Abstract
Although fungi cause a recognized problem during storage of recalcitrant seeds of many tropical species, there are no data to date on defence strategies of these seeds against fungal attack. To ascertain whether recalcitrant seeds of Avicennia marina elaborate compounds that might suppress fungal proliferation during hydrated storage, the production and efficacy of β-1,3-glucanase (EC 3.2.1.39) and chitinase (EC 3.2.1.14) were studied in relation to histopathological changes. Freshly harvested seeds had low β-1,3-glucanase and chitinase activities and fluorescence microscopy revealed progressive deterioration of the internal tissues of these seeds associated with fungal infection during hydrated storage. In seeds treated to minimize associated fungi (clean seeds), β-1,3-glucanase and chitinase activities increased significantly during 10 d of hydrated storage. Similar high levels of activity were observed when these seeds were experimentally infected with Fusarium moniliforme and subjected to further storage. The histopathological observations indicated delayed disease development in the 10-d clean-storage period, although the hypersensitive response was not observed. The results suggest that, although the recalcitrant seeds of A. marina elaborate some antifungal enzymes, there is a lack of effective defence strategies that might lead to successful responses against fungal infections.
Key words: Avicennia marina; chitinase; fluorescence microscopy; Fusarium moniliforme; β-1,3-glucanase; hydrated storage; recalcitrant seeds
INTRODUCTION
A number of economically important tropical and subtropical crops, e.g. tea (Berjak et al., 1993), mango, litchi and commercial rubber (Chin and Roberts, 1980), as well as forest and horticultural species, are characterized by producing recalcitrant seeds. Recalcitrant seeds are not only hydrated when they are shed, but are continuously metabolically active. As such, most are akin to seedlings, and bear little resemblance to the familiar quiescent, desiccated orthodox seed (Berjak et al., 1989; Berjak and Pammenter, 2001). Recalcitrant seeds are highly sensitive to desiccation and some are also chilling sensitive, necessitating storage under high relative humidity conditions and at ambient temperatures. This approach, termed wet or hydrated storage is, however, conducive to fungal proliferation that severely curtails the post-harvest lifespan of seeds (Calistru et al., 2000). Although the role of fungi in deterioration during storage of recalcitrant seeds has been the focus of several reports (Mycock and Berjak, 1990, 1995; Pongapanich, 1990; Singh and Singh, 1990; Berjak, 1996; Calistru et al., 2000; Sutherland et al., 2002), there are no published data about the defence strategies of such seeds against fungal attack.It is well known that the plants react to microbial infection with a broad range of defence mechanisms, which may restrict or prevent pathogen growth. Among these are cell wall reinforcement (Hahn et al., 1989), accumulation of antimicrobial proteins (Fritig et al., 1998), production of phytoalexins (Ebel, 1986) and the hypersensitive response (Hammond-Kosak and Jones, 1997), which are all important strategies contributing towards a successful resistance response. Genetic deficiencies in the ability of the plant to activate these various pathways are commonly associated with enhanced susceptibility.The present contribution is a part of an ongoing study on the mechanisms associated with deterioration, and the nature of the responses of recalcitrant seeds to experimental inoculation with Fusarium moniliforme (the most deleterious of the local recalcitrant-seed-associated fungal species). The highly recalcitrant seeds of Avicennia marina (Forssk.) Vierh. lose viability within 14–16 d in wet storage (Berjak et al., 1989) and within 7 d if inoculated with F. moniliforme immediately after harvest. However, if wet-stored for 4 d before inoculation, the seeds become less susceptible to the depredation associated with the presence of this fungus (Calistru et al., 2000), but the effect is transient. The present study was undertaken to assess histopathologically the location and extent of the infection caused by F. moniliforme in A. marina seeds at different stages during hydrated storage and also to ascertain if, and when, these recalcitrant seeds elaborate compounds that might suppress fungal proliferation during storage. The role of two pathogenesis-related (PR) proteins [β-1,3-glucanase (EC 3.2.1.39) and chitinase (EC 3.2.1.14)] was evaluated and related to the short-term storage responses of these seeds. The antifungal properties of β-1,3-glucanases and chitinases have been discussed extensively (for reviews, see Bol and Linthorst, 1990; Stintzi et al., 1993; Van Loon, 1997; Fritig et al., 1998; Van Loon and Van Strien, 1999). Both enzymes have been reported from a variety of monocotyledonous and dicotyledonous plant species, in different tissues and life-cycle stages, including seeds of tobacco (Leubner-Metzger et al., 1995, 1996), tomato (Wu et al., 2001), pea ((Petruzzelli et al., 1999), barley (Jacobsen et al., 1990), maize (Neucere et al., 1991; Huynh et al., 1992) and cucumber (Majeau et al., 1990). However, their occurrence in recalcitrant seeds has not been investigated.
MATERIALS AND METHODS
Seed material and sampling procedures
Seeds were obtained from a single population of A. marina trees in the Beachwood Mangroves Nature Reserve, Durban, South Africa. The mature seeds (propagules that had dropped from the tree within the previous 24 h) were collected during March and early April and the entire study was conducted over two seasons. In the laboratory, the pericarps were removed by soaking the propagules in water for 30 min. All seeds were then surface-sterilized in 1 % sodium hypochlorite for 20 min. After brief rinsing with sterile distilled water (three changes), the seeds were subjected to hydrated storage under the following conditions: 30 seeds were immediately inoculated with an identified strain of F. moniliforme and stored for 8 d, while 180 seeds were maintained clean for a total of 18 d in storage [fungal contamination was kept minimal to zero by an initial application of 2·5 ml l–1 of a fungicide (Previcur N®; AgrEvo, Pietermaritzburg, South Africa)]. From this point, the seeds were referred to as infected-stored and clean seeds, respectively. After 4 and 10 d of storage, 20 clean seeds were removed from the initial containers and inoculated with F. moniliforme. These seeds were then stored for a further period of 8 d (referred to as clean-stored infected seeds). All seeds were stored at 25 °C in a monolayer, on plastic mesh grids suspended about 100 mm over sterile distilled water, within sealed, sterile plastic buckets. To facilitate reporting, the codes (after Calistru et al., 2000) used for each sample are listed in Table 1. The embryonic axes were excised from all samples. Five axes per treatment were immediately processed for histopathology, while the remainder were stored in liquid nitrogen for further use in enzyme assays.
Table 1.
Sample codes
| Sample | Code |
| Fresh seeds (0 d clean, 0 d infected) | 0c0i |
| Seeds stored clean for 4 d | 4c0i |
| Seeds stored clean for 10 d | 10c0i |
| Seeds stored clean for 12 d | 12c0i |
| Seeds stored clean for 18 d | 18c0i |
| Seeds stored infected for 8 d | 0c8i |
| Seeds stored clean for 4 d, infected and re-stored for 8 d | 4c8i |
| Seeds stored clean for 10 d, infected and re-stored for 8 d | 10c8i |
Inoculation
Fusarium moniliforme (subsequently identified by the Plant Protection Research Institute, Pretoria, South Africa), was isolated from freshly harvested A. marina seeds and maintained on potato dextrose agar at 25 °C. Fungal mycelium was harvested after 14 d. The seeds were inoculated with the fungus by aseptically placing small aliquots of the mycelium on the abaxial surface of the cotyledons in the immediate proximity of the embryonic axis.
Fluorescence microscopy
Hypocotyl tips were excised from the embryonic axes, cleared and fixed in a mixture of ethanol/dichloromethane (3 : 1, v/v) containing 0·15 % trichloroacetic acid. The specimens were then stained according to Rohringer et al. (1977), except that 0·1 % Uvitex 2B (Ciba-Geigy, Basel, Switzerland) in 0·1 m Tris–HCl buffer (pH 5·8) was used in place of Calcofluor. Uvitex 2B stain is an optical brightener that specifically binds to the chitin of fungal cell walls. Specimens were stored in 50 % glycerol (containing a trace of lactophenol as preservative) until they were examined with a Nikon E-400 microscope equipped with epifluorescence optics. The filter combination UV-2A (excitation filter 330–380 nm and barrier filter 420 nm) was used in order to visualize the fungal structures by their light-blue fluorescence, and the B-2A combination (excitation filter 450–490 nm and barrier filter 520 nm) used for autofluorescence assessment. Observations were carried out at ×40 and ×100 magnifications.
Protein extraction and determination
To obviate adverse effects of polyphenolics, a method modified as follows from Farrant et al. (1992a) was used. Frozen axes of A. marina (0·5 g) were ground in liquid nitrogen to a fine powder. One gram of insoluble polyvinylpyrrolidone (Sigma, St Louis, MO, USA) was mixed with the sample, which was then transferred into a centrifuge tube and suspended in 7 ml of 50 mm Tris–HCl buffer (pH 7·3), containing 10 mm β-mercaptoethanol and 2 mm phenylmethylsulfonyl fluoride. The suspension was mixed thoroughly for 1 min and incubated on ice for 10 min to extract the soluble proteins. After centrifugation for 30 min at 15 000 g, the supernatant, which contained the total soluble protein, was collected.To remove the interference caused by low-molecular mass sugars, which are a major form of carbohydrate reserve in the seeds (Farrant et al., 1992b), the supernatant was purified by gel filtration on a PD-10 column packed with Sephadex G-25 (Pharmacia, Germany) and then used for enzyme determination.The protein concentration was determined in triplicate according to Bradford (1976), using Bradford reagent (Bio-Rad, Philadelphia, PA, USA) and bovine gamma globulin (Bio-Rad) as the standard.
β-1,3-glucanase assay (Fink et al., 1988)
β-1,3-Glucanase activity was assayed by measuring the rate of reducing sugar production with laminarin (Sigma) as substrate. A standard curve relating absorbance at 540 nm to glucose concentration was used to calculate specific β-1,3-glucanase activity, which was expressed as mg glucose mg–1 protein min–1.
Chitinase assay (Wirth and Wolf, 1990)
Chitinase activity was determined spectrophotometrically with dye-labelled CM-Chitin-RBV (Loewe Biochemica GmbH, Sauerbach, Germany) as substrate. The absorbance was measured at 550 nm and the chitinase activity was expressed as A550 nm mg–1 protein min–1.
All enzyme assays were performed in triplicate and the means ± standard deviation calculated.
RESULTS
The present study was undertaken on the hypocotyl tips of the embryonic axes of A. marina seeds, because during storage this is the region of the embryo that first shows fungal proliferation. The mature propagule of A. marina has an unusually well-developed embryonic axis, with the whole structure resembling a seedling, rather than a seed [see Farrant et al. 1993) where the structure of the A. marina seed is illustrated diagrammatically]. The hypocotyl tip has five (or rarely, more) meristematic root primordia that are enclosed by a thin layer of hypocotyl tissue, from which a thick mass of bristle-like hairs protrudes. Microscopical examination for the fungal infection was performed on the bristles, adjacent hypocotyl surface and the internal tissue of root primordia.
Epifluorescent examination of Uvitex 2B-stained hypocotyl tips excised from freshly harvested seeds (0c0i) showed no evidence of fungal proliferation on the hypocotyl surface and internal tissue of root primordia, although isolated fungal structures could occasionally be seen on the bristles (Fig. 1A–C). No signs of fungal infection could be seen either internally or externally on the bristles or on the hypocotyl surface of A. marina seeds after 4 d clean storage (4c0i; Fig. 1D–F). However, fungal contaminants were observed on the bristles and the surface of the root primordium of seeds stored clean for 10 d (10c0i) and fungal penetration, confined to the epidermal cells, was also noticed (Fig. 1G–I), indicating that the treatment used was fungistatic in the short term, rather than fungicidal.
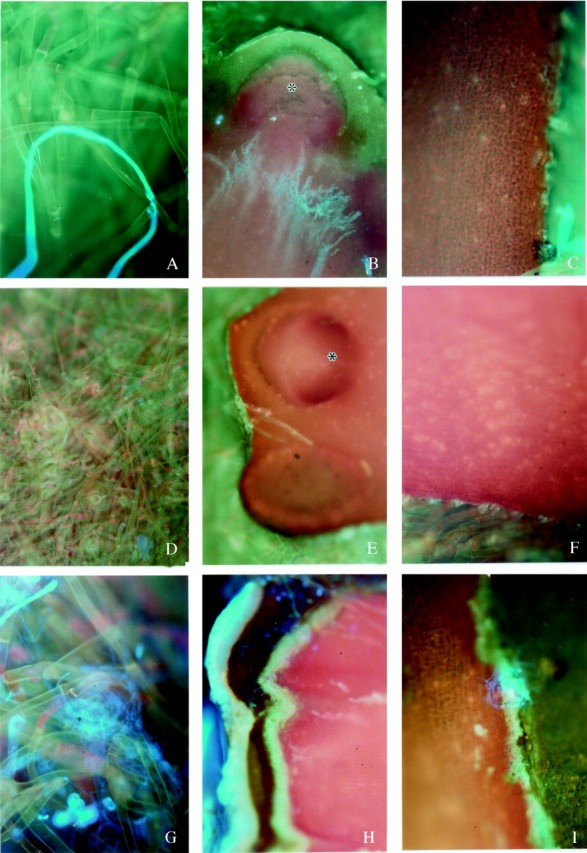
Fig. 1. Epifluorescence micrographs of the distal hypocotyl tip of excised embryonic axes taken from (A–C) freshly harvested seeds (0c0i); (D–F) seeds stored clean for 4 d (4c0i); (G–I) seeds stored clean for 10 d (10c0i). In A–C, the bright blue fluorescence reveals chitin and hence fungal structures. Although the occasional bright-light-blue fluorescing chitin, indicating a fungal structure, could be seen on the bristles (A) there was no evidence of fungal infection on the hypocotyl surface (B) or within the tissue of a root primordium (C). A and C, ×100; B, ×40. In D–F, no signs of fungal infection could be seen on the bristles (D), the surface of the hypocotyl tip (E), or internal tissues (F). D and E, ×40; F, ×100. In G–I, fungal structures revealed by the bright blue fluorescence of chitin could be observed on the bristles (G) and the surface of the root primordium (H). Fungal penetration into the root primordium tissues (I) was mainly confined to the epidermal cells. G and I, ×100; H, ×40. * indicates a root primordium.
Fusarium moniliforme produced abundant aerial mycelium on the bristles of seeds inoculated when fresh and then stored for 8 d (0c8i) (Fig. 2A). The adjacent surface of the hypocotyl tip, as well as the internal tissue, was colonized by the fungus, and advanced deterioration of regions of the internal tissue was also observed (Fig. 2B and C). In contrast, after 4 d of clean storage, inoculation and further storage for 8 d (4c8i) the hyphae were mainly confined to the bristles and no signs of infection on the hypocotyl surface or internal tissue were observed (Fig. 2D–F). The hypocotyl tips excised from seeds stored clean for 10 d, inoculated and then stored for 8 d (10c8i) showed abundant fungal mycelium on the bristles, the hypocotyl surface and under the cuticle (Fig. 2G and H). Some fungal hyphae were revealed deep within the internal tissue, although colonies were not evident (Fig. 2I).
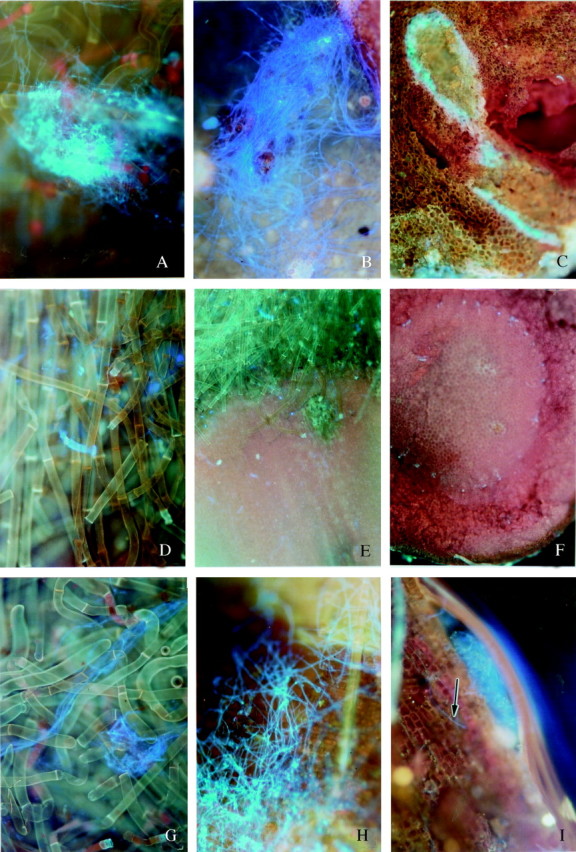
Fig. 2. Epifluorescence micrographs of the distal hypocotyl tip of excised embryonic axes taken from (A–C) seeds inoculated when fresh and then stored for 8 d (0c8i); (D–F) seeds stored clean for 4 d, inoculated and then stored for 8 d (4c8i); (G–I) seeds stored clean for 10 d, inoculated and then stored for 8 d (10c8i). In A–C, most of the fungal mass, revealed by bright blue fluorescence of chitin, was confined to the bristles (A) and the adjacent surfaces of the hypocotyls tip (B); however, localized but substantial regions of the internal tissue were colonized by fungus (C), and degradation of the internal tissue was also apparent. A–C, ×100. In D–F, although some fungal mycelium, revealed by the bright blue fluorescence of chitin, can be seen on the bristles (D), the hypocotyl surface (E) and internal tissue was clean (F). D, 100×; E and F, 40×. In G–I, fungal mycelium, revealed by the bright blue fluorescence of chitin, was common on the bristles (G), on and below the hypocotyl surface (H), and the occasional hypha (arrow) could also be discerned in the internal tissue (I). G and H, ×100; I, ×200.
All specimens showing fungal proliferation were examined in parallel for the presence of necrotic spots, as indicated by orange-yellow autofluorescence with the B-2A filter combination (Rohringer et al., 1977). Such necrotic lesions would be indicative of the occurrence of a hypersensitive response within the host tissues. No such signs of necrosis, however, were observed.
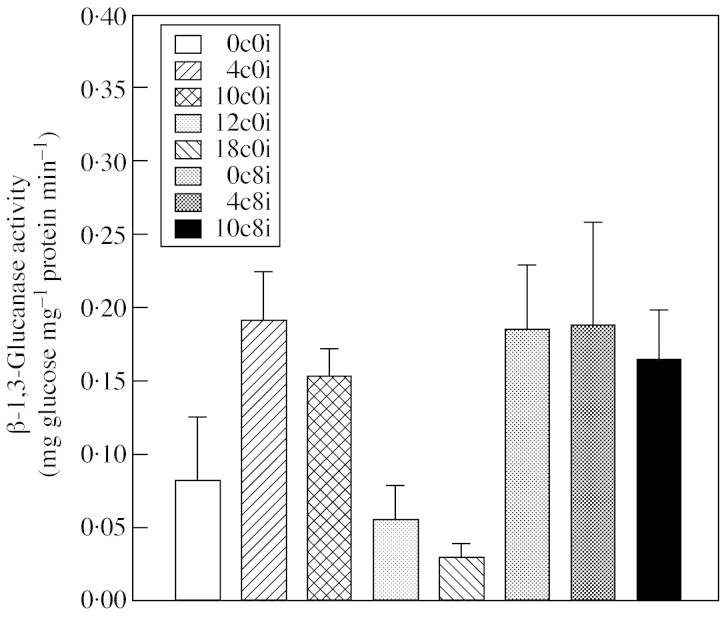
Activity of β-1,3-glucanase was expressed even in axes of freshly harvested seeds (0c0i) (Fig. 3). However, after 4 d of clean hydrated storage of the seeds (4c0i) the activity had increased more than two-fold and remained relatively high during 10 d of storage (10c0i). After that period, however, a significant decrease in activity occurred, and after 12 and 18 d (12c0i and 18c0i) of storage the values were two and three times lower, respectively, compared with the level after 4 d. High levels of activity were observed when the seeds were infected with F. moniliforme prior to, or after, 4 and 10 d clean storage and subjected to a further 8 d of storage (samples 0c8i, 4c8i and 10c8i).
Fig. 3. Changes in the total β-1,3-glucanase activity in the axes of recalcitrant seeds of Avicenia marina. Data presented are the means of three replicates. Error bars represent positive s.d. Data from the first experiment only are presented, but similar trends were obtained in confirmatory studies. 0c0i, Fresh seeds; 4c0i, seeds stored clean for 4 d, 10c0i, seeds stored clean for 10 d; 0c8i, seeds stored infected for 8 d; 4c8i; seeds stored clean for 4 d, infected and re-stored for 8 d; 10c8i, seeds stored clean for 10 d, infected and re-stored for 8 d.
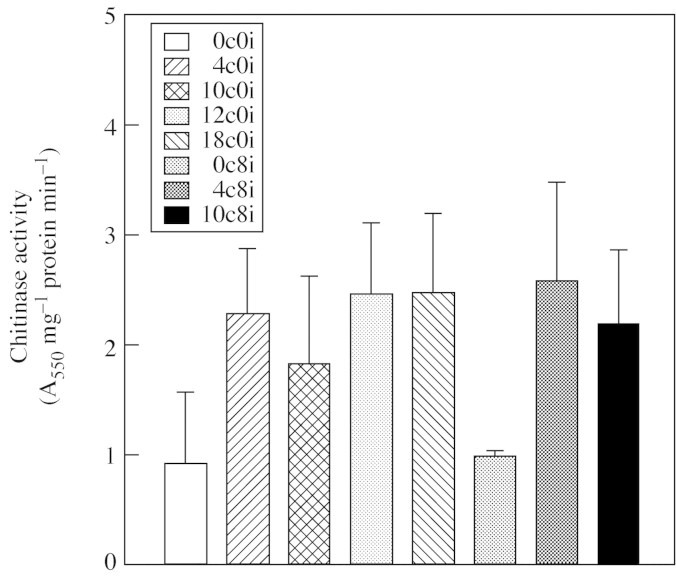
The changes of chitinase activity in axes of freshly harvested and clean-stored infected and non-infected seeds are shown in Fig. 4. The trends of this activity in seeds stored clean for up to 10 d (10c0i) were similar to those of β-1,3-glucanases. In contrast to the β-1,3-glucanases, however, chitinase activity remained high after 12 and 18 d of clean storage (12c0i and 18c0i, respectively). Importantly, there was no difference between the level of chitinase activity in fresh seeds (0c0i) and after their inoculation with F. moniliforme and a subsequent period of 8 d in storage (0c8i), as was the case for β-1,3-glucanases.
Fig. 4. Changes in the total chitinase activity in axes of recalcitrant seeds of Avicenia marina. Data presented are the means of three replicates. Error bars represent positive s.d. Data from first experiment only are presented as similar trends were obtained in confirmatory studies.
DISCUSSION
Extending the storage lifespan of recalcitrant seeds demands urgent attention to help conserve the genetic resources of many important tropical species. The role of microflora and, particularly, of fungi is emerging as an important factor in determining seed lifespan, especially as the seeds must be subjected to hydrated storage.
Freshly harvested (0c0i) and 4-d clean-stored (4c0i) A. marina seeds contained no internal fungal structures. The difference between these two seed samples, however, become apparent when the seeds were experimentally infected with F. moniliforme and maintained for 8 d in storage (0c8i and 4c8i, respectively). While considerable fungal proliferation had occurred in 0c8i seeds, there were no signs of fungal infection in 4c8i seeds. The first signs of fungal proliferation during clean storage were observed when the seeds were inoculated after being stored for 10 d and then re-stored for 8 d (10c8i). However, in this case the extent of fungal proliferation and the symptoms of infection were significantly delayed compared with the seeds inoculated when fresh (0c8i). Thus, the microscopical observations clearly show that fungal proliferation is considerably suppressed if the seeds are subjected to hydrated clean-storage prior to infection. Similar results were previously obtained by Calistru et al. (2000) using different experimental approaches. It was suggested by these authors that the decreased susceptibility of wet-stored A. marina seeds to fungal infection might be a result of inherent, and/or inducible, defence mechanisms developed by these metabolically active seeds, in which germinative events have been shown to be well underway after 4 d in hydrated storage (Farrant et al., 1986; Berjak et al., 1989).
The biochemical studies showed that, although β-1,3-glucanases and chitinases are present in the seeds of A. marina when they are shed (Figs 3 and 4; sample 0c0i), the activity levels significantly increased when fresh seeds were subjected to 4 d (4c0i) and 10 d (10c0i) clean storage. From our histopathological study (Fig 1D–I) as well as ultrastructural studies (Calistru et al., 2000) it is clear that 4c0i and 10c0i seeds do not contain internal fungal contaminants. Therefore, it appears that the β-1,3-glucanase and chitinase activities in these seeds were not induced during storage as a result of fungal infection. It has been shown that the induction of PR proteins is not specifically restricted to pathogen attack but that they are also induced during different physiological and developmental processes, including seed germination (reviewed by Leubner-Metzger and Meins, 1999; Neuhaus, 1999).
Seeds of A. marina are known to initiate germinative metabolism and become more metabolically active during 4 d of hydrated storage (Farrant et al., 1986; Berjak et al., 1989; Calistru et al., 2000). Thus, it appears that both enzymes are induced during hydrated seed storage in association with ongoing germinative metabolism. The important question is what the physiological function might be, of this induction in uninfected seeds? It has been suggested that class I β-1,3-glucanase is involved in the germination of tobacco seeds by promoting endosperm rupture and radicle protrusion (Leubner-Metzger et al., 1995, 1996), but this cannot be the case for A. marina seeds, which are exendospermous and without obvious mechanical barriers to root protrusion (Farrant et al., 1993). Thus, the high level of β-1,3-glucanase and chitinase activity in A. marina seeds during short-term wet storage may be part of a strategy to create an antimicrobial environment prior to microbial infection, rather than a requirement for protrusion of the roots developing from the primordia during hydrated storage. A similar antifungal role for these enzymes has been suggested for germinating pea (Petruzzelli et al., 1999) and tomato (Wu et al., 2001) seeds.
Chitinase and β-1,3-glucanase activities remained high even when A. marina seeds were infected and subjected to further storage for 8 d (4c8i and 10c8i); therefore induction of new isoenzymes cannot be precluded. These observations are in accordance with the findings that, for up to 14 d of clean storage, seeds of A. marina were less susceptible to fungal attack than those in the newly harvested state (Calistru et al., 2000; and our histopathological data). However, after this period A. marina seeds become more susceptible to the effects of the fungus than those in the newly harvested condition (Calistru et al., 2000). It should be noted that the β-1,3-glucanase activity decreased markedly with prolonged storage (samples 12c0i and 18c0i; Fig. 3), in contrast to the chitinase activity which remained relatively unchanged (Fig. 4). Only in combination are these two enzymes highly effective in limiting fungal growth, especially that of Fusarium spp. (Mauch et al., 1988). Fusarium hyphal cell walls consist of randomly orientated chitin microfibrils embedded in a carbohydrate matrix consisting mainly of glucans (Griffin, 1981). Apparently, β-1,3-glucanase and chitinase must act simultaneously for complete digestion of the fungal cell walls and thus destruction of the pathogen. Thus, high activities of both enzymes might be necessary for the successful defence response of A. marina seeds against F. moniliforme infection. This might may explain the high susceptibility of 0c8i seeds in which β-1,3-glucanase activity was apparently induced in association with fungal infection, but chitinase activity remain unchanged compared with the fresh seeds (Figs 3 and 4). At present, however, it is not known whether the same classes of β-1,3-glucanases and chitinases are present in fresh seeds and in those during storage and experimental infection.
The present results suggest that the decreased susceptibility of A. marina seeds during short-term clean storage relies on the ability to create an antifungal environment prior to infection, which would also be an effective strategy during germination in the natural environment. Although the defensive role of β-1,3-glucanases and chitinases in these seeds requires elucidation, it is likely that these enzymes offer some protection against F. moniliforme.
All recalcitrant seeds stored hydrated ultimately die (Pammenter et al., 1994). However, if fungal activity is minimized (Calistru et al., 2000) or apparently effectively curtailed (Motete et al., 1997), the hydrated storage lifespan of A. marina seeds is significantly extended. These authors suggested that one critical factor contributing to the very curtailed hydrated storage lifespan of recalcitrant seeds that harbour fungal inoculum is the absence of some vital defence factor and/or the defence compounds present not being effective. The possible basis of the vulnerability of the seeds to F. moniliforme might be the inability for the hypersensitive response as indicated by our histopathological studies. This strongly suggests that A. marina seeds lack resistance genes that could interact with the pathogen avirulence genes (Hammond-Kosak and Jones, 1997).
As far as is known, this is the first report on the defence mechanisms of recalcitrant seeds against fungal infection. It provides an appropriate framework for future studies on recalcitrant seed antimicrobial strategies. In the natural environment, such strategies are suggested to be adequate to enable seedling establishment, whereas they are inadequate under the mild but prolonged stress conditions suggested to accompany germinative metabolism in storage, where no extraneous liquid water source is available (Berjak et al., 1989; Pammenter et al., 1994; Motete et al., 1997).
Received: 4 April 2003; Returned for revision: 16 April 2003; Accepted: 2 June 2003Published electronically: 24 July 2003
References
- BerjakP.1996. The role of microorganisms in deterioration during storage of recalcitrant and intermediate seeds. In: Ouedraogo AS, Poulsen K, Stubsgaard F, eds. Intermediate/recalcitrant tropical forest tree seeds Rome: IPGRI, 121–126. [Google Scholar]
- BerjakP, Pammenter NW.2001. Seed recalcitrance – current perspectives. South African Journal of Botany 67: 79–89. [Google Scholar]
- BerjakP, Farrant JM, Pammenter NW 1989. The basis of recalcitrant seed behaviour. In: Taylorson RB, ed. Recent advances in the biology and germination of seeds New York: Plenum Press, 89–108 [Google Scholar]
- BerjakP. Vertucci CW, Pammenter NW.1993. Effects of developmental status and dehydration rate on characteristics of water and desiccation-sensitivity in recalcitrant seeds of Camellia sinensis Seed Science Research 3: 155–166. [Google Scholar]
- BolJF, Linthorst HJM.1990. Plant pathogenesis-related proteins induced by virus infection. Annual Review of Phytopathology 28: 113–138. [Google Scholar]
- BradfordMM.1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 248–254. [DOI] [PubMed] [Google Scholar]
- CalistruC, McLean M, Pammenter NW, Berjak P.2000. The effects of mycofloral infection on the viability and ultrastructure of wet-stored recalcitrant seeds of Avicennia marina (Forssk.) Vierh. Seed Science Research 10: 341–353. [Google Scholar]
- ChinHF, Roberts EH.1980.Recalcitrant crop seeds. Kuala Lumpur, Malaysia: Tropical Press. [Google Scholar]
- EbelJ.1986. Phytoalexin synthesis: the biochemical analysis of the induction process. Annual Review of Phytopathology 24: 235–264. [Google Scholar]
- FarrantJM, Pammenter NW, Berjak P.1986. The increasing desiccation sensitivity of recalcitrant Avicennia marina seeds with storage time. Physiologia Plantarum 67: 291–298. [Google Scholar]
- FarrantJM, Berjak P, Pammenter NW.1992a. Proteins in development and germination of desiccation sensitive (recalcitrant) seed species. Plant Growth Regulation 11: 257–265. [Google Scholar]
- FarrantJM, Pammenter NW, Berjak P.1992b. Development of the recalcitrant (homoiohydrous) seeds of Avicennia marina: anatomical, ultrastructural and biochemical events associated with development from histodifferentiation to maturation. Annals of Botany 70: 75–86. [Google Scholar]
- FarrantJM, Pammenter NW, Berjak P.1993. Seed development in relation to desiccation tolerance: a comparison between desiccation-sensitive (recalcitrant) seeds of Avicennia marina and desiccation-tolerant types. Seed Science Research 3: 1–13. [Google Scholar]
- FinkW, Liefland M, Mendgen K.1988. Chitinases and β-1,3-glucanases in the apoplastic compartment of oat leaves (Avena sativa L.). Plant Physiology 88: 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FritigB, Thierry H, Legrand M.1998. Antimicrobial proteins in induced plant defense. Current Opinion in Immunology 10: 16–22. [DOI] [PubMed] [Google Scholar]
- GriffinGJ.1981. Physiology of conidium and clamidospore germination in Fusarium In: Nelson PE, Toussoun TA, Cook RJ, eds. Fusarium: diseases, biology, and taxonomy. University Park, PA: The Pennsylvania State University Press, 331–339. [Google Scholar]
- HahnMG, Bucheli P, Cervone F, Doares SH, O’Neill RA, Darvill A, Albersheim P.1989. Roles of cell wall constituents in plant-pathogen interactions. In: Kosuge T, Nester EW, eds. Plant–microbe interactions. Molecular and genetic perspectives. Vol. 3 New York, McGraw–Hill, 131–181. [Google Scholar]
- Hammond-KosakKE, Jones JDG.1997. Plant disease resistance genes. Annual Review of Plant Physiology and Plant Molecular Biology 48: 575–607. [DOI] [PubMed] [Google Scholar]
- HuynhQK, Hironaka CM, Levine EB, Smith CE, Borgmeyer JR, Shah DM.1992. Antifungal proteins from plants. Purification, molecular cloning and antifungal properties of chitinases from maize seed. Journal of Biological Chemistry 267: 6635–6640. [PubMed] [Google Scholar]
- JacobsenS, Mikkelsen JD, Hejgaard J.1990. Characterization of two antifungal endochitinases from barley grain. Physiologia Plantarum 79: 554–562. [Google Scholar]
- Leubner-MetzgerG, Meins F Jr.1999. Functions and regulation of plant β-1,3-glucanases (PR-2). In: Datta SK, Muthukrishnan S, eds. Pathogenesis-related proteins in plants Boca Raton, FL: CRC Press, 49–76. [Google Scholar]
- Leubner-MetzgerG, Fründt C, Meins F Jr.1996. Effects of gibberellins, darkness and osmotica on endosperm rapture and class I β-1,3-glucanase induction in tobacco seed germination. Planta 199: 282–288. [Google Scholar]
- MajeauN, Trudel J, Asselin A.1990. Diversity of cucumber chitinase isoforms and characterization of one seed basic chitinase with lysozyme activity. Plant Science 68: 9–16. [Google Scholar]
- MauchF, Mauch-Mani B, Boller T.1988. Antifungal hydrolases in pea tissue. II. Inhibition of fungal growth by combinations of chitinase and β-1,3-glucanase. Plant Physiology 88: 936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MoteteN, Pammenter NW, Berjak P, Frédéric JC.1997. Response of the recalcitrant seeds of Avicennia marina to hydrated storage: events occurring at the root primordia. Seed Science Research 7: 169–178. [Google Scholar]
- MycockDJ, Berjak P.1990. Fungal contaminants associated with several homoiohydrous (recalcitrant) seed species. Phytophylactica 22: 413–418. [Google Scholar]
- MycockDJ, Berjak P.1995. The implication of seed-associated mycoflora during storage. In: Kigel J, Galili G, eds. Seed development and germination New York: Marcel Dekker, 747–766. [Google Scholar]
- NeucereJN, Cleveland TC, Dischinger C.1991. Existence of chitinase activity in mature corn kernels (Zea mays L.). Journal of Agricultural and Food Chemistry 39: 1326–1328. [Google Scholar]
- NeuhausJ-M.1999. Plant chitinases. In: Datta SK, Muthukrishnan S, eds. Pathogenesis-related proteins in plants Boca Raton, FL: CRC Press, 77–105. [Google Scholar]
- PammenterNW, Berjak P, Farrant J M, Smith MT, Ross G.1994. Why do stored hydrated recalcitrant seeds die? Seed Science Research 4: 187–191. [Google Scholar]
- PetruzzelliL, Kunz C, Waldvogel R, Meins F Jr, Leubner-Metzger G.1999. Distinct ethylene- and tissue-specific regulation of β-1,3-glucanases and chitinases during pea seed germination. Planta 209: 195–201. [DOI] [PubMed] [Google Scholar]
- PongapanichK.1990. Fungi associated with forest tree seeds in Thailand. In: Anon, ed. Pests and diseases of forest plantation in the Asia-Pacific Region Bangkok: RAPA, 114–121. [Google Scholar]
- RohringerR, Kim WK, Samborski DJ, Howes NK.1977. Calcofluor: an optical brightener for fluorescence microscopy of fungal plant parasites in leaves. Phytopathology 67: 808–810. [Google Scholar]
- SinghD, Singh KG.1990. Occurrence of fungi in rubber seeds of Malaysia. Journal of Natural Rubber Research 3: 64–65. [Google Scholar]
- StintziA, Heitz T, Prasad V, Wiedemann-Merdinoglu S, Kauffmann S, Geoffroy P, Legrand M, Fritig B.1993. Plant ‘pathogenesis-related’ proteins and their role in defense against pathogens. Biochimie 75: 687–706. [DOI] [PubMed] [Google Scholar]
- SutherlandJR, Diekmann M, Berjak P.2002.Forest tree seed health for germplasm conservation. Rome: IPGRI. [Google Scholar]
- Van LoonLC.1997. Induced resistance in plants and the role of pathogenesis-related proteins. European Journal of Plant Pathology 103: 753–765. [Google Scholar]
- Van LoonLC, Van Strien EA.1999. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiological and Molecular Plant Pathology 55: 85–97. [Google Scholar]
- WirthSJ, Wolf GA.1990. Dye-labelled substrates for the assay and detection of chitinase and lysozyme activity. Journal of Micro biological Methods 12: 197–205. [Google Scholar]
- WuC-T, Leubner-Metzger G, Meins F Jr, Bradford KJ.2001. Class I β-1,3-glucanase and chitinase are expressed in the micropilar endosperm of tomato seeds prior to radicle emergence. Plant Physiology 126: 1299–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]