Abstract
Changes in photochemical activity induced by water deficit were investigated in Talinum triangulare, an inducible CAM plant. The aim was to analyse the interactions between C3 photosynthesis, induction and activity of CAM, photosynthetic energy regulation and the mechanisms responsible for photoprotection and photoinhibition under water stress. Gas exchange, chlorophyll a fluorescence, titratable acidity, carotenoid composition and relative contents of the PSII reaction centre protein (D1) were measured. A decrease in xylem tension (ψ) from –0·14 to –0·2 MPa substantially decreased daytime net CO2 assimilation and daily carbon gain, and induced CAM, as shown by CO2 assimilation during the night and changes in titratable acidity; a further decrease in ψ decreased nocturnal acid accumulation by 60 %. Non-photochemical quenching of chlorophyll a fluorescence (NPQ) increased with water deficit, but decreased with a more severe drought (ψ below –0·2 MPa), when CAM activity was low. NPQ was lower at 0900 h (during maximum decarboxylation rates) than at 1400 h, when malate pools were depleted. Down-regulation of PSII activity related to the rise in NPQ was indicated by a smaller quantum yield of PSII photochemistry (ΦPSII) in droughted compared with watered plants. However, ΦPSII was larger at 0900 h than at 1400 h. The de-epoxidation state of the xanthophyll cycle increased with drought and was linearly related to NPQ. Intrinsic quantum yield of PSII (FV/FM) measured at dusk was also lower in severely stressed plants than in controls. Under maximum photosynthetic photon flux and high decarboxylation rates of organic acids, the D1 content in leaves of droughted plants showing maximal CAM activity was identical to the controls; increased drought decreased D1 content by more than 30 %. Predawn samples had D1 contents similar to leaves sampled at peak irradiance, with no signs of recovery after 12 h of darkness. It is concluded that under mild water stress, early induction of CAM, together with an increased energy dissipation by the xanthophyll cycle, prevents net degradation of D1 protein; when water deficit is more severe, CAM and xanthophyll cycle capacities for energy dissipation decline, and net degradation of D1 proceeds.
Key words: CAM, D1 protein, photoinhibition, Talinum triangulare, water deficit, xanthophylls.
INTRODUCTION
Inducible CAM is a mechanism whereby a switch from C3 photosynthetic CO2 fixation to nocturnal organic acid accumulation in darkness, greatly decreases water loss during drought due to stomatal closure in the light when atmospheric humidity and leaf temperature favour water loss (Lüttge, 1997). Once CAM is induced, decarboxylation of organic acids in the day during phase III, increases internal CO2 and reduces stomatal conductance (Osmond, 1978). The carboxylation of RuBP behind closed stomata (Lüttge, 1997) maintains linear electron flow through PSII reaction centres.
It has been widely reported that induction of CAM is accompanied by important changes in PSII photochemistry (Adams et al., 1987; Adams and Osmond, 1988; Mattos and Lüttge, 2001). However, attempts to understand the photochemical mechanisms underlying the changes in PSII photochemistry during the induction of CAM by water deficit are few. Most have concentrated on the operation of the xanthophyll cycle and its relation to CAM-modulated changes in chlorophyll a fluorescence parameters (Winter et al., 1990; Winter and Lesch, 1992; Maxwell et al., 1994; Barker and Adams, 1997).
Photoinhibition, the light-induced persistent decrease in the efficiency of open PSII reaction centres, is a complex process extensively debated (Krause, 1988; Osmond, 1994; Osmond and Grace, 1995). Damage and breakdown of the PSII reaction centre protein, D1, is considered one of the main causes of photoinhibition (Aro et al., 1993), since D1 has the fastest rate of turnover among the proteins within the thylakoid membrane and its cycle is light dependent (Mattoo et al., 1984). Mechanisms which provide protection against photoinhibition and degradation of D1 protein operate in the thylakoid membrane. A very important photo-protective process is the xanthophyll cycle. This cycle is responsible for the de-excitation of excess energy by non-radiative dissipation in the pigment bed of the light-harvesting chlorophyll–protein complexes (Demmig-Adams and Adams, 1996). The cycle involves the enzymatic de-epoxidation of violaxanthin (V) to zeaxanthin (Z) via antheraxanthin (A) and re-epoxidation of Z to V via A. In low light or darkness, the thylakoid proton gradient is small and epoxidation of Z to V takes place, whereas at high light, low pH in the lumen promotes the conversion of V to Z (Yamamoto et al., 1962).
Inducible CAM is a very important biochemical and physiological adaptation, by which many plants adapt to dry, hot conditions often with large photon fluxes. However, the xanthophyll cycle is also of great importance. The balance between these processes is examined in Talinum triangulare (Jacq.) Wild. (Portulacaceae), a perennial shrub with succulent leaves commonly found in Venezuela from sea level up to 300 m in both exposed and shaded areas. Plants with a good water supply perform typical C3 photosynthesis. However, a few days after withholding watering, CAM is induced but decreases with prolonged drought; photosynthetic rate returns to control values less than 24 h after re-watering (Herrera et al., 1991). These observations suggest that leaves of T. triangulare have particularly efficient mechanisms of defence against photoinhibition which may explain its ecological distribution and adaptability. Therefore, in this study, chlorophyll a fluorescence parameters and two main processes associated with changes in PSII activity, i.e. the activity of the xanthophyll cycle and relative contents of the PSII core protein D1, in the inducible CAM plant Talinum triangulare subjected to drought, are examined.
MATERIALS AND METHODS
Plant material and growth conditions
Plants of T. triangulare collected at Meseta de Mamo (Vargas State, Venezuela) during April 2000, were grown in 10-l pots containing commercial compost in the glasshouse under natural light. Plants were acclimated to glasshouse conditions for 30 d before the experiments. During acclimation the plants were fully watered every day and fertilized weekly with commercial N : P : K (15 : 15 : 15) fertilizer. Day length was 12 h (0600–1800 h), mean maximum daily (between 0900 h and 1430 h) photosynthetic photon flux (PPF) was 1507 ± 22 µmol photons m–2 s–1, mean air temperature 32 ± 5 °C and relative humidity 60 ± 10%. Water deficit was imposed by withholding watering over 26 d to 20 plants, 35 d after transplanting. The youngest fully expanded leaf was used for measurements. In all cases, five leaves from different plants (either droughted or irrigated) were sampled, except for instantaneous gas exchange measurements, where three leaves per treatment were used. For 24-h courses of gas exchange, one leaf was used, and courses were recorded in two different plants each day.
Water relations
Xylem tensions were measured with a pressure chamber (PMS, Corvallis, OR, USA) in branches taken at dawn. Relative water content (RWC) was determined as RWC = (FW – DW)/(TW – DW), where FW is the leaf fresh weight, TW is the turgid weight after 3 h of re-hydration in distilled water and DW is the dry weight of the leaf after being oven-dried for 48 h.
Gas exchange and CAM activity
Gas exchange was measured in the glasshouse. Daily maximum photosynthetic rate (A) between 0900 and 1400 h was measured at a PPF of approx. 1300 µmol m–2 s–1 with an infra-red gas analyser (CIRAS 1; PP Systems, Hitchin, UK); the CO2 concentration of the gas stream entering the leaf chamber was 350 ± 10 µmol mol–1. During measurements, air temperature and relative humidity were 30 ± 2 °C and 45 ± 5 %, respectively. Gas exchange was measured at night throughout the drought cycle; night temperature, relative humidity and CO2 concentration within the leaf chamber were 20 ± 4 °C, 60 ± 8 % and 350 ± 10 µmol mol–1, respectively.
Titratable acidity was determined in leaf samples taken at hourly intervals from dawn to dusk and immediately frozen in liquid nitrogen. Samples were ground to a fine powder in liquid nitrogen and extracted in 50 ml of deionized water. The extracts were boiled for 5 min and titrated with 10 mol m–3 KOH to pH 7 (Nobel, 1988). Nocturnal acid accumulation (ΔH+) was calculated as the difference between dawn and dusk values. The relative recycling of CO2 by CAM was calculated for the plants on which 24 h gas exchange courses were done, according to Griffiths (1988). The percentage of CO2 recycling = 100 × [(0·5 × ΔH+) – integrated night-time A)]/(0·5 × ΔH+), taking ΔH+ on an area basis.
Chlorophyll a fluorescence
Chlorophyll a fluorescence was measured with a Mini PAM 101 fluorometer (Walz, Effeltrich, Germany) at 0530, 0900, 1400 and 1900 h with sunlight as actinic light, as for photosynthesis measurements. The following fluorescence parameters were calculated: maximum efficiency of open PSII reaction centres, FV/FM = (FM – FO)/FM; quantum yield of PSII photochemistry, ΦPSII = (FM′ – FS)/FS (Genty et al., 1989), and -non-photochemical quenching, NPQ = (FM/FM′) – 1; where FO is the minimum fluorescence signal of dark adapted leaves, FM is the maximum fluorescence signal of dark adapted leaves after a pulse of saturating light, FS is the minimum fluorescence signal of an illuminated leaf, and FM′ is the maximum fluorescence signal of an illuminated leaf after a pulse of saturating light.
Pigment analysis
Leaf samples taken before dawn and at 0900 h were freeze clamped and immersed in liquid nitrogen until analysis. Samples were ground in 100 % acetone under liquid nitrogen and centrifuged at 9000 g and 4 °C for 10 min. The supernatant was removed and stored and the pellets extracted with 100 % acetone until they were colourless. Extracts were combined and filtered through a nylon membrane (0·45 µm pore size) and the xanthophylls analysed by HPLC. The HPLC system comprised a controller (Waters 600S; Waters, Milford, MA, USA) and a solvent pump (Waters 626) connected to a photodiode array (PDA) (Waters 996). A non-end-capped C18 column (Resolve Waters, Milford, MA, USA) was used for the separation of pigments. Elution of pigments was by a linear solvent gradient from 100 % of a mixture of acetonitrile:water (90 : 10) v/v to 100 % of a mixture of ethylacetate : methanol (80 : 20) v/v over 30 min and the pigments were detected at 445 nm. Conversion of peak areas from leaf extracts to pigment amounts was done using pure standards prepared by separation of bands of individual pigments by thin layer chromatography. Extinction coefficients published by Davies (1976) were used. Chlorophylls a and b were determined as in Lichtenthaler and Wellburn (1983). The de-epoxidation state of the xanthophyll cycle was calculated as DEPS = (Z + 0·5A)/(Z + A + V).
D1 determination
Leaves from droughted and irrigated plants were taken at dawn, during the hours of maximum PPF and at dusk and were immersed in liquid nitrogen until analysis. Extraction of D1 protein was as described by Baena-González et al. (1999), except that 300 mol m–3 sucrose was used instead of sorbitol. Ground samples were centrifuged at 7000 g and 4 °C for 5 min, the supernatant discarded and the pellet re-suspended in 1 ml shocking buffer (10 mol m–3 HEPES pH 7·5, 5 mol m–3 sucrose, 5 mol m–3 MgCl2 and 10 mol m–3 NaF), extracts were centrifuged again at 8000 g and 4 °C for 3 min and the supernatant discarded. The pellet was re-suspended in 200 µl suspension buffer (10 mol m–3 HEPES pH 7·5, 100 mol m–3 sucrose, 5 mol m–3 NaCl, 10 mol m–3 MgCl2 and 10 mol m–3 NaF). Extraction was done under dim light.
SDS–PAGE and Western blotting
Samples were solubilized according to Laemmli (1970). Amounts of solubilized protein equivalent to 1 µg chlorophyll were loaded into each well of 15 % (w/v) polyacrylamide gels containing 6 kmol m–3 urea. Proteins were blotted onto a PVDF membrane in a semi-dry blotting system and the membrane incubated overnight with anti-D1 protein antibody raised against the D–E loop of D1 from Synechocystis spp., provided by Prof. Eva Mari Aro (University of Turku, Finland). Bands corresponding to D1 protein were detected by chemioluminescence after exposure of the PVDF membrane to x-ray films and quantified densitometrically. Values were expressed as a percentage of the value for irrigated plants sampled at dawn (control).
Data were analysed with a one-way ANOVA, and statistical significance was assumed when P < 0·05.
RESULTS
Xylem tension decreased with drought in plants of T. triangulare (Fig. 1); RWC was 0·95 in watered plants and decreased to 0·89 and 0·78 after 7 and 26 d of drought, respectively (data not shown). Maximum daytime A decreased from 14·0 to about 2·0 μmol CO2 m–2 s–1 with a small decrease in xylem tension (from –0·14 to –0·2 MPa); thereafter, daytime net CO2 uptake remained at approx. 2·0 μmol CO2 m–2 s–1 until day 25, when daytime CO2 uptake was lower than 1·0 µmol CO2 m–2 s–1, during phases II and IV of CAM (Fig. 2). Stomatal conductance (gs) followed the same trend as A throughout the experiment. No differences in gas exchange patterns were observed between irrigated plants measured after 26 d of experiment and those sampled at the beginning of the drought treatment (data not shown); the same is true for other variables measured (see below). A xylem tension of –0·2 MPa induced nocturnal CO2 assimilation in T. triangulare which continued with a further decrease in ψ; net CO2 exchange during the night was nearly zero in severely droughted plants (Fig. 2). Drought progressively decreased total carbon gain, being smallest in severely stressed plants (Table 1). This decrease was the result of a diminished CO2 assimilation rate during the day and an increase in night-time carbon gain due to CAM induction, which also decreased after 25 d of drought.
Fig. 1. Time-course of changes in xylem tensions of plants of T. triangulare subjected to drought. Open circle, plants irrigated throughout the experiment and measured on day 26. Values are means ± s.e. of n = 3. When not evident, error bars are smaller than the symbols.

Fig. 2. Changes in CO2 assimilation (filled circles) and stomatal conductance (open circles) in plants of T. triangulare measured on different days during a drought cycle. The black bar on the abscissa indicates the dark period.
Table 1.
Changes with drought in daytime and night-time total carbon gain and relative internal CO2 recycling (%R)
| Carbon gain (24 h) (mmol m–2) | |||
| Time under drought (d) | Daytime | Night-time | %R |
| 0 | 179·2 | 0·0 | 100 |
| 7 | 38·9 | 5·4 | 63 |
| 14 | 20·7 | 5·4 | 43 |
| 25 | 7·0 | 1·5 | 82 |
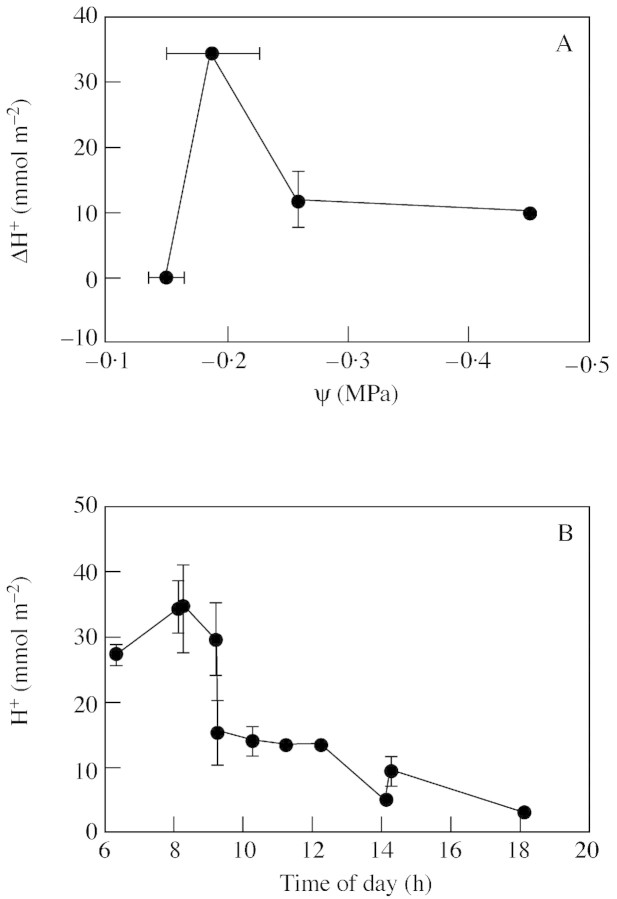
Watered plants showed no daily oscillations of titratable acidity, while the highest ΔH+ occurred on the seventh day of drought at ψ = –0·2 MPa and declined by approx. 60 % when xylem tensions were lowest (Fig. 3A). Decarb oxylation, measured as the decrease in H+ content, in plants droughted for 7 d was fastest between 0800 and 0900 h and completed at 1400 h (Fig. 3B). Internal recycling of CO2 was high throughout the drought cycle, and was larger in severely than in moderately droughted plants, when the daily carbon gain was minimal (Table 1).
Fig. 3. A, Changes in nocturnal acid accumulation as a function of xylem tension in plants of T. triangulare subjected to drought. Acid accumulation was calculated as the difference between dawn and dusk values. B, Daily course of titratable acidity measured in plants droughted for 7 d (ψ = –0·2 MPa). Values are means ± s.e. of n = 5.
Combining data from Figs 1 and 2 and Table 1, it was calculated that on day 7 of drought, during phase III of CAM, between three and 18 times more CO2 was produced in the mesophyll by the decarboxylation of malate than assimilated from air. In severely droughted plants, CO2 from decarboxylation was three times as high as that fixed by a low photosynthetic rate at 0900 h.
Maximum quantum yield of PSII, FV/FM, in severely droughted plants did not recover to control values in the evening (after 1–2 h of darkness) (Fig. 4A). Both pre-dawn and evening values of FO decreased with the onset of drought, more severe drought increased FO at dusk, compared with plants at a xylem tension of –0·2 MPa (Fig. 4B). Values of ΦPSII decreased by approx. 85 % at a ψ = –0·2 MPa, remaining stable until the end of the drought cycle (Fig. 4C). However, during the hours of maximum decarboxylation (0900 h) ΦPSII was larger than at 1400 h, when most of the malate had been consumed. No differences between 0900 and 1400 h were observed in severely stressed plants. Non-photochemical quenching of chlorophyll fluorescence increased at a ψ = –0·2 MPa compared with well-watered plants; afterward, NPQ decreased slightly but remained higher than in irrigated plants (Fig. 4D). In irrigated plants and in plants with maximum CAM activity (ψ = –0·2 MPa), NPQ was smaller when measured at 0900 h than at 1400 h; these differences were not detected in severely stressed plants.

Fig. 4. Changes in photochemical parameters of leaves of T. triangulare at different xylem water tensions (ψ): A, maximum quantum yield of PSII before dawn and in the evening (after 1–2 h of darkness); B, minimum fluorescence before dawn and in the evening (after 1–2 h of darkness); C, quantum yield of PSII at maximum decarboxylation rates (0900 h); and after malate was exhausted (1400 h); D, non-photochemical quenching at maximum decarboxylation rates (0900 h) and after malate was exhausted (1400 h). Empty symbols are of plants irrigated throughout the experiment and measured on day 26. Values are means ± s.e. of n = 5.
Chlorophyll and carotenoid contents remained constant during the experiment until plants showed a xylem tension of –0·45 MPa, when a significant increase, relative to irrigated plants, in chlorophyll content was observed (Fig. 5A). This was due to leaf compaction, as dry mass per unit area increased 1·5 times from 3·6 ± 0·3 to 5·3 ± 0·3 mg cm–2. The chlorophyll a/b ratio did not change, remaining at 3·08 ± 0·2. Composition of the xanthophyll cycle changed markedly in response to water deficit (Fig. 5B); at a ψ = –0·2 MPa, V content decreased and A + Z was higher than in watered plants, corresponding to an increase in DEPS of 30 % compared with watered plants (Fig. 5C), in samples taken at peak PPF (phase III of CAM). The overnight retention of Z and A in T. triangulare occurred in watered plants and increased with drought in samples taken at 0530 h (Fig. 5C). There was a linear, positive correlation between NPQ and DEPS (r2 = 0·97); the correlations between NPQ and ΔH+, and between DEPS and ΔH+, were also linear and positive (r2 = 0·99 and 0·95, respectively). Amounts of D1 protein in leaf samples taken during phase III of CAM were unchanged by 7 d of drought (ψ = –0·2 MPa) but decreased more than 35 % in severely droughted plants; pre-dawn samples showed the same trend as leaves sampled at peak irradiance, with no sings of recovery after 12 h of darkness (Fig. 5D).
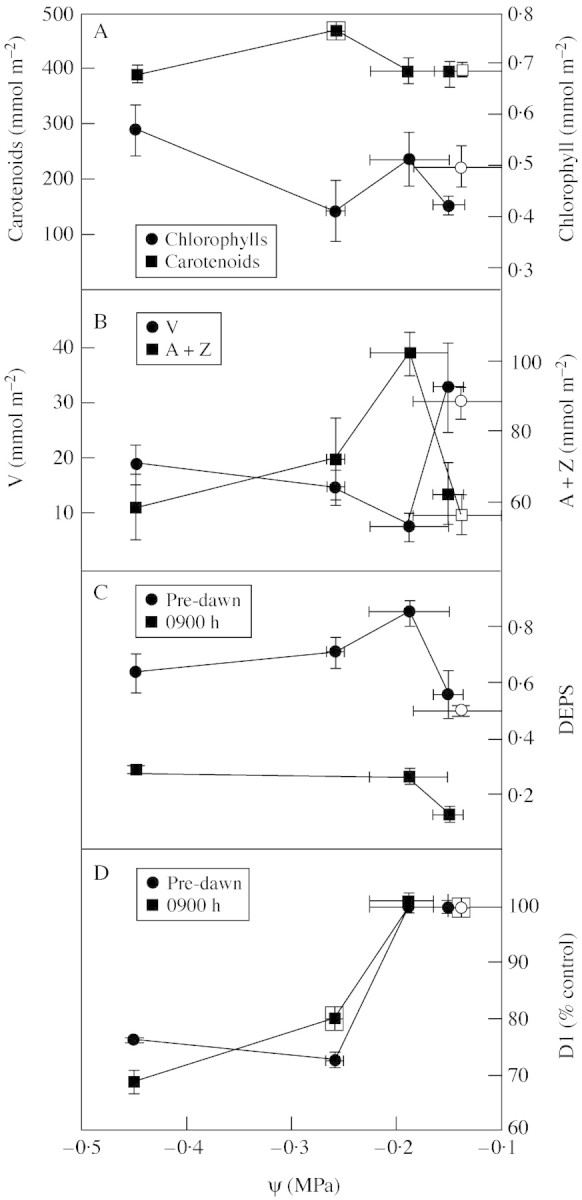
Fig. 5. Changes in pigment composition of leaves from T. triangulare plants with xylem tension: A, chlorophyll content and total carotenoid content; B, contents of violaxanthin and antheraxanthin plus zeaxanthin; C, de-epoxidation state of the xanthophyll cycle at 0900 h and before dawn; D, contents of PSII reaction centre protein (D1), as a percentage of the value of irrigated plants taken at 0530 h, at the time of maximum decarboxylation rate (0900 h) and before dawn. Empty symbols, plants irrigated throughout the experiment and measured on day 26. Values are means ± s.e. of n = 5.
DISCUSSION
Water deficit decreased diurnal net CO2 uptake by 90 % and induced CAM, shown by organic acid accumulation and nocturnal CO2 assimilation, in plants of T. triangulare, as reported previously (Herrera et al., 1991). Maximum decarboxylation rates occurred between 0800 and 0900 h, when irradiance was high. Upon induction of CAM, major changes in photochemical reactions within PSII were observed. The larger NPQ and the lower ΦPSII recorded with the onset of drought, compared with watered plants, indicated a greater non-radiative energy dissipation and suggests that the supply of CO2 from decarboxylation of night-time accumulated acid was not as efficient in providing C3 photosynthesis and allowing it to use excitation energy to carry out non-cyclic electron transport, as CO2 via the stomata. This is also supported by the progressive decrease in total carbon gain with drought. The low efficiency for linear electron transport to CO2 derived from CAM activity is consistent with the relatively small organic acid accumulation observed for T. triangulare in this study. However, at the time of maximum decarboxylation rate (0900 h), NPQ was smaller and ΦPSII was larger, than at 1400 h, when malate pools from nocturnal CO2 uptake had been exhausted. More over, at ψ = –0·45 MPa (day 26), when nocturnal CO2 uptake and the daily carbon gain were minimal, there were no differences in photochemical efficiency between 0900 and 1400 h. These results illustrate how availability of CO2 derived from CAM can modulate photosynthetic efficiency during drought in this inducible species.
The increase in NPQ correlated with the down-regulation of PSII photochemistry. Interestingly, even though ΦPSII decreased drastically after 7 d of drought when xylem tensions fell from –0·14 to –0·2 MPa, and remained low until the end of the experiment, NPQ decreased only when drought stress was more severe, coinciding with a diminished CAM activity (smaller ΔH+ and nocturnal CO2 assimilation). This was rather unexpected, compared with results shown by Adams and Osmond (1988), since a smaller ΔH+ means a lower CO2 availability during phase III of CAM and therefore more excess energy to be dissipated.
An explanation for the decrease in NPQ when ΔH+ was minimal and C3 photosynthesis largely inhibited, is that NPQ is almost entirely determined by the rate constant of energy dissipation, qE (Demmig-Adams and Adams, 1994; Demmig-Adams et al., 1996) with minimal contribution from other components (qT or qI) of NPQ. This seems to be the case in our experiment as a strong positive, linear relationship was found between NPQ and DEPS. In droughted plants of the CAM species Mesembryanthemum crystallinum (Keiller et al., 1994) and Clusia minor (Mattos et al., 1999; Mattos and Lüttge, 2001), qE and qI were inversely related during phase III of CAM, illustrating that once qE is saturated the photoinhibitory component (qI) increases. At ψ below –0·2 MPa, photoinhibition in T. triangulare became important, as there was a decreased capacity for energy dissipation by the xanthophyll cycle and less CO2 accumulated during darkness. At peak irradiance, not only NPQ but also diurnal DEPS decreased and nocturnal DEPS increased. In contrast, more severe drought (ψ below –0·2 MPa) did not increase DEPS further before dawn, and FO rose well above values of plants at ψ = –0·2 MPa, when D1 content started to decrease. These results suggest that the increased xanthophyll cycle activity and the induction of CAM by drought are linked, in an as yet unknown manner, and may be triggered by similar cellular conditions elicited by water deficit. They also suggest that the drought-induced decrease in ΦPSII could be ascribed to different processes within PSII depending on the intensity of the stress. Decreased FO indicates energy dissipation in the antennae, which is capable of quenching not only FM but also FO, whilst an increased FO indicates acceptor-side photoinhibition due to double reduction of QA (Mishra et al., 1994; Osmond and Grace, 1995). Accumulation of doubly reduced QA leads to production of reactive oxygen species (Mishra et al., 1994), which consequently degrade D1 protein.
During phase III of CAM, photosynthesis occurs under highly oxidative conditions (Osmond et al., 1999), due to stomatal closure and therefore elevated intercellular O2 concentrations. In the case of T. triangulare this situation could have been exacerbated because of its intrinsic low organic acid accumulation capacity, which could result in an intercellular CO2 concentration smaller than in obligate CAM plants, despite the high internal CO2 recycling observed in this study. The results presented here support this hypothesis, as net degradation of D1 at peak PPF (phase III of CAM) was found at ψ below –0·2 MPa, when CAM activity had diminished. Additionally, accelerated de-phosphorylation of D1 due to the relatively high temperatures (above 30 °C) which predominated in this experiments (see Materials and Methods) could have also contributed to the enhanced degradation in droughted plants, as has been shown for PSII core proteins, including D1 (Rokka et al., 2000).
The results presented here suggest that, under water deficit, CAM activity in T. triangulare plays a central role in the protection of the photosynthetic machinery from photoinhibition. At maximum CAM activity, a relatively high intercellular CO2 concentration and the capacity for energy dissipation by the xanthophyll cycle are sufficient to prevent damage to and net degradation of D1. When CAM activity is limited and the capacity for energy dissipation by the xanthophyll cycle is exhausted after prolonged drought, inactive reaction centres accumulate with the subsequent degradation of D1.
ACKNOWLEDGEMENTS
We thank Professor E. M. Aro for the kind donation of D1 antibody. The assistance of Susana El Souki is highly appreciated. IVIC receives grant-aided support from the Ministry of Science and Technology of Venezuela. This project was partly supported by a postdoctoral fellowship from CONICIT No. 99001085 to A.J.P.
Received: 30 April 2003; Returned for revision: 29 May 2003; Accepted: 2 June 2003Published electronically: 24 July 2003
References
- AdamsWW III, Osmond B. 1988. Internal CO2 supply during photosynthesis of sun and shade grown plants in relation to photoinhibition. Plant Physiology 86: 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AdamsWW III, Osmond B, Sharkey T. 1987. Responses of two CAM species to different irradiances during growth and susceptibility to photoinhibition by high light. Plant Physiology 83: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AroE-M, Virgin I, Andersson B.1993. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochimica et Biophysica Acta 1143: 113–134. [DOI] [PubMed] [Google Scholar]
- Baena-GonzálezE, Barbato E, Aro E-M.1999. Role of phosphorylation in repair cycle and olygomeric structure of photosystem two. Planta 208: 196–204. [Google Scholar]
- BarkerDH, Adams III WW.1997. The xanthophyll cycle and energy dissipation in differently oriented faces of the cactus Opuntia macrorhiza Oecologia 109: 353–361. [DOI] [PubMed] [Google Scholar]
- DaviesBH.1976. Carotenoids. In Goodwing TW, ed. Chemistry and biochemistry of plant pigments New York: Academic Press, 150–153. [Google Scholar]
- Demmig-AdamsB, Adams WW III.1994. Capacity for energy dissipation in the pigment bed in leaves with different xanthophyll cycle pool. Australian Journal of Plant Physiology 21: 575–588. [Google Scholar]
- Demmig-AdamsB, Adams WW III.1996. Xanthophyll cycle and light stress in nature: a uniform response to excess direct sunlight among higher plant species. Planta 198: 460–470. [Google Scholar]
- Demmig-AdamsB, Adams WW III, Barker DH, Logan BA, Bowling DR, Verhoeven AS.1996. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiologia Plantarum 98: 253–264. [Google Scholar]
- GentyB, Briantais J-M, Baker NR.1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990: 87–92. [Google Scholar]
- GriffithsH 1988. Crassulacean acid metabolism: a re-appraisal of physiological plasticity in form and function. Advances in Botanical Research 15: 43–92. [Google Scholar]
- HerreraA, Delgado J, Paraguatey I.1991. Occurrence of inducible Crassulacean acid metabolism in leaves of Talinum triangulare (Portulacaceae). Journal of Experimental Botany 42: 493–499. [Google Scholar]
- KeillerDR, Slocombe SP, Cockburn W.1994. Analysis of chlorophyll a fluorescence in C3 and CAM forms of Mesembryanthemum crystallinum Journal of Experimental Botany 45: 325–334. [Google Scholar]
- KrauseGH.1988. Photoinhibition of photosynthesis. An evaluation of damaging and protective mechanisms. Physiologia Plantarum 74: 566–574. [Google Scholar]
- LaemmliUK.1970. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- LichtenthalerHK, Wellburn AR.1983. Determination of carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochemical Society Transactions 11: 591–592. [Google Scholar]
- LüttgeU.1997.Physiological ecology of tropical plants. Berlin: Springer-Verlag. [Google Scholar]
- MattooAK, Hoffman-Falk H, Marder JB, Edelman M.1984. Regulation of protein metabolism: coupling of photosynthetic electron transport to in vivo degradation of the rapidly metabolised 32-kilodalton protein of the chloroplast membrane. Proceedings of the Natural Academy of Science USA 81: 1380–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MattosEA, Lüttge U.2001. Chlorophyll fluorescence and organic acid oscillations during transitions from CAM to C3 photosynthesis in Clusia minor L. (Clusiaceae). Annals of Botany 88: 457–463. [Google Scholar]
- MattosEA, Herzog B, Lüttge U.1999. Chlorophyll fluorescence during CAM-phases in Clusia minor L. under drought stress. Journal of Experimental Botany 50: 253–261. [Google Scholar]
- MaxwellC, Griffiths H, Young AJ.1994. Photosynthetic acclimation to light regime and water stress by the C3-CAM epiphyte Guzmania monostachia: gas-exchange characteristics, photochemical efficiency and the xanthophyll cycle. Functional Ecology 8: 746–754. [Google Scholar]
- MishraNP, Francke C, Van Gorkom HJ, Demetrios DF.1994. Destructive role of singlet oxygen during aerobic illumination of the photosystem II core complex. Biochimica et Biophysica Acta 1186: 81–90. [Google Scholar]
- NobelPS.1988.Environmental biology of agaves and cacti. Cambridge: Cambridge University Press. [Google Scholar]
- OsmondB, Maxwell K, Popp M, Robinson S.1999. On being thick: fathoming apparently futile pathways of photosynthesis and carbohydrate metabolism in succulent CAM plants. In: Bryant JA, Burrell MM, Kruger NJ, eds. Plant carbohydrate biochemistry Oxford: Bios Scientific Publishers, 183–200. [Google Scholar]
- OsmondCB.1978. Crassulacean acid metabolism: a curiosity in context. Annual Review of Plant Physiology 29: 379–414. [Google Scholar]
- OsmondCB.1994. What is photoinhibition? Some insights from comparison of shade and sun plants. In: Baker NR, Boyer JR, eds. Photoinhibition: molecular mechanisms to the field Oxford: Bios Scientific Publications, 1–24. [Google Scholar]
- OsmondCB, Grace SC.1995. Perspectives on photoinhibition and photorespiration in the field: quintessential inefficiencies of the light and dark reactions of photosynthesis? Journal of Experimental Botany 46: 1351–1362. [Google Scholar]
- RokkaA, Aro E-M, Herrmann RG, Andersson B, Vener AV.2000. Dephosphorylation of photosystem II reaction centre proteins in plant photosynthetic membranes as an immediate response to abrupt elevation of temperature. Plant Physiology 123: 1525–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WinterK, Lesch M.1992. Diurnal changes in chlorophyll a fluorescence and carotenoids composition in Opuntia ficus-indica, a CAM plant, and in three C3 species in Portugal during summer. Oecologia 91: 505–510. [DOI] [PubMed] [Google Scholar]
- WinterK, Díaz M, Lesch M.1990. Changes in the xanthophyll cycle components and in fluorescence yield in leaves of a Crassulacean acid metabolism plant, Clusia rosea Jacq. throughout a 12-hour photoperiod of constant irradiance. Planta 182: 181–185. [DOI] [PubMed] [Google Scholar]
- YamamotoHY, Nakayama TOM, Chichester CO.1962. Studies on the light and dark interconversions of leaf xanthophylls. Archives of Biochemistry and Biophysics 97: 168–173. [DOI] [PubMed] [Google Scholar]