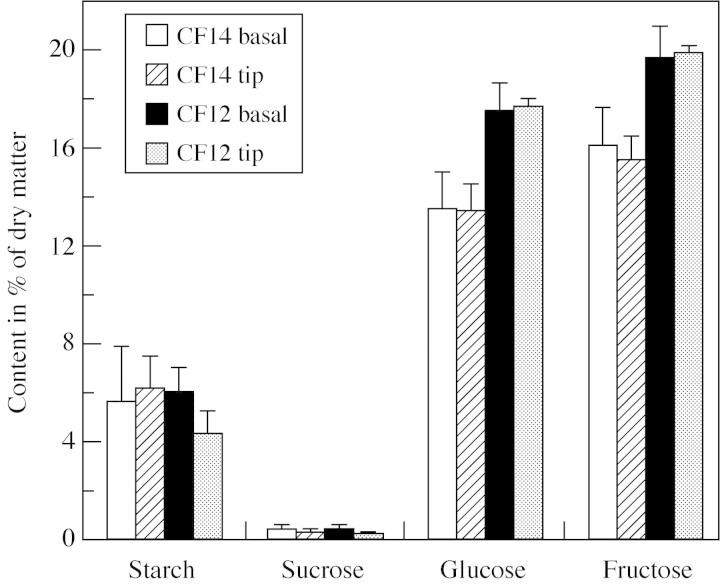
The final content of starch, glucose, fructose and sucrose was measured in the pruned experiment (Fig. 7). The two tomato lines significantly differed in their soluble carbohydrate contents: CF12-C fruits were richer than CF14-L ones in glucose (P = 0·02), fructose (P = 0·03) and in total soluble carbohydrates (P = 0·024; 29·7 % of dry matter for the CF14-L and 37·8 % of dry matter for CF12-C). Independently of the line, basal fruits were richer than tip fruits in sucrose (P = 0·013). Starch was present in small amounts ranging from 4 to 7 % of dry matter, but it was not significantly different among treatments.
Fig. 7. Starch, sucrose, glucose and fructose content as a percentage of dry matter measured in CF14-L and CF12-C tomatoes at basal and tip fruit positions in the pruned experiment. Data are means of four MG fruits and vertical bars represent 95 % confidence intervals.
DISCUSSION
Interaction between the genetic control of fruit size and the level of competition
In the genetic characterization of CF12-C and CF14-L lines, QTL effects represent differences in fruits from six successive unthinned trusses (Lecomte et al., 2003). Results of the present study suggested that at least one of the QTLs of fruit size is likely to involve genes controlling cell division. Indeed, in the competition experiment, the pericarp of CF14-L fruits had more cell layers and more cells than CF12-C fruits, both at anthesis and at the MG stage (Table 1). Since cell size never differed between the two lines, cell number appeared to be the main determinant of genetic differences in fruit size, as found in tomato mutants by Bohner and Bangerth (1988b). Actually, a gene coding for a b2-cyclin is localized within the confidence interval of the fresh weight QTL (M. Causse, pers. comm.). Further experiments should test its involvement in the cell number difference between the two genotypes.
Interestingly, truss pruning totally masked the QTL effects on fruit size and the hypothetical genetic control of cell division, but induced a decrease in the number of cells and cell layers, especially in the CF14-L fruits, and a global increase in mean cell size in both lines. Why ovary removal tended to limit cell division of the remaining ovary of the same inflorescence is unclear, but the CF14-L line was more sensitive to this unknown factor. In that case the period of cell expansion appeared to exert the main control on final fruit size. Similar growth of CF12-C and CF14-L fruits on pruned trusses suggested that the detected QTL of fruit size did not involve any control of fruit growth during the cell expansion phase.
The relationship between fruit size and pericarp cell number held only for young fruits (Fig. 4), probably because the importance of cell size in the control of fruit size is lessened when there is moderate or low competition among sinks. Observations of upper trusses may have increased the differences in fruit size between the two lines and improved the relationship between fruit size and cell number. In the present experiment only the first truss was observed, whereas the QTL analysis was performed on fruits sampled on many upper trusses (Causse et al., 2002) when the competition among sinks is more accentuated. Another hypothesis is that cell division activity before anthesis exerts the main control on potential fruit size (Ho, 1996a) and that final fruit size would be better related to the cell number at anthesis than to the final number of cells in the fruit.
Different responses of basal and tip fruits to truss pruning
Generally, when they grow on the same inflorescence, the first induced fruits at the basal position are bigger and contain more cells than tip fruits (Bangerth and Ho, 1984; Bohner and Bangerth, 1988a). The same was observed in the competition experiment, though the significant differences measured at anthesis were later attenuated (Table 1). The absence of a significant gradient in cell number within the first inflorescence was already observed on round tomatoes (Bertin et al., 2002), probably due to the low number of competing sinks at this stage of plant development. Indeed the number of cells in tip fruits is more sensitive than in basal fruits to the intra-plant competition as shown, for instance, after plant defoliation (Bohner and Bangerth, 1988a).
Comparison of competition and pruned experiments indicated that basal fruits hardly responded to the decrease in truss size, whereas the growth of tip fruits reached that of basal fruits (Fig. 3) mainly by an increase of cell size (Table 1). Pruning the basal flowers removed the dominance exerted on tip ovaries during the period of cell expansion. Auxin (IAA), which affects cell enlargement rather than cell division (Bohner and Bangerth, 1988b), can be assumed to be involved in the response of tip fruits. Indeed Gruber and Bangerth (1990) demonstrated that removal of the dominant fruit in a tomato truss induced an increased of IAA diffusion by the remaining fruits. Interestingly in the pruned experiment, from anthesis to the end of the division period, the cell number increased on average 2·5-fold in basal organs and 4-fold in tip organs, with respective division rates of about 1·3 and two cell generations. The higher division rate in tip ovaries, but with a similar number of cells at both positions, indicated a shorter cell division period in tip fruits, as observed on IAA-induced fruits (Bünger-Kibler and Bangerth, 1983). Thus different responses of basal and tip fruits to the early reduction of truss size could mainly result from induced changes in hormonal signals that determine the dominance of basal organs on the intact inflorescence.
Absence of a direct relationship between fruit or cell size and endoreduplication
Nuclei DNA content was assumed to be a pertinent variable in the control of fruit size and sugar metabolism. In the tomato pericarp, small cells localized around the vascular bundles and in the hypodermis present a low endoreduplication level (4C), whereas cell enlargement is accompanied by a considerable increase in DNA content (Bünger-Kibler and Bangerth, 1983). In arabidopsis a relationship between endoreduplication and cell size was reported in epidermis cells (Melaragno et al., 1993). More generally the level of endoreduplication was suggested to set the size limit of a cell (Traas et al., 1998). However, in fruit tissue, there is little experimental evidence of any direct relationship between endoreduplication and fruit size or carbohydrate metabolism.
The present study did not supply any conclusive results to support these hypotheses. Indeed, fruit size and carbohydrate content were significantly affected through genetic and internal plant controls, rather than by endoreduplication. For instance, the growth of tip fruits was promoted by truss pruning through a large increase of cell size (Table 1), without significant changes in the endoreduplication pattern (Fig. 6). Similarly, Bünger-Kibler and Bangerth (1983) could not find any effect of hormonal treatment on endoreduplication when cell size was increased by four- to five-fold. A reasonable hypothesis is that treatments modifying cell expansion do not necessarily affect the duration of the cell division period. Then the time of the switch from mitosis to endoreduplication would be the main determinant of the final level of ploidy reached by a given cell. This hypothesis would justify higher levels of endoreduplication in small fruit genotypes than in round or beef tomatoes (Bergervoet et al., 1996; N. Bertin, pers. comm.), in parallel to about ten times fewer cells in small fruit genotypes (Bohner and Bangerth, 1988b; Bertin et al., 2002), partly due to a shorter cell division period. This switch is regulated by both developmental cues, endogenous and environmental signals (Traas et al., 1998; Joubès and Chevalier, 2000).
Final fruit composition is affected by genetics rather than by fruit position
Differences in fruit composition mainly resulted from genetic differences. CF12-C fruits presented a higher content in dry matter and carbohydrates than CF14-L fruits (Fig. 7). The average surplus of dry matter accumulation in CF12-C fruits represented less than 0·30 % of fruit fresh weight, whereas the surplus of carbohydrates concerned about 8 % of the fruit dry matter. These results are in accordance with the QTL analysis. The higher dry matter content may result from different phloem and xylem fluxes or different sap concentration (Guichard et al., 2001). Similar starch content in CF12-C and CF14-L fruits, in parallel with higher contents in glucose and fructose in CF12-C tomatoes may result from many enzymatic steps controlling sucrose hydrolysis and starch accumulation, which in turn may also affect the assimilate import into the fruit (Ho, 1996b). The dynamic accumulation of individual compounds during fruit development would help to understand which mechanism was involved in the genetic differences.
In accordance with results reported by Ho et al. (1983), fruit position and truss pruning hardly affected the fruit composition except that there was a slight, but non-significant decrease of dry matter content from basal to tip fruits.
Conclusion
The present analysis is an original approach to define some physiological functions underlying QTLs. Although several physiological and environmental factors interact in the control of fruit size, the cell number appeared to be the main determinant factor of differences in fruit size between the near isogenic lines CF12-C and CF14-L. Thus, the corresponding fruit weight QTL could be assumed to be essentially linked to cell division, excluding cell expansion and endoreduplication. An important interaction between the genetic potential expression and the cultural conditions was demonstrated, since truss pruning masked the QTL effect by promoting cell expansion. Such a result highlights the importance of considering the climatic and culture conditions under which plants are grown for genetic analysis, together with the intrinsic properties of individual fruits.
Received 14 March 2003; Returned for revision: 30 April 2003; Accepted: 17 May 2003Published electronically: 9 July 2003
References
- BangerthF, Ho LC.1984. Fruit position and fruit set sequence in a truss as factors determining final size of tomato fruits. Annals of Botany 53: 315–319. [Google Scholar]
- BergervoetJHW, Verhoeven HA, Gilissen LJW, Bino RJ.1996. High amounts of nuclear DNA in tomato (Lycopersicon esculentum Mill.) pericarp. Plant Science 116: 141–145. [Google Scholar]
- BertinN, Gautier H, Roche C.2002. Number of cells in tomato fruit depending on fruit position and source-sink balance during plant development. Plant Growth Regulation 36: 105–112. [Google Scholar]
- BohnerJ, Bangerth F.1988a. Effects of fruit set sequence and defoliation on cell number, cell size and hormone levels of tomato fruits (Lycopersicon esculentum Mill.) within a truss. Plant Growth Regulation 7: 141–155. [Google Scholar]
- BohnerJ, Bangerth F.1988b. Cell number, cell size and hormone levels in semi-isogenic mutants of Lycopersicon pimpinellifolium differing in fruit size. Physiologia Plantarum 72: 316–320. [Google Scholar]
- Bünger-KiblerS, Bangerth F.1983. Relationship between cell number, cell size and fruit size of seeded fruits of tomato (Lycopersicon esculentum Mill.), and those induced parthenocarpically by the application of plant growth regulators. Plant Growth Regulation 1: 143–154. [Google Scholar]
- CausseM, Saliba-Colombani V, Lecomte L, Duffé P, Rousselle P, Buret M.2002. QTL analysis of fruit quality in fresh tomato: a few chromosome regions control the variation of sensory and instru mental traits. Journal of Experimental Botany 53: 2089–2098. [DOI] [PubMed] [Google Scholar]
- CowanAK, Moore-Gordon CS, Bertling I, Wolstenholme BN.1997. Metabolic control of avocado fruit growth. Plant Physiology 114: 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’AmatoF.1964. Endopolyploidy as a factor in plant tissue development. Caryologia 17: 41–52. [Google Scholar]
- D’AmatoF.1984. Role of polyploidy in reproductive organs and tissues. In: Johri BM, ed. Embryology of Angiosperms. Berlin: Springer-Verlag, 519–566. [Google Scholar]
- GalbraithDW, Harkins KR, Knapp S.1991. Systemic endopolyploidy in Arabidopsis thaliana Plant Physiology 96: 985–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GillaspyG, Ben-David H, Gruissem W.1993. Fruits: a developmental perspective. The Plant Cell 5: 1439–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GomezL, Rubio E, Augé M.2002. A new procedure for extraction and measurement of soluble sugars in ligneous plants. Journal of the Science of Food and Agriculture 82: 360–369. [Google Scholar]
- GomezL, Rubio E, Lescourret F.2003. Critical study of a procedure for the assay of starch in ligneous plants. Journal of the Science of Food and Agriculture (in press). [Google Scholar]
- GruberJ, Bangerth F.1990. Diffusible IAA and dominance phenomena in fruits of apple and tomato. Physiologia Plantarum 79: 354–358. [Google Scholar]
- GuichardS, Bertin N, Leonardi C, Gary C 2001. Tomato fruit quality in relation to water and carbon fluxes. Agronomie 21: 385–392. [Google Scholar]
- HigashiK, Hosoya K, Ezura H.1999. Histological analysis of fruit development between two melon (Cucumis melo L. reticulatus) genotypes setting a different size of fruit. Journal of Experimental Botany 50: 1593–1597. [Google Scholar]
- HoLC.1996a. Tomato. In: Zamki E, Shaffer AA, eds. Photoassimilate distribution in plant and crops New York: Marcel Dekker, 709–728. [Google Scholar]
- HoLC.1996b. The mechanism of assimilate partitioning and carbohydrate compartmentation in fruit in relation to the quality and yield of tomato. Journal of Experimental Botany 47: 1239–1243. [DOI] [PubMed] [Google Scholar]
- HoLC, Hewitt JD.1986. Fruit development. In: Atherton JG, Rudich J, eds. The tomato crop London: Chapman and Hall, 201–240. [Google Scholar]
- HoLC, Sjut V, Hoad GV.1983. The effect of assimilate supply on fruit growth and hormone levels in tomato plants. Plant Growth Regulation 1: 155–171. [Google Scholar]
- JoubèsJ, Chevalier C.2000. Endoreduplication in higher plants. Plant Molecular Biology 43: 735–745. [DOI] [PubMed] [Google Scholar]
- JoubèsJ, Phan T-H, Just D, Rothan C, Bergounioux C, Raymond P, Chevalier C.1999. Molecular and biochemical characterization of the involvement of cyclin-dependent kinase A during the early development of tomato fruit. Plant Physiology 121: 857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JullienA, Munier-Jolain NG, Malézieux E, Chillet M, Ney B.2001. Effect of pulp cell number and assimilate availability on dry matter accumulation rate in a banana fruit [Musa sp. AAA group ‘Grande Naine’ (Cavendish subgroup)]. Annals of Botany 88: 321–330. [Google Scholar]
- LeBotJ, Adamowicz S, Robin P, Andriolo JL, Gary C.1998. Modelling nitrate uptake by greenhouse tomato crops at the short and long time scales. Acta Horticulturae 456: 237–245. [Google Scholar]
- LecomteL, Saliba-Colombani V, Gautier A, Gomez-Jimenez MC, Duffé P, Buret M, Causse M.2003. Fine mapping of QTLs for the fruit architecture and composition in fresh market tomato, on the distal region of the long arm of chromosome 2. Molecular Breeding (in press). [Google Scholar]
- MapelliS, Frova C, Torti G, Soressi GP.1978. Relationship between set, development and activities of growth regulators in tomato fruits. Plant and Cell Physiology 19: 1281–1288. [Google Scholar]
- MelaragnoJE, Mehrotra B, Coleman AW.1993. Relationship between endopolyploidy and cell size in epidermal tissue of Arabidopsis. The Plant Cell 5: 1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba-ColombaniV, Causse M, Gervais L, Philouze J.2000. Efficiency of AFLP, RAPD and RFLP markers for the construction of an intraspecific map of the tomato genome. Genome 43: 29–40. [PubMed] [Google Scholar]
- Saliba-ColombaniV, Causse M, Langlois D, Philouze J, Buret M.2001. Genetic analysis of organoleptic quality in fresh market tomato. 1. Mapping QTLs for physical and chemical traits. Theoretical and Applied Genetics 102: 259–272. [Google Scholar]
- ThompsonDS.2001. Extensiometric determination of the rheological properties of the epidermis of growing tomato fruit. Journal of Experimental Botany 52: 1291–1301. [PubMed] [Google Scholar]
- TraasJ, Hülskamp M, Gendreau E, Höfte H.1998. Endoreduplication and development: rule without dividing. Current Opinion in Plant Biology 1: 498–503. [DOI] [PubMed] [Google Scholar]