Abstract
The morphology, anatomy and histology of mature green vanilla beans were examined by light and transmission electron microscopy. Beans have a triangular cross-section with a central cavity containing seeds. Each angle is lined with tubular cells, or papillae, while the cavity sides consist of placental laminae. The epicarp and endocarp are formed by one or two layers of very small cells, while the mesocarp contains large, highly vacuolarized cells, the cytoplasm being restricted to a thin layer along the cell walls. The radial distributions of glucovanillin and β-glucosidase activity, measured on p-nitrophenyl-β-glucopyranoside and glucovanillin, are superimposable and show how β-glucosidase activity increases from the epicarp towards the placental zone, whereas glucovanillin is exclusively located in the placentae and papillae. Subcellular localization of β-glucosidase activity was achieved by incubating sections of vanilla beans in a buffer containing 5-bromo-4-chloro-3-indolyl-β-d-glucopyranoside as a substrate. Activity was observed in the cytoplasm (and/or the periplasm) of mesocarp and endocarp cells, with a more diffuse pattern observed in the papillae. A possible mechanism for the hydrolysis of glucovanillin and release of the aromatic aglycon vanillin involves the decompartmentation of cytoplasmic (and/or periplasmic) β-glucosidase and vacuolar glucovanillin.
Key words: Orchidaceae, Vanillaplanifolia Andrews, vanilla bean structure, β-d-glucosidase, glucovanillin localization
INTRODUCTION
Vanilla (Vanilla planifolia Andrews), a monocotyledonous orchid native of Central America, is grown for the attractive aroma produced by its fruit. The yearly world production averages 2000 t (Todd, 1998) and most of it is used as hydroalcoholic extracts by the agro-food industry.
Green vanilla beans contain aroma precursors, primarily vanillin β-d-glucoside (or glucovanillin) and minor glycosides of p-hydroxybenzaldehyde, p-hydroxybenzoic acid, vanillic acid (Leong et al., 1989) and p-hydroxybenzylalcohol (Kanisawa, 1993). These precursors are hydrolysed by β-d-glucosidase activity upon ripening on the vine or during the curing process (Arana, 1943; Hanum, 1997; Odoux, 2000), resulting in the release of aromatic aglycons and the generation of a strong vanilla flavour.
The hydrolysis of glucovanillin occurs either at a very late stage of pod maturation (Arana, 1943) or during the initial thermal steps of the traditional curing process which involves killing–sweating sequences to prevent bean dehiscence upon drying (Odoux, 2000). At the usual harvesting time of beans ready to be cured (mature green stage), before the pod becomes dehiscent, both glucovanillin and β-d-glucosidase coexist in the bean without hydrolysis taking place, and so these beans do not possess the characteristic vanilla flavour (Arana, 1943; Wild-Altamirano, 1969; Ranadive et al., 1983; Kanisawa, 1993; Brodélius, 1994; Havkin-Frenkel et al., 1999). This phenomenon has not been explained yet, although several hypotheses can be proposed. First, the enzyme and substrate might not be located in the same tissues or, if so, they might be in different subcellular compartments, e.g. cytoplasm and vacuole. Alternatively, the enzyme and substrate might be in contact but other factors (e.g. β-d-glucosidase inhibitor) prevent hydrolysis. Localizations of β-glucosidase and glucovanillin previously reported in the vanilla bean have been controversial (De Lanessan, 1886; Arana, 1943). Moreover, there are few data concerning vanilla β-glucosidase and only a few papers mention the level of activity upon ripening (Arana, 1943; Wild-Altamirano, 1969; Ranadive et al., 1983), during the curing process (Hanum, 1997; Odoux, 2000), or in extracts obtained under various conditions (Dignum et al., 2001a). Most recently, a vanilla bean β-glucosidase was successfully purified and some of its characteristics studied (Odoux et al., 2003).
The hydrolysis step is of major importance to the final aromatic quality of the finished product. To understand better the events leading to that hydrolysis, and because formerly published data on the morphology, anatomy and histology of vanilla bean tissues are old, scarce and poorly illustrated (De Lanessan, 1886; Villiers et al., 1909; Swamy, 1947; Simony and Duquesnoy, 1953), a detailed examination of vanilla bean structure by light and transmission electron microscopy was made. The radial quantitative distributions of glucovanillin and β-glucosidase in the various tissues of green mature vanilla beans were also determined. Finally, the in situ subcellular localization of β-glucosidase activity in the various constitutive tissues was examined by light microscopy. From these data, a possible mechanism is proposed by which glucovanillin is brought into contact with β-glucosidase activity leading to subsequent hydrolysis and flavour development.
MATERIALS AND METHODS
Plant material
Several batches of mature green vanilla beans (i.e. ready to be cured) were harvested in the Democratic Republic of Madagascar between June and August 2000, then immediately air-freighted to our laboratory and treated upon arrival.
Chemicals
All reagents were of analytical grade. p-Nitrophenyl-β-glucopyranoside (pNPG), 5-bromo-4-chloro-3-indolyl-β-d-glucopyranoside and glucono-δ-lactone were from Sigma (St Louis, MO, USA).
Purification of glucovanillin
Vanilla beans (160 g) were ground with a Waring blender in 96 % ethanol (500 ml). The slurry was boiled for 30 min, and then the medium was filtered on Whatman filter paper and brought to dryness in a vacuum rotary evaporator. The residue was dissolved in distilled water and extracted with methylene chloride (4 × 100 ml). The aqueous phase was injected onto an XAD-2 column (5 × 20 cm) (Rohm and Haas France S.A., Chauny, France), the column washed with water (500 ml), then ethanol (250 ml), and the medium was then brought to dryness. The residue was dissolved in methanol (30 ml) and the solution kept at –18 °C for 48 h. After crystallization of glucovanillin, the methanolic supernatant was discarded. Crystals were then washed on a fritted crucible with ethyl acetate, redissolved in distilled water (50 ml), and the solution passed through a Supelclean LC-18 SPE column (6 ml) (Supelco, Bellefonte, PA, USA). The solution was brought to dryness and the glucovanillin crystals dried overnight in a vacuum oven (50 °C). After total hydrolysis with sweet almond β-glucosidase, the purity was estimated to be about 96 % by measuring the vanillin content using HPLC (Odoux, 2000).
β-Glucosidase assay
β-Glucosidase activity was determined by incubating 0·2 ml of 4 mm p-nitrophenyl-β-glucopyranoside or glucovanillin in 0·1 m sodium phosphate buffer (pH 7·0) with 0·2 ml of enzyme diluted in the same buffer (<0·2 nkat in the reaction medium) for 20 min at 40 °C. [1 nkat of β-glucosidase activity is the amount of enzyme that hydrolyses 1 nanomole of substrate per second at pH 7·0 (40 °C).] When assayed with pNPG, the reaction was stopped by the addition of 1·0 ml of 0·5 M NaOH and the absorbance was read at 400 nm. When assayed with glucovanillin, vanillin released by the enzyme was measured by HPLC (Odoux, 2000). β-Glucosidase activity was also measured on both substrates in the presence of 1·7 mm glucono-δ-lactone.
Dissection of vanilla bean
Cross-sections (0·3 cm thick) of mature green vanilla beans were obtained using a razor blade under a stereomicroscope. Using a scalpel, pieces of flesh (approx. 3 mm3) were carefully dissected from the outer part of the beans (zone 1) to the placental area (zone 4) (Fig. 1C). Additional pieces were also obtained from the three corners of the internal cavity (papillae area; zone 5). Seeds were separated from the placental tissue by gentle brushing. Mature green vanilla beans were also frozen overnight (–20 °C) after which cross-sections (0·3 cm thick) were prepared and pieces of flesh (approx. 3 mm3; zones 6 and 7) (Fig. 1D) carefully dissected by hand.
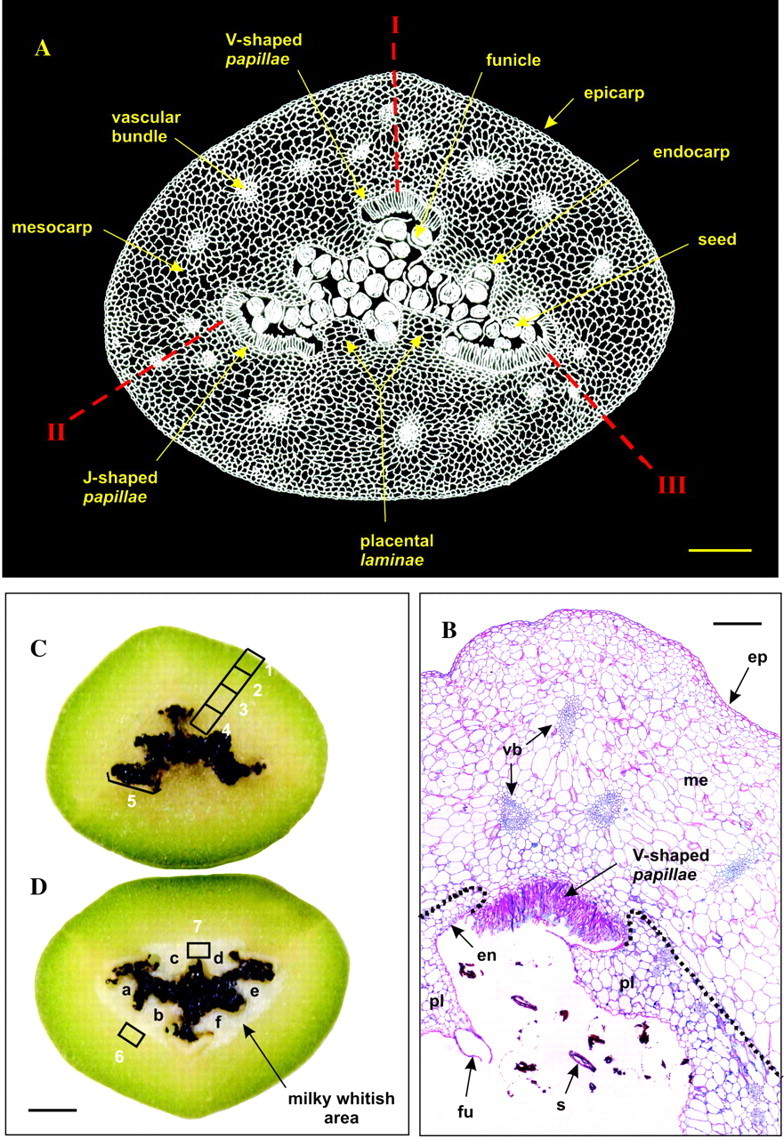
Fig. 1. General views of a transverse equatorial section of a mature green vanilla bean. A, Cross-section drawing of a mature vanilla bean (negative image). Notice the carpel suture lines (I, II, III) and the longitudinal dehiscence splits (II, III). B, Thin cross-section (5 µm thick) of a mature vanilla bean after staining with periodic acid–Schiff (PAS)–Naphthol Blue Black. ep, Epicarp; me, mesocarp; en, endocarp; pl, placental lamina; fu, funicle; s, seed remnants; vb, vascular bundle. The dotted line represents the boundary demarcating the milky whitish area visible in D. C, Cross-section of a fresh, mature green vanilla bean. D, Cross-section of a frozen, mature green vanilla bean. In C and D, zones 1–7 were hand-dissected and analysed for glucovanillin and β-glucosidase contents. Notice that freezing led to the appearance of a frosted, milky whitish area clearly separated from the outer part of the bean by a sharp boundary. a–f, Placental laminae. Bars = 0·15 cm (A), 400 µm (B) and 0·3 cm (C, D).
Pieces (approx. 10 mg) were placed in Eppendorf tubes with 1 ml of 0·1 m phosphate buffer (pH 7·0), homogenized using a micro-Potter Elvejhem and diluted 20 × with the same phosphate buffer. After centrifugation (9000 g, 5 min), diluted extracts were then analysed for β-glucosidase and glucovanillin content as follows. To determine β-glucosidase, the activity was measured as described above on 20 × diluted extracts and expressed as nkat g–1 (f. wt basis). To determine glucovanillin, 12 ml of methanol containing 0·1 m phosphoric acid was added to 8 ml of the 20 × diluted extract. After filtration, extracts were directly analysed by HPLC (Odoux, 2000) using glucovanillin as an external standard. Glucovanillin concentration was expressed as mm, taking into account the water content of the different zones of approx. 85 %.
Light microscopy
Transverse slices (1 mm thick) were dipped in a fixative containing 0·1 m phosphate buffer (pH 7·0), 1 % glutaraldehyde, 2 % paraformaldehyde and 1 % caffeine. The medium was put under vacuum for 15 min, and sections left to incubate for 24 h at 4 °C. Samples were then dehydrated by successively dipping them in 70 % ethanol for 30 min, 95 % ethanol (2 × 30 min) and ethanol for 8 h. They were then pre-impregnated in a medium containing Technovit 7100 resin (100 ml; Kulzer, Wehrheim, Germany) to which had been added Technovit 7100 accelerator (1 g), Technovit polyethylene glycol (PEG 400; 2 ml) and triethylene glycol dimethacrylate (0·4 ml) diluted 2 × with ethanol for 2 h at 4 °C. Samples were impregnated for 24 h at 4 °C in the undiluted impregnation medium before they were finally embedded at 37 °C in the impregnation medium (15 ml) with 1 ml of Technovit 7100 hardener. Sections (5 µm) were obtained using a Historange LKB microtome, then oxidized for 5 min in 1 % periodic acid, washed with distilled water and stained for 10 min in the dark with Schiff reagent. This reagent was prepared by dispersing basic fuchsin (CI 42500; 1 g) in boiling water (200 ml), filtering the solution once it had cooled to 50 °C, adding sodium metabisulfite (2 g) and 1 m HCl (20 ml), before finally, after 24 h in the dark, activated charcoal (0·5 g) was added and the medium filtered. Sections were washed with distilled water until the washing liquid was colourless and then stained with Naphthol Blue Black (Fisher, 1968) [CI 20470; 1 g in a mixture of acetic acid (7 ml) and distilled water (93 ml)] for 5 min at 60 °C. Sections were quickly washed with distilled water, treated with 7 % acetic acid and finally dried for 15 min at 60 °C. Sections were examined under a DMRXA Leica microscope.
In situ localization of β-glucosidase activity
Transverse slices (0·5 cm thick) were obtained by cutting beans using a scalpel and then dipping them for 30 min at room temperature in 0·1 m phosphate buffer (pH 7·0) containing 4 % paraformaldehyde. Thick sections (100 or 300 µm) were obtained using a vibratome. Sections were dipped for 30–45 min at 28 °C in the following medium: 1 m Na2HPO4 (150 µl), 1 m NaH2PO4 (100 µl), dimethyl sulfoxide (500 µl), N,N′-dimethylformamide (100 µl), 50 mm potassium ferricyanide (25 µl), 50 mm potassium ferrocyanide (25 µl), 5-bromo-4-chloro-3-indolyl-β-d- glucopyranoside (5 mg), distilled water (4·1 ml) (Guivarch’ et al., 1996). After preparation, sections (100 µm thick) were immediately examined under a DMRXA Leica microscope. Sections (300 µm thick) were dipped, dehydrated, impregnated and embedded as described above. Thin sections (5 µm thick) were obtained with a Historange LKB microtome and examined under a DMRXA Leica microscope.
Controls were run as follows: heat denaturation, transverse sections were dipped in a boiling water bath for 5 min then treated as above; β-glucosidase inhibition, after the preliminary fixation step, sections (100 or 300 µm thick) were dipped in 0·1 m phosphate buffer (pH 7·0) containing 1 % glucono-δ-lactone then treated as above.
Transmission electron microscopy
Samples (1 mm3) were fixed in 0·1 m cacodylate buffer (pH 7·2) containing 4 % glutaraldehyde for 12 h at 4 °C, then rinsed in cacodylate buffer and finally postfixed in the dark for 2 h in 0·1 m cacodylate buffer (pH 7·2) containing 1 % osmium tetroxide and 3 % sucrose. Subsequently, they were dehydrated in a graded ethanol series, then embedded in Spurr’s resin. Sections (80 nm thick) were obtained using an ultramicrotome and stained in the dark for 5 min with 2·8 % uranyl acetate in 50 % methanol. Sections were then rinsed with methanol, methanol/distilled water (1/1), distilled water and dried. Sections were observed with a JEOL 1200EX2 electron microscope at 70 keV.
RESULTS
Morphology, anatomy and histology of vanilla bean
The flower of Vanilla planifolia has a unilocular inferior ovary with three united carpels which, after fertilization, develops into a ‘bean’ (or pod), so named because of its characteristic shape (see location of carpel suture lines, I, II and III in Fig. 1A). Upon maturation on the vine, the pod dehisces along two longitudinal splits (II and III) (Fig. 1A) (Swamy, 1947). The pod has a roughly triangular transverse section with a central cavity containing black seeds (Fig. 1C and D). In mature green vanilla beans, the outer fleshy portion of the pod is green pigmented while the inner portion surrounding the central cavity is yellowish (Fig. 1C). Embedded in this tissue are vascular bundles that have been systematically observed as a triangularly arranged triplet at the three corners of the bean (Fig. 1A and B); triplets of vascular bundles are also seen on each side of the bean. Each side of the pod bears a placenta divided into two placental longitudinal laminae (a–f) (Fig. 1A, B and D) bearing funicles to which are attached seeds. On cross-sectioning, each pair of placental laminae appear as finger-shaped lobes bent inside the central cavity. At the three corners of the triangular central cavity are seen layers of very elongated (length/diameter = 10) tubular cells (Fig. 1A and B), the papillae (De Lanessan, 1886; Villiers et al., 1909; Swamy, 1947). The papillae are systematically arranged as a ‘V’ at the non-dehiscent carpel suture line (I) while they form a ‘J’ at the level of dehiscence splits (II and III).
The epicarp consists of one layer of tabular cells (Fig. 1B) and, when seen from above, has an elongated, polygonal shape running parallel to the long axis of the bean. Their thick cell walls (cellulose, hemicelluloses, pectic substances) are stained an intense fuchsia red by the periodic acid–Schiff (PAS) reagent. The endocarp is made of one or two layers of small cells. The mesocarp is a well-developed parenchyma with cells (Figs 1A and B and 2A and B) of increasing volume from the epicarp or endocarp side towards the middle mesocarp where cell size reaches 150–300 µm. In the placental laminae (Figs 1B and 2C) regular piles of large cells are visible. PAS staining of mesocarp cell walls is less intense than that of the epicarp. Proteinaceous material stained greenish-blue by Naphthol Blue Black can be seen at the periphery of the cells (Fig. 2A–C), indicating that most of the intracellular volume is filled by the vacuole. This observation is confirmed by transmission electron microscopy (Fig. 3A): the cytoplasm appears to be restricted to a thin layer along the cell wall (0·5 µm thick on average), representing a cytoplasmic volume of less than 3 % of the intracellular volume. Inside the cytoplasm are mitochondria and endoplasmic reticulum. These organelles are predominantly seen in the placental laminae, suggesting high protein synthesis activity. The presence of aleurone protein bodies (Fig. 3B) also supports this hypothesis and could be related to the stronger staining by the Naphthol Blue Black of the laminae compared with that of the outer mesocarp (Fig. 1B). The hair-like cells (the papillae) are intensely stained by the PAS reagent (Figs 1B and 2D). In agreement with previous observations (De Lanessan, 1886), papillae are also intensely stained by Sudan Red indicating the presence of a high concentration of lipids (data not shown). Some proteinaceous material, stained greenish-blue by Naphthol Blue Black, can be seen in the papillae and in the central cavity in the immediate vicinity of the papillae apical ends (Fig. 2D).
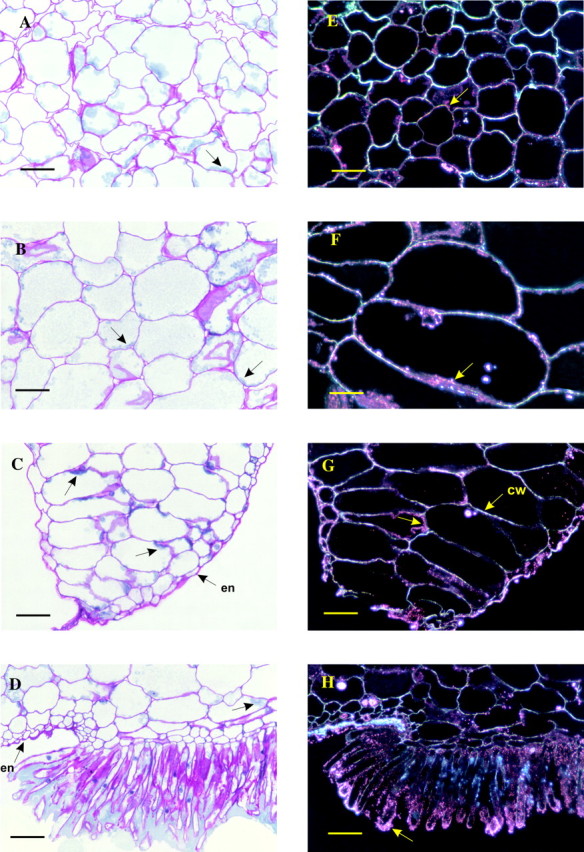
Fig. 2. Light microscopy images of the different portions of a transverse equatorial section of a green mature vanilla bean. A–D, Thin cross-sections (5 µm thick) of a mature vanilla bean after staining with periodic acid–Schiff (PAS)–Naphthol Blue Black: A, outer mesocarp; B, inner mesocarp; C, placental lamina; D, papillae area. en, Endocarp; arrows, cytoplasmic material. E–H, Thin cross-sections (5 µm thick) of a mature vanilla bean after staining for β-glucosidase activity (black field): E, outer mesocarp; F, inner mesocarp; G, placental lamina (cw, cell wall); H, papillae area. Arrows, Positive cytoplasmic material. Bars = 100 µm.
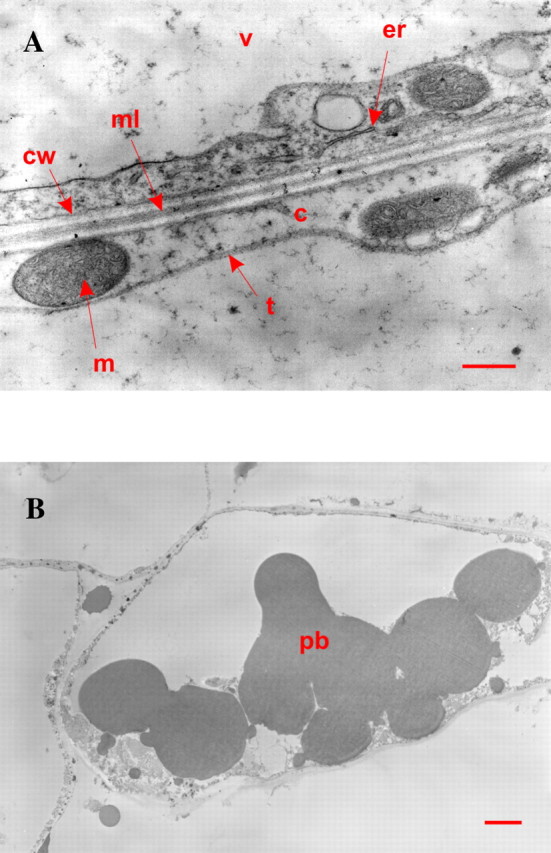
Fig. 3. Transmission electron micrographs of cells from the placental laminae. A, View of a cell edge. c, Cytoplasm; m, mitochondrion; er, endoplasmic reticulum; v, vacuole; t, tonoplast; cw, cell wall; ml, middle lamella. Bar = 500 nm. B, View of a cell exhibiting aleurone protein bodies. Bar = 2 µm.
Radial distribution of glucovanillin and β-glucosidase activity in vanilla bean
Having carefully hand-dissected pieces of flesh from the outer part of beans to the placental area (zones 1–4) and from the papillae area (zone 5) (Fig. 1C), their glucovanillin content and β-glucosidase activity were analysed. The results are presented in Fig. 4.
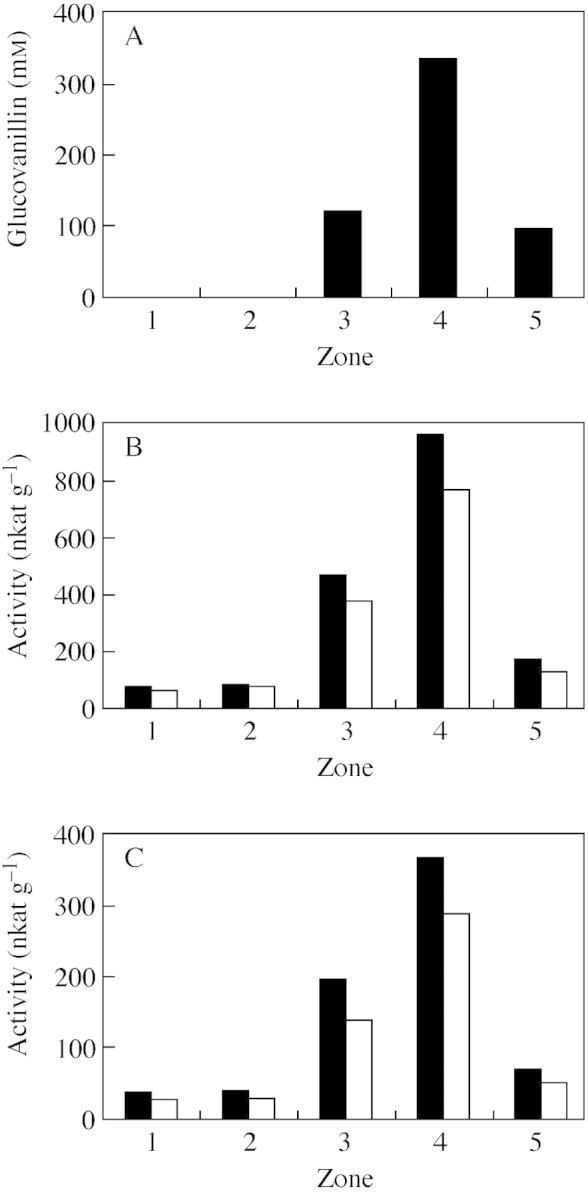
Fig. 4. Distribution of glucovanillin and β-d-glucosidase activity in zones 1–5 (see Fig. 1C) of a transverse equatorial section of a green mature vanilla bean. A, Glucovanillin (mm). B, β-d-Glucosidase (nkat g–1, f. wt): pNPG as substrate (filled squares), with the presence of 1·7 mm glucono-δ-lactone (open squares). C, β-d-Glucosidase (nkat g–1, f. wt): glucovanillin as substrate (filled squares), with the presence of 1·7 mm glucono-δ-lactone (open squares).
Glucovanillin was absent from the epicarp and the outer mesocarp area (zones 1 and 2) (Fig. 4A). It was detected in the inner mesocarp (zone 3) and its concentration found to be much higher in the placental zone (approx. 300 mm) (zone 4). The papillae area (zone 5) had a lower concentration of glucovanillin than the placentae.
Since glucovanillin was found concentrated in the placentae and because it was noticed that a 100 mm glucovanillin solution in distilled water had turned to a milky solid medium after standing overnight at 4 °C, it was decided to freeze beans in order to visualize the radial glucovanillin gradient. Freezing beans leads to the appearance of a milky whitish area separated from the mesocarp by a clear boundary and corresponding to the placental zone (Fig. 1D; compare with Fig. 1C). The papillae also became more distinguishable after freezing. Measuring glucovanillin in zones from the milky whitish area (zone 7) and from the non-whitish zone immediately adjacent to the boundary (zone 6) showed that glucovanillin is only present in the milky whitish zone (approx. 300 mm). Because of the high concentration of glucovanillin in the placentae, freezing led to it becoming insoluble. Although glucovanillin was detected in zone 3, it seems likely that it is restricted exclusively to the placentae and the papillae (zones 4, 5 and 7). Hand-dissection was performed on fresh vanilla beans (Fig. 1C) to measure β-glucosidase activity since freezing beans drastically reduces this activity (Dignum et al., 2001; E. Odoux, pers. comm.). As the clear boundary between the placentae and mesocarp was not visible, it is likely that zone 3 included a little of the placental zone. Results obtained from frozen vanilla beans were clearer with respect to the apparently restricted localization of glucovanillin to the placentae and the papillae of green mature vanilla beans. Finally, it must be mentioned that seeds do not contain glucovanillin.
The activity of β-glucosidase, the enzyme responsible for hydrolysis of glucovanillin into vanillin and glucose, was measured with two substrates, synthetic p-nitrophenyl-β-d-glucopyranoside and natural glucovanillin. Using both substrates, the β-glucosidase was found to have a distribution very similar to that of glucovanillin (Fig. 4B and C; compare with Fig. 4A): weak activity was measured in zones 1 and 2 but it increased enormously from zones 3 to 4. Once again, as for glucovanillin, the papillae area (zone 5) was poorer in β-glucosidase than the placentae. Furthermore, the relative proportions of activity in the different zones were similar using both substrates. When activity was measured in the presence of glucono-δ-lactone, a competitive inhibitor of β-glucosidases, the inhibition percentages for one or other substrate were constant whatever the zone. All these results strongly suggest that the β-glucosidase activity measured with p-nitrophenyl-β-d-glucopyranoside is the same as the activity measured with glucovanillin.
In situ localization of β-glucosidase activity in vanilla bean cells
The β-glucosidase activity was demonstrated by incubating pre-fixed mesocarp sections with the substrate 5-bromo-4-chloro-3-indolyl-β-d-glucopyranoside. Upon hydrolysis this substrate gives 5-bromo-4-chloro-indoxyl, which in turn undergoes oxidative dimerization to give 5,5′-dibromo-4,4′-dichloro-indigo that appears pink in a black field (Fig. 2E–H). A positive reaction was restricted to the peripheral zone of the cells from mesocarp, placental laminae and endocarp (Fig. 2E–H). Most of the intracellular volume of the cells was devoid of any positive reaction. Although the stain seemed to coincide with the cell walls, it can be seen in Fig. 2E–H that in many places cell walls are not stained but appear as bright white structures, suggesting that the β-glucosidase activity is not wall-bound. Finally, we examined the papillae (Fig. 2H) which were also densely marked for β-glucosidase activity, especially at their apical ends. Controls were run by exposing bean sections to a β-glucosidase inhibitor, glucono-δ-lactone, or to heat (100 °C for 5 min). In both cases (inhibition or heat denaturation), no β-glucosidase activity was detected.
DISCUSSION
The results presented here show that, in mature green vanilla beans, the vanillin precursor, glucovanillin, is primarily located in the placentae and, to a lesser degree, in the papillae. The rest of the bean (i.e. the outer fleshy portion) is devoid of this compound. The presence of glucovanillin in the central part of the bean around the seeds (placentae and papillae) could be related to the recognized role of phenolic compounds in seed germination (Kuras et al., 1999) and would agree with the hypothesized role of papillae in the biosynthesis of glycosylated aroma precursors that would then be excreted into the medium surrounding seeds (Swamy, 1947; Havkin-Frenkel et al., 2002).
The tissue distribution of β-glucosidase activity is remarkably similar to that of glucovanillin, although small amounts of activity were detected in the outer fleshy portion where glucovanillin was not detectable. The enzyme is primarily located in the placental laminae and, to a lesser extent, in the papillae where the cells show some of the characteristics of intense protein synthesis (nucleus size, intense staining by Naphthol Blue Black).
All these findings are in disagreement with those of Arana (1943),who stated that glucovanillin is distributed throughout the outer portion (i.e. zones 1–3; 60 –80 % of total glucovanillin) and the central portion (i.e. zone 4 and seeds; 20–40 % of total glucovanillin) including placental tissue and seeds. Arana (1943) also stated that β-glucosidase was exclusively found in the outer parts of the bean, the placental tissue being devoid of activity. For the vanillin to be released from its natural precursor during the curing process, she concluded that during drying glucovanillin must be able to diffuse in water from the central part of the bean to the outer part containing the hydrolytic enzyme. In fact, as the β-glucosidase and its natural substrate glucovanillin exhibit very similar localizations in the bean, there appears to be no need for the substrate to diffuse from the inner part to the outer part of the bean for hydrolysis to take place. In agreement with our observations, it has been reported (De Lanessan, 1886) that when vanilla beans are cut longitudinally into strips from the outer to the inner parts of the bean, only the inner strip (i.e. the placental zone containing both enzyme and substrate) develops a vanilla aroma after drying. Mangin (1886) also reported that vanilla beans contain an inner aromatic pulp (i.e. the placental zone) while the rest of the bean is odourless.
In addition, β-glucosidase activity and its substrate coexist at the same stages of pod development, while the spontaneous hydrolysis of glucovanillin occurs only at a very late stage when beans turn black (Arana, 1943; E. Odoux, pers. comm.). Indeed, glucovanillin is synthesized from the 15th week after pollination, reaching its maximum between 25 and 30 weeks (Kanisawa, 1993; Brodélius, 1994; Havkin-Frenkel et al., 1999), whereas the β-glucosidase activity is present at various levels throughout the life of the fruit (Wild-Altamirano, 1969; Ranadive et al., 1983; Kanisawa, 1993). Therefore, a regulation mechanism must exist to prevent glucovanillin hydrolysis at the green mature stage.
The data presented here show that β-glucosidase activity is unlikely to be bound to either the cell wall or to be of vacuolar origin. The many plant β-glucosidases that are cell wall bound (Nagahashi et al., 1992; Konno et al., 1996) require high ionic strength to become soluble, whereas vanilla β-glucosidase activity is freely soluble in dilute buffers (Hanum, 1997; Dignum et al., 2001; Odoux et al., 2003). An indirect argument in favour of the non-vacuolar origin of the activity is that the pH of a bean extract (i.e. consisting primarily of the vacuolar juice) is 5·0–5·5, a pH value at which vanilla β-glucosidase activity is very unstable (complete loss of activity after 4 h at room temperature) (Odoux et al., 2003). It seems, therefore, that the β-glucosidase activity, localized by light microscopy, is cytoplasmic and likely to be characteristic of many β-glucosidases from monocotyledons (Esen and Stetler, 1993; Leah et al., 1995; Sarry, 2001), and/or periplasmic.
Although glucovanillin was not localized, it is probably in the vacuole as this is the most common storage compartment for secondary metabolites (Kuras et al., 1999; Wink, 1999; Beckman, 2000). Moreover, the concentration found in the placental laminae (300 mm) is incompatible with cytoplasmic localization because a cytoplamic volume of less than 3 % of the intracellular volume implies a 10 m concentration of cytoplasmic glucovanillin which would be insoluble.
Finally, as both enzyme and substrate are located in the same tissues, although probably present in two different cellular compartments (cytoplasmic and/or periplasmic β-glucosidase and vacuolar glucovanillin), one has to understand why vanillin is not released until beans are cured or ripened on the vine. The hydrolysis of glucovanillin occurs at a very late stage of maturation when beans turn black, and could be due to a decompartmentation by minor or major alterations to the tonoplast, the cytoplasmic membrane and the cell walls. Indeed, black beans ripened on the vine lose the turgescence they show during the mature green stage and can be fully flexed between the fingers. This change in texture also occurs during the traditional curing process and probably reflects a destructuralization of the tissues at the membrane and cell wall levels.
Future goals will be to demonstrate the fine subcellular localization of β-glucosidase (cytoplasm, plastids, reticulum, periplasm) by raising antibodies against purified vanilla β-glucosidase (Odoux et al., 2003), labelling these antibodies, and applying them to sections for examination in transmission electron microscopy. Subcellular localization of glucovanillin in cells will also need to be studied by appropriate techniques (e.g. cryofixation, confocal microscopy, preparation of vacuoles from protoplasts). Finally, possible alterations of the tonoplast, cytoplasmic membrane and cell walls at a late stage of maturity and during the initial steps of curing will be examined by transmission electron microscopy.
Received: 31 March 2003; Returned for revision: 16 April 2003; Accepted: 20 May 2003Published electronically: 18 July 2003
References
- AranaFE.1943. Action of a β-glucosidase in the curing of vanilla. Food Research 288: 343–351. [Google Scholar]
- BeckmanH.2000. Phenolic-storing cells: keys to programed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiological and Molecular Plant Pathology 57: 101–110. [Google Scholar]
- BrodéliusPE.1994. Phenylpropanoid metabolism in Vanilla planifolia Andr. V. High performance liquid chromatographic analysis of phenolic glycosides and aglycones in developing fruits. Phyto chemical Analysis 5: 27–31. [Google Scholar]
- De LanessanJ-L.1886. Vanille. In: De Lanessan J-L, ed. Les plantes utiles des colonies françaises Paris: Imprimerie Nationale, 62–74. [Google Scholar]
- DignumMJW, Kerler J, Verpoorte R.2001. β-Glucosidase and peroxidase stability in crude enzyme extracts from green beans of Vanilla planifolia Andrews. Phytochemical Analysis 12: 174–179. [DOI] [PubMed] [Google Scholar]
- EsenA, Stetler DA.1993. Subcellular localization of maize β-glucosidase. Maize Genetics Cooperation Newsletter 67: 19–20. [Google Scholar]
- FisherDB.1968. Protein staining of ribboned epon sections for light microscopy. Histochemie 16: 92–96. [DOI] [PubMed] [Google Scholar]
- Guivarch’A, Caissard JC, Azmi A, Elmayan T, Chriqui D, Tepfer M.1996.In situ detection of expression of the gus reporter gene in transgenic plants: ten years of blue genes. Transgenic Research 5: 281–288. [Google Scholar]
- HanumT.1997. Changes in vanillin and activity of β-glucosidase and oxidases during post harvest processing of vanilla beans (Vanilla planifolia). Bulletin Teknologia dan Industri Pangan 8: 46–52. [Google Scholar]
- Havkin-FrenkelD, Podstolski A, Witkowska E, Molecki P, Mikolajczyk M.1999. Vanillin biosynthetic pathways: an over view. In: Fu TJ, Singh G, Curtis WR eds. Plant cell and tissue culture for the production of food ingredients New York: Kluwer Academic, Plenum Publisher. 35–43. [Google Scholar]
- Havkin-FrenkelD, Pak FE, French J.2002. The botany and ecobotany of vanilla. Abstracts of the XXVIth International Horticultural Congress, Toronto, August 2002: abstract 1540-1600 SO6-O-15, 181–182. [Google Scholar]
- KanisawaT.1993. Flavor development in vanilla beans. Kouryou 180: 113–123. [Google Scholar]
- KanisawaT, Tokoro K, Kawahara S.1994. Flavour development in the beans of Vanilla planifolia. In: Kurihara K, Suzuki N, Ogawa H, eds. Olfaction Taste XI, Proceedings of the International Symposium Tokyo: Springer, 268–270. [Google Scholar]
- KonnoH, Yamasaki Y, Katoh K.1996. A β-glucosidase associated with cell walls from cell suspension cultures of carrot. Phytochemistry 43: 1157–1161. [Google Scholar]
- KurasM, Stefanowska-Wronka M, Lynch JM, Zobel AM.1999. Cytochemical localization of phenolic compounds in columella cells of the root cap in seeds of Brassica napus – change in the localization of phenolic compounds during germination. Annals of Botany 84: 135–143. [Google Scholar]
- LeahR, Kigel J, Svendsen I, Mundy J.1995. Biochemical and molecular characterization of a barley seed β-glucosidase. Journal of Biological Chemistry 26: 15789–15797. [DOI] [PubMed] [Google Scholar]
- LeongG, Archavlis A, Derbesy M.1989. Research on the glucoside fraction of the vanilla bean. Journal of Essential Oil Research 1: 33–41. [Google Scholar]
- ManginA.1886. Les vanilliers. In: Mame A and sons, eds. Les plantes utiles Tours, 134–141. [Google Scholar]
- NagahashiG, Lassiter GD, Patterson DL.1992. Unique properties of cell wall-associated β-glucosidase. Plant Science 81: 163–168. [Google Scholar]
- OdouxE.2000. Changes in vanillin and glucovanillin concentrations during the various stages of the process traditionally used for curing Vanilla fragrans beans in Réunion. Fruits 55: 119–125. [Google Scholar]
- OdouxE, Chauwin A, Brillouet JM.2003. Purification and characterization of vanilla bean (Vanilla planifolia Andrews) β-D-glucosidase. Journal of Agricultural and Food Chemistry 51: 3168–3173. [DOI] [PubMed] [Google Scholar]
- RanadiveAS, Szkutnica K, Guerrera JG, Frenkel C.1983. Vanillin biosynthesis in vanilla beans. In: Proceedings 9th International Congress on Essential Oils, Singapore, Malaysia, 147–154. [Google Scholar]
- SarryJE.2001.Etude biochimique et moléculaire de β-D-glucoside hydrolases de la baie de raisin (Vitis vinefera L.). PhD Thesis, Université Montpellier II, France. [Google Scholar]
- SimonyR, Duquénois P.1953. Compléments à la connaissance anatomique et histochimique des fruits de vanilliers cultivés, en particulier dans les départements et territoires français d’outre-mer. Etudes d’Outre-Mer 36: 129–134. [Google Scholar]
- SwamyBGL.1947. On the life history of Vanilla planifolia Botanical Gazette 108: 449–456. [Google Scholar]
- Todd H.1998. The North American market for vanilla. Perfumer and Flavorist 23: 23–25. [Google Scholar]
- VilliersA, Collin E, Fayolle M.1909. Vanille. In: Doin O and Sons eds. Traite des falsifications et altérations des substances alimentaires. II Aliments principaux et condiments Paris: Dion O and sons, 339–349. [Google Scholar]
- Wild-AltamiranoC.1969. Enzymic activity during growth of vanilla fruit. 1. Proteinase, glucosidase, peroxidase and polyphenoloxidase. Journal of Food Science 34: 235–238. [Google Scholar]
- WinkM.1999. Compartmentation of secondary metabolites and xenobiotics in plant vacuoles. Advances in Botanical Research 25: 141–169. [Google Scholar]


