Abstract
This study presents the pollen aperture morphology of 148 out of approx. 200 species in 16 genera of the Detarieae s.s. and 13 related genera, investigated with light microscopy, scanning electron microscopy and transmission electron microscopy, using various staining techniques. Features of detarioid legume pollen apertures are described, illustrated and discussed in relation to function and phylogeny. Protruding apertures in the mature pollen grains of taxa in the Detarieae s.s are associated with underlying structures composed of pectic substances called ‘Zwischenkörper’. This is the first report of Zwischenkörper in legume pollen. A review of previous literature on Zwischenkörper, evidence of how they differ from onci, and a discussion of developmental origins, terminology and function, is given. Zwischenkörper occur in pollen of Daniellia, Eurypetalum, Eperua, Augouardia, Stemonocoleus, Baikiaea, Copaifera, Pseudosindora, Detarium, Sindora, Sindoropsis, Tessmannia, Gilletiodendron, Hylodendron, Hymenaea, Peltogyne and Guibourtia of the Detarieae s.s. clade of recent molecular analyses. Exinous projections and/or bridges are present over the centre of apertures in the pollen of Sindora, Copaifera, Detarium, Pseudosindora, Hylodendron and Sindoropsis, and may cover the Zwischenkörper of live pollen. Zwischenkörper also occur in the closely related genus Barnebydendron, the sister genus to the Detarieae s.l. clade Goniorrhachis, and also Cercis of the sister clade to the Detarieae s.l. plus Goniorrhachis. A modified form of Zwischenkörper occurs in the pollen of Schotia. Zwischenkörper were not detected in the genera Colophospermum, Prioria, Gossweilerodendron, Oxystigma, Kingiodendron and Hardwickia of the Prioria clade, or in Endertia, Lysidice and Saraca of the Amherstieae clade. None of these taxa have protruding apertures.
Key words: Pollen aperture morphology, Zwischenkörper, Leguminosae, Caesalpinioideae, Detarieae, Alcian blue stain, systematics
INTRODUCTION
Of the three subfamilies in the Leguminosae, the pollen of subfamily Caesalpinioideae is the most morphologically diverse when compared with the Papilionoideae and Mimosoideae, and the Detarieae s.l. has the highest floral and pollen morphological diversity within the legume family (Graham and Barker, 1981; Muller, 1981, 1984; Ferguson, 1987; Guinet and Ferguson, 1989; Banks and Klitgaard, 2000). There are perforate, rugulate, striate, reticulate, gemmate, verrucate and psilate ornamentation types; however, systematically informative characters have only been delimited from aperture morphology and wall structure (colporate or porate apertures; aperture membrane type; apocolpium present, syncolporate or parasyncol porate; presence or absence of aperture membrane or exinous bridges; presence or absence of prominent aperture margins; presence or absence of a foot layer, additional infratectal layer or supratectal structures) (Banks and Klitgaard, 2000). This paper examines the pollen aper ture structures present in the Detarieae s.s. with particular reference to underlying structures that are usually removed by standard preparation techniques (termed ‘Zwischenkörper’), and assesses these data in the context of the systematics of the group.
Designation of the Detarieae s.s.
Of the three subfamilies that are widely recognized within the Leguminosae, recent molecular analyses have demonstrated that the Caesalpinioideae is paraphyletic, with subfamilies Papilionoideae and Mimosoideae nested within it (Doyle et al., 1997, 2000, based on rbcL data; Bruneau et al., 2000, 2001, based on trnL data). Taxonomy in subfamily Caesalpinioideae has remained problematical due to difficulties in finding clear characters to differentiate the taxa. A summary of taxonomic history is given by Bruneau et al. (2001). The Detarieae s.l. [Detarieae and Amherstieae (Cowan and Polhill, 1981) or Detarieae and Macrolobieae (Breteler, 1995)] form a well-supported monophyletic clade in the chloroplast trnL analysis of Bruneau et al. (2001). This clade can be recognized by a combination of morphological characters, and the presence of a large insertion in the trnL intron is a synapomorphy for the Detarieae s.l. clade (Bruneau et al., 2001). It comprises approx. 84 genera (Bruneau et al., 2000), which is just over half the 161 genera of subfamily Caesalpinioideae (Lewis et al., 2003), and is both economically and ecologically important. Within the Detarieae s.l., there are three well-supported clades: the Detarieae s.s., the Prioria clade and the ‘Amherstieae’ clade; however, relationships among these three clades are not resolved. The Prioria clade share many morphological characteristics with members of the Detarieae s.s. (gland-dotted leaves, early caducous stipules, tendency towards apetaly, ten free stamens), but are distinguished by having an ovary with one ovule (Bruneau et al., 2001). The Amherstieae clade includes all members of the tribe Macrolobieae (Breteler, 1995) as well as some of the Amherstieae tribe of Cowan and Polhill (1981). Morphological characters include leaves that are not gland-dotted, persistent intrapetiolar stipules, some apetalous species, and a reduction in stamen number in several species (Bruneau et al., 2001). A group of ten genera was circumscribed as the Detarium group in tribe Detarieae (Table 1) by Cowan and Polhill (1981). In the trnL analysis of Bruneau et al. (2001), eight of these ten Detarium group genera form a clade together with eight other genera, and this is designated the Detarieae s.s. (Fig. 1; Table 1). Of the two genera present in the Detarium group sensu Cowan and Polhill (1981) but not in the Detarieae s.s. of Bruneau et al. (2000, 2001), the Brazilian monotypic Goniorrhachis is sister to the Detarieae s.l. clade (Fig. 1), and Pseudosindora was not sampled by Bruneau et al. The other eight members of the Detarieae s.s. comprise three genera from the Crudia group (Augouardia, Stemonocoleus and Guibourtia), two from the Hymenostegia group (Daniellia and Eurypetalum), one from the Brownea group (Eperua) and two from the Hymenaea group (Hymenaea and Peltogyne). Six further genera of the Crudia group form the Prioria clade (Colophospermum, Prioria, Gossweilerodendron, Oxystigma, Kingiodendron and Hardwickia).
Table 1.
Aperture structures of the Detarieae s.s.
| Genus | Total no. of species | No. of species with acetolysed pollen examined | No. of species with pollen stained with Alcian blue | Figures | Exinous bridge across meso-aperture | Exinous projections over centre of aperture | Apertures protrude, especially when pollen is dehydrated | Presence of fastigium | Response to Alcian blue stain |
| Detarieae s.s. | |||||||||
| Daniellia Benn. | 9 | 3 | 1 | 5B | x | x | + | ? | + |
| Eurypetalum Harms | 3 | 2 | 1 | – | x | x | + | ? | + |
| Eperua Aubl. | 14 | 14 | 4 | – | x | x | +12 of 14 species, x in 2 | +/? | + |
| Augouardia Pellegr. | 1 | 1 | 1 | – | x | x | x | ? | + |
| Stemonocoleus Harms | 1 | 1 | 1 | – | x | x | + | + | + |
| Baikiaea Benth. | 4 | 4 | 2 | 3D, 4B and D | x | x | + | + | + |
| Copaifera L. | 25–30 | 11 | 3 | 2E, 4C, 5D | +/x | + | + | + | + |
| Pseudosindora Symington | 1 | 1 | 3 | 3B | x | + | + | ? | + |
| Detarium Juss. | 3 | 3 | 1 | 5A and C | x | ? | ? | ? | + |
| Sindora Miq. | 18–20 | 10 | 2 | 2A–D, and F, 3F | +/x | + | + | + | + |
| Sindoropsis J. Léonard | 1 | 1 | 1 | 3E | x | + | + | ? | + |
| Tessmannia Harms | 11 | 7 | 2 | – | x | x | + | + | + |
| Gilletiodendron Vermoesen | 5 | 5 | 2 | – | x | x | ? | ? | + |
| Hylodendron Taub. | 1 | 1 | 3 | – | x | + | + | + | + |
| Hymenaea L. | 15 | 10 | 5 | 4A | x | x | + | + | + |
| Peltogyne Vogel | 23 | 7 | 1 | 3A and C | x | x | + | ? | + |
| Guibourtia Benn. | 16–17 | 8 | 2 | – | x | x | + | + | + |
| Schotia Jacq. | 6 | 6 | 1 | 5E | x | x | + | ? | + |
| Prioria clade | |||||||||
| Colophospermum J. Léonard | 1 | 1 | 1 | 5F | x | x | x | x | x |
| Prioria Griseb. | 1 | 1 | 1 | – | x | x | x | x | x |
| Gossweilerodendron Harms | 2 | 2 | 1 | – | x | x | x | x | x |
| Oxystigma Harms | 5 | 4 | 1 | – | x | x | x | x | x |
| Kingiodendron Harms | 6 | 4 | 1 | – | x | x | x | x | x |
| Barnebydendron J.H. Kirkbr. | 2 | 2 | 2 | – | x | x | + | x | + |
| Goniorrhachis Taub. | 1 | 1 | 2 | – | x | x | x/+ | ? | + |
| Amherstieae clade | |||||||||
| Endertia Steenis & deWit | 1 | 1 | 1 | – | x | x | x | x | x |
| Lysidice Hance | 1 | 1 | 1 | – | x | x | x | x | x |
| Saraca L. | 8 | 3 | 1 | – | x | x | x | x | x |
| Cercideae | |||||||||
| Cercis L. | 6 | 1 | 1 | – | x | + | x | ? | + |
Layout follows the phylogeny of Bruneau et al. (2001) (Fig. 1). Genera in bold indicate that they are members of the Detarium Group of Cowan and Polhill (1981).
x, absent; +, present; ?, data are missing or inconclusive.
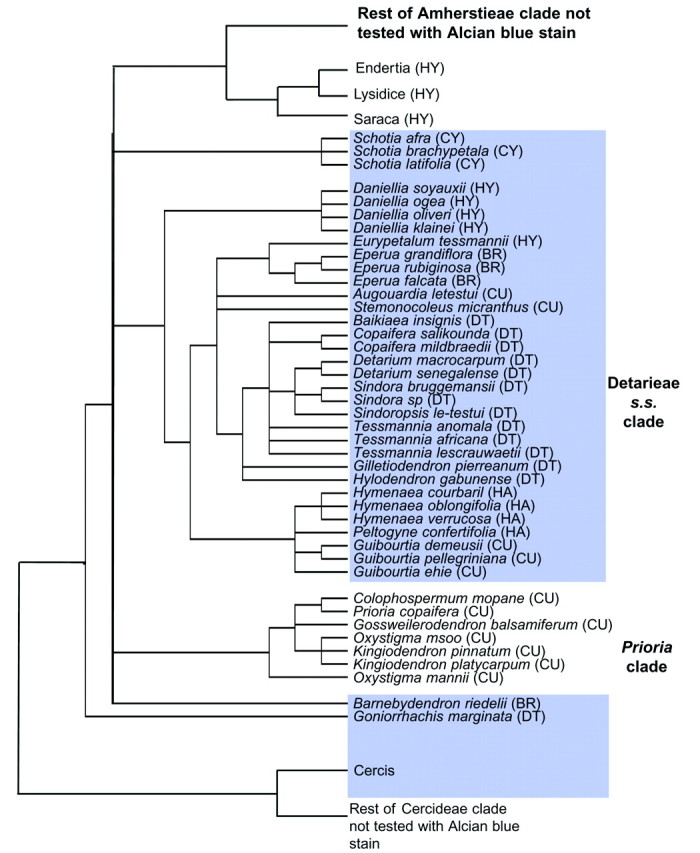
Fig. 1. Phylogeny of Detarieae s.l. inferred from trnL intron sequences (based on analyses of Bruneau et al., 2001, with permission). The distribution of a positive response to Alcian blue stain (presence of Zwischenkörper) is shown shaded. In parentheses following species names are the generic groups of Cowan and Polhill (1981). CY, Cynometra group; HY, Hymenostegia group; BR, Brownea group; CU, Crudia group; DT, Detarium group; HA, Hymenaea group.
Pollen morphology of the Detarieae s.s.
Although pollen of taxa in the Detarium group has been described by many authors, relatively little attention has been paid previously to aperture structure. For example, Fasbender (1959) carried out a detailed survey of caesalpinioid genera using light microscopy (LM), but only described ‘some consticticolpi’ in Detarium, and Tessmannia pollen as being crassimarginate. Although pollen samples of Copaifera and Sindora were examined, the survey did not include species with exinous bridges. A survey of the Caesalpinioideae using scanning electron microscopy (SEM) (Graham and Barker, 1981) concentrated mainly on surface ornamentation of the group; and another using transmission electron microscopy (TEM) (Ferguson, 1987) concentrated on exine stratification. However, apertures are often one of the most important features of pollen with regard to providing informative characters for systematic analysis (e.g. Blackmore and Crane, 1998). A Sindora pollen type, distinct due to paired endoapertures in the colpi (Fig. 2A–D), is probably the earliest record of Leguminosae pollen from the Maastrichtian of Siberia, Canada and Colombia (Muller, 1981; Herendeen et al., 1992). Despite such an early appearance (around 70 million years bp) of this pollen type in the fossil record, apertures separated by a broad bridge of exine across the centre of the aperture at the equator, creating two distinct endoapertures in the apertures (Fig. 2C and D), have only been reported previously to occur in the pollen of the genus Sindora. Within the Caesalpinioideae, the exinous bridge structure has only been reported in taxa of the Detarieae s.s. Within the Leguminosae, Dumasia villosa DC. in subfamily Papilionoideae (Ferguson and Skvarla, 1981) is the only other species so far found to have an exinous bridge across the centre of the aperture, in this case separating two pores.
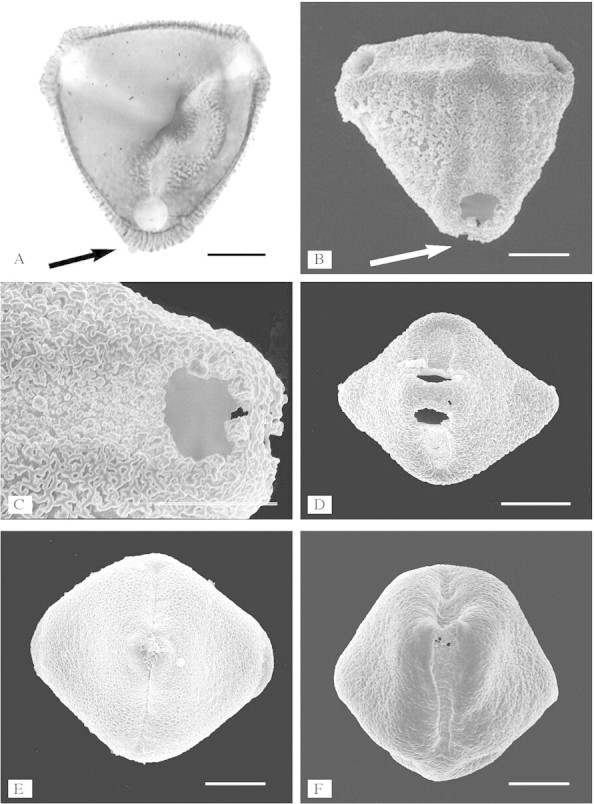
Fig. 2. Aperture structure of Sindora and Copaifera illustrated using acetolysed pollen. A, Sindora maritima: polar view showing columellate exinous bridges across the centre of the apertures (arrow, LM) (compare with B showing a grain in similar orientation illustrated using SEM, and Fig. 5A where no exinous bridge is present). B, Sindora coriacea: polar view showing a grain with exinous bridges across the centre of the colpori (arrow) in oblique polar view (SEM). C, Sindora coriacea: detail of exinous bridge (SEM). D, Sindora maritima: equatorial view showing exinous bridges across centre of the aperture (SEM). E, Copaifera multijuga: equatorial view showing exinous projections, rather than exinous bridges, across centre of the apertures (SEM). F, Sindora klaineana: equatorial view showing exinous projections, rather than exinous bridges, across the centre of the apertures (SEM). Scale bars = 10 µm in A–C, E and F, 20 µm in D.
In some genera of the former Crudia group (Table 1; Fig. 1), Banks and Gasson (2000) showed that the apertures do not buckle inwards in dehydrated pollen, as in most other legume pollen (Heslop-Harrison, 1976; Banks and Gasson, 2000; Banks and Klitgaard, 2000), but appear to be protruding and fixed open. Consequently the mesocolpial wall buckles inwards (Figs 2F and 3A–C) (see also Crane, 1986, p. 186; Banks and Klitgaard, 2000, Figs 47 and 48). This could be significant to the wall structure. Instead of a convex bend to the wall associated with tensile forces exerted on the tectum and infratectum, created by apertural areas buckling inwards, a convex bend caused by mesocolpial wall buckling inwards will be associated with compressive forces on the tectum and infratectum (Crane, 1986).
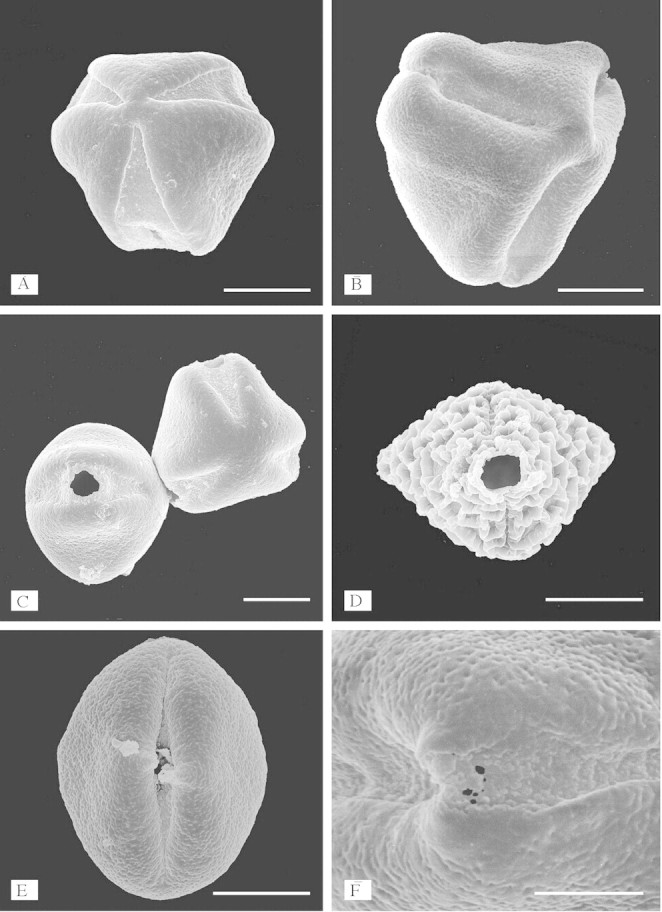
Fig. 3. Protruding apertures and exinous projections. A, Peltogyne paniculata: polar view showing protruding apertures compared with mesocolpial areas (SEM). B, Pseudosindora palustris: oblique view showing protruding apertures (SEM). C, Two grains of Peltogyne lecointei showing protruding apertures (SEM). D, Baikiaea suzannae: equatorial view showing protruding apertures (SEM). E, Sindoropsis letestui: equatorial view showing protruding apertures and exinous projections over the centre of the apertures (SEM). F, Sindora klaineana: close-up of aperture showing exinous projections (SEM). Scale bars = 10 µm in A–E, 5 µm in F.
MATERIALS AND METHODS
Pollen material was obtained from the herbarium of the Royal Botanic Gardens, Kew (K) (Table 1). A full list of samples examined can be obtained from the author. Anthers were taken from mature, unopened buds. Standard acetolysis techniques were employed to initially survey 360 samples of 83 detarioid genera (Banks and Klitgaard, 2000). This study presents pollen aperture morphology of 148 species out of approx. 200 in 29 genera representing the Detarieae s.s. and relatives (Table 1). Mature, unopened buds were dissected in a 1 % solution of Libsorb using a Leica Wild M8 micro scope. Pollen was acetolysed using the method of Erdtman (1960), and prepared for light microscopy (LM) by mounting in glycerol jelly. Measurements were recorded using a Nikon Labophot LM. Acetolysed pollen exines in 95 % ethanol were allowed to air-dry onto specimen stubs, sputter-coated with platinum, and examined in a Hitachi S-2400 SEM. All acetolysed samples were examined using LM and scanning electron microscopy (SEM).
Selected samples of acetolysed material were fixed with a 2 % solution of osmium tetroxide in cacodylate buffer, pre-stained with 0·5 % uranyl acetate, embedded in Epon–Araldite resin following the method of Skvarla (1966), thin-sectioned, post-stained with uranyl acetate and lead citrate, then examined using a Hitachi H-300 transmission electron microscope (TEM).
For examination of the protruding apertures, pollen samples of Baikiaea plurijuga Harms and Detarium senegalense J.F. Gmel. were taken from mature anthers and stained with Alexander’s stain (Alexander, 1969). After warming gently for about 30 s, the pollen was examined and photographed using a Nikon Labophot LM.
Forty-six samples of pollen from 29 detarioid genera were taken from mature anthers and stained with Alcian blue (Table 1). Where possible, samples were selected for this pollen study to match the species used for the molecular analysis of Bruneau et al. (2001) (Fig. 1). It should be noted, however, that the staining response in pollen taken from some older specimens was not as good as that of younger specimens. After 5–10 min, the pollen was examined and photographed using a Nikon Labophot LM.
Unacetolysed pollen of Copaifera baumiana Harms from spirit material was embedded in Epon–Araldite resin following the method of Skvarla (1966) and thin-sectioned using a diamond knife. Some sections were stained with Alcian blue and examined using a Nikon Labophot LM, other sections were post-stained with uranyl acetate and lead citrate in an LKB 2168 Ultrostainer. Sections were examined using a Hitachi H-300 TEM.
Terminology follows Punt et al. (1994).
RESULTS
This study found apertures with exinous bridges over the endoaperture region at the equator in the pollen of eight out of the 18–20 species in Sindora (of 13 species examined), and one species out of the 25–30 in Copaifera (of 16 species examined) (Fig. 2A–D). Pollen of the other species within these two genera has apertures with exinous projections over the centre of the aperture (Fig. 2E and F). This type of aperture structure is also found in the pollen of Detarium, Pseudosindora (Fig. 3B), Hylodendron and Sindoropsis (Fig. 3E). The pollen of Baikiaea (Fig. 3D), Copaifera (Fig. 2E), Sindora (Fig. 2B and D), and Tessmannia have greatly protruding apertures that form diamond-shaped pollen (Figs 2D and E and 3D), and the pollen of other taxa in the Detarieae s.s. have protruding apertures that are more pronounced in dehydrated pollen. There were no structures present in acetolysed pollen to account for the protruding apertures illustrated in Figs 2 and 3. No thickening of the exine was observed around the apertures, in fact thin section images of acetolysed pollen show that a cavity or fastigium is present in the endoaperture area in some taxa (Table 1). On either side of the endoaperture, a separation of the ectexine from the endexine occurs in the wall structure (Fig. 4). In unacetolysed pollen stained with Alexander’s stain (Fig. 5A), exine is stained turquoise and cytoplasm magenta. A lens-shaped structure in the endoapertural area can be seen, although it is not stained. TEM sections of unacetolysed pollen also show a structure present overlying the endexine (Fig. 4C). Alcian blue was found to stain these structures preferentially (Fig. 5B–E). The structures observed fit the definition of a Zwischenkörper described by Rowley (1964), Heslop-Harrison and Heslop-Harrison (1980, 1981), Heslop-Harrison et al. (1986), El-Ghazaly and Jensen (1987), Heslop-Harrison and Heslop-Harrison (1991) and El-Ghazaly (2000). The presence of this structure causes the apertures to protrude. Removal of the structure by acetolysis leaves a fastigium in the endoaperture area, and wedge-shaped separation of the ectexine from the endexine in the endoaperture margins (Figs 4A–D; Table 1). Fastigia are present in all pollen that stain positively with Alcian blue except for Barnebydendron. However, sections have to be cut precisely across the endoaperture area in order to see whether fastigia are present, and it is possible that in this case the sectioning was not in exactly the right region.
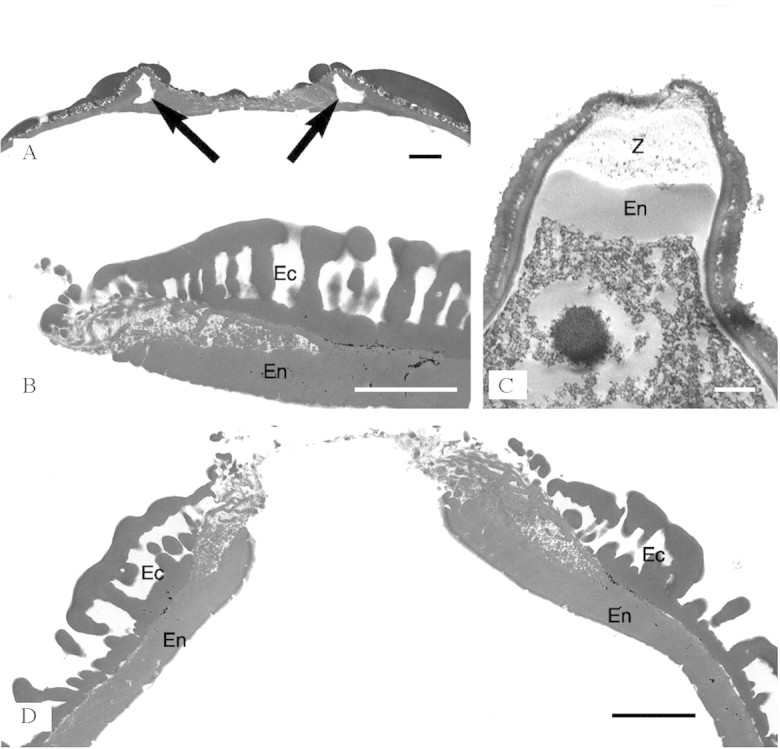
Fig. 4. Zwischenkörper in TEM. A, Hymenaea velutina: transverse section through edge of endoaperture area showing fastigium; arrows show separation of ectexine from endexine (TEM). B, Baikiaea plurijuga: transverse section of the pollen wall one side of the endoaperture area showing a wedge-shaped separation of the ectexine (Ec) and endexine (En) (TEM). C, Copaifera baumiana: section through the endoaperture of non-acetolysed pollen showing Zwischenkörper (Z) beneath the endoaperture above the thickened endexine (En) (TEM). D, Baikiaea plurijuga: section through the endoaperture area showing fastigium and some separation of endexine (En) and ectexine (Ec). All parts show that the ectexine becomes thinner at the aperture margins. The endexine is slightly thickened at the aperture margins, but not enough to account for the prominence of the endoapertures. Scale bars = 2 µm.
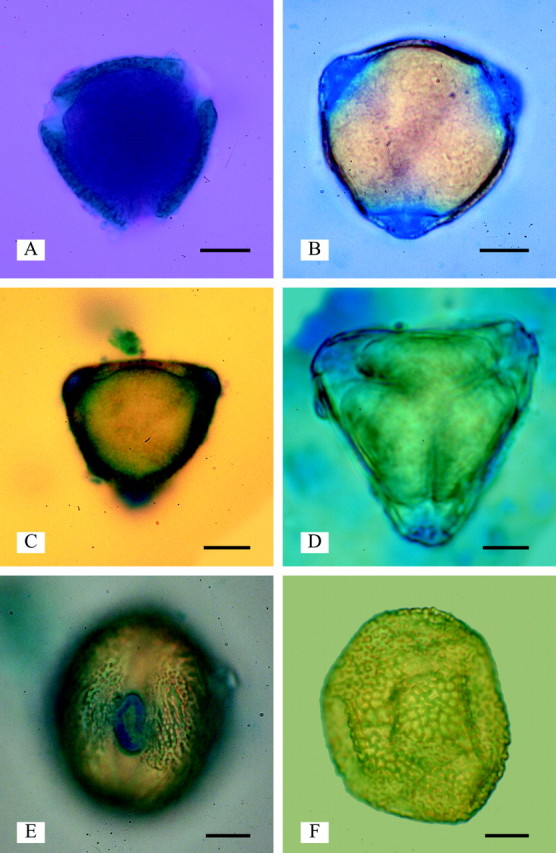
Fig. 5. Zwischenkörper below endoapertures (LM). A, Detarium senegalense pollen showing dark red cytoplasm and green-blue exine stained with Alexander’s stain. The area below the endoapertures (Zwischenkörper) has not stained, showing that it is not composed of exine or cytoplasm (LM). B, Daniellia oliveri: Zwischenkörper stained with Alcian blue (LM). C, Detarium senegalense: Zwischenkörper stained with Alcian blue (LM). D, Copaifera cnearensis. E, Schotia brachypetala: an area encircling the endoaperture is stained with Alcian blue (LM). F, Colophospermum mopane showing no staining with Alcian blue. Scale bars = 10 µm.
In pollen from the closely related Prioria clade, the apertures are not protruding (Fig. 1), and Alcian blue did not preferentially stain the aperture areas (e.g. Colophospermum, Fig. 5F). In the one sample of Schotia examined, a protruding ring is present around the endoaperture (Banks and Klitgaard, 2000, Fig. 30) and only this area stains positively with Alcian blue (Fig. 5E). It has the appearance of an annulus, but the exine is not thickened. This suggests that the protrusion is created by the underlying structure that stains in response to Alcian blue.
DISCUSSION
Distribution of Zwischenkörper
This is the first report of Zwischenkörper in legume pollen. Projecting apertures with underlying Zwischenkörper are present in all genera that form the Detarieae s.s. clade plus Schotia, Barnebydendron and Goniorrhachis (Table 1; Fig. 1), and Cercis which occurs in a basal position in the sister group (tribe Cercideae) in the molecular phylogeny of Bruneau et al. (2001). Alcian blue stain tests indicate that Zwischenkörper are also present in putative sister families Quillajaceae and Surianaceae (F. Claxton, pers. comm., 2002). Zwischenkörper are absent from the Prioria clade and three genera in the Amherstieae clade that form the sister group to the rest of the Amherstieae (Endertia, Lysidice and Saraca) in the molecular phylogeny of Bruneau et al. (2001). Zwischenkörper may have been either lost twice from the Amherstieae and Prioria groups, or there could have been one loss for both clades; however, this remains ambivalent, since there is currently lack of resolution at this node, resulting in a polytomy (Fig. 1). Zwischenkörper were not detected in pollen that does not have protruding apertures or exinous projections, although a more extensive survey would be required to confirm a correlation. However, results so far suggest that exinous projections are present where the apertures protrude due to the presence of Zwischenkörper (Table 1; Fig. 3F). Exine that projects over the centre of the apertures is present in the pollen of other groups of the Leguminosae (Banks and Klitgaard, 2000; Banks et al., 2003) and in putative sister families Quillajaceae and Surianaceae (F. Claxton, pers. comm., 2002).
Definition of Zwischenkörper
The term ‘Zwischenkörper’ was first used by Fritzche (1837), and translated by Beer (1906) as ‘interstitial body’. Zwischenkörper and onci have been described previously in grass and Corylus pollen (Rowley, 1964; Heslop-Harrison and Heslop-Harrison, 1980, 1981, 1991; Heslop-Harrison et al., 1986; El-Ghazaly and Jensen, 1986a, b, 1987). There has been ongoing confusion concerning terminology; both ‘oncus’ and ‘Zwischenkörper’ have been used interchangeably to describe structures present under the apertures. Some literature (e.g. El-Ghazaly, 1999b) suggests that Zwischenkörper are always present in microspores, but by maturation of the pollen grains they are replaced by an intinous oncus.
Blackmore and Crane (1988) described endoapertures as being formed during development where the presence of onci disrupts endexine formation. Onci determine pollen size and shape during dehydration and rehydration (Frenguelli et al., 1997). Punt et al. (1994) defined an oncus as ‘a lens-shaped structure that is not resistant to acetolysis and occurs beneath the apertures of many kinds of pollen grains’. Zwischenkörper had the same description, but with the comment that, although it resembles an oncus it is ‘treated as distinct because some pollen grains have both features’. Zwischenkörper comprise a layer of well-developed pectic substances, and physically overlie and seal off the middle part of the intine (Heslop-Harrison and Heslop-Harrison, 1991).
A review and advice on the confused terminology applied to structures underlying pollen apertures are given by Rodríguez-García and Fernández (1988). These authors state that both onci and Zwischenkörper have different origins, and that it is yet to be determined whether both have similar or different roles in development and germination. They suggest that it is appropriate to use the term ‘oncus’ for both, ‘as long as the origin is clearly stated as intinous or exinous’. However, the structures are not only different in origin (Rodríguez-García and Fernández, 1988), but are also different histochemically (Heslop-Harrison and Heslop-Harrison, 1980; El-Ghazaly and Jensen, 1987). Intine stains with Aniline blue-black, Zwischenkörper do not stain with Aniline blue-black (El-Ghazaly and Jensen, 1987). Zwischenkörper stain with Alcian blue, intine does not stain with Alcian blue (Heslop-Harrison and Heslop-Harrison, 1980). Rowley (1975) compared aperture formation in Silene, Tradescantia, Nelumbo and Epilobium, and found several possibly distinct apertural induction processes.
Because the first use of the term ‘Zwischenkörper’ was applied to the structure that lies above the intine and stains with Alcian blue (Fritzche, 1837; Beer, 1906; Rowley, 1964; Heslop-Harrison and Heslop-Harrison, 1980), it is continued to be used it here to apply to those analogous structures in detarioid legumes. The term ‘onci’ is used for those structures that are intinous in origin and do not stain with Alcian blue.
Zwischenkörper function
Blackmore and Barnes (1986) pointed out that similar needs for harmomegathy and germination are met through extremely diverse mechanisms in different species. It is probable that much of this diversity has arisen through compromises with selection for other functions, such as mechanical efficiency, economy of materials, adaptations related to storage and release of exine and intine held substances, or adaptations for dispersal, all constrained by ontogenetic inheritance.
Although reported to be present during early development, Zwischenkörper are not always present in mature pollen grains (El-Ghazaly, 1999b). In the pollen of Olea the Zwischenkörper are greatly reduced at grain maturity and replaced by an oncus developed from intinous material (Rodríguez-García and Fernández, 1988). Zwischenkörper have been described as structures that assist germination of the pollen tube. Heslop-Harrison and Heslop-Harrison (1991) and Heslop-Harrison et al. (1986) related Zwischenkörper function to hydration, activation, germination and early pollen tube growth. Of the layers present in the structure of the endoapertures, both papers described a ‘central protein-bearing layer’, which at germination discharges its proteins into the stigma during early hydration. It is suggested that these proteins could be associated with recognition processes, and also present are enzymes that assist with pollen tube growth. Secondarily, gelatinizing pectins hydrate, expand and push out of the aperture along with any operculum or encapsulating sporopollenin. The hydration of Zwischenkörper functions even in dead pollen, the germination process ceases at the point where the pollen tube tip should differentiate. Other instances are found of intine functions in osmoregulation, e.g. Cresti and Tiezzi (1990) describe the same pollen hydration and germination process as described by Heslop-Harrison and Heslop-Harrison (1980, 1981), although in this case the structure comprises ‘the outer layer of the intine’. Zwischenkörper in the pollen of Betula are described and illustrated, using chemical fixation and rapid-freeze fixation techniques, in El-Ghazaly (2000). This study suggests that the helical units within the Zwischenkörper observed by freeze-substitution fixation support the hypothesis that the Zwischenkörper has a transportation function. A good review of the development of Zwischenkörper in Betula is given in El-Ghazaly (1999b), and of Olea in Rodríguez-García and Fernández (1988). El-Ghazaly (1999a) suggest that Betula allergen studies provide histochemical evidence that the intine differs from the Zwischenkörper, that the timing of formation and protein within the structures is different, and that the two layers have different functions.
Systematic information from pollen morphology
In the Detarieae s.l., varied pollen surface ornamentation types can provide useful taxonomic information, but the presence of mixtures of patterns, intermediate forms, and similar surfaces with differing underlying structure, prevent surface ornamentation from being defined for use as characters for cladistic analysis (Banks and Klitgaard, 2000). Aperture morphology and wall structure provide a better source for additional characters to further resolve the phylogeny. The presence of Zwischenkörper in the Detarieae s.s. clade, plus Schotia, Barnebydendron, Goniorrhachis and Cercis, suggests a closer relationship between the Prioria clade and Amherstieae clade than between the Detarieae s.s. and Amherstieae clade. Other pollen characters that could provide further systematic information towards resolving relationships include the extra infratectal layer found in the wall structure of Augouardia, Stemonocoleus and most species of Eperua; a similar but more granular structure is also seen in Prioria and Gossweilerodendron. Some species of Sindora and Copaifera have exinous bridges across the centre of the apertures; additionally, Detarium, Pseudosindora, Hylodendron and Sindoropsis have exinous projections over the centre of the aperture. Psilate aperture membranes occur in 13 genera of the Detarieae s.l. including all genera of the Prioria clade. Aperture number may be a useful character in higher level systematic studies. It is intended that the pollen characters discussed here (and in Banks et al., 2003) will be added to other morphological and molecular datasets for future phylogenetic analyses.
ACKNOWLEDGEMENTS
Thanks to Carol Furness, Bente Klitgaard, C. E. Lewis, Gwilym Lewis, Paula Rudall and one anonymous reviewer for comments on the manuscript, and to Frances Claxton for information on the pollen morphology of related families.
Received: 3 February 2003; Returned for revision: 6 April 2003; Accepted: 24 June 2003Published electronically: 24 July 2003
References
- AlexanderMP.1969. Differential staining of aborted and nonaborted pollen. Stain Technology 44: 117–122. [DOI] [PubMed] [Google Scholar]
- BanksH, Gasson, P.2000. Pollen morphology and wood anatomy of the Crudia group (Leguminosae, Caesalpinioideae, Detarieae). Botanical Journal of the Linnean Society 134: 19–59. [Google Scholar]
- BanksH, Klitgaard BB.2000. Palynological contribution to the systematics of detarioid legumes (Leguminosae: Caesalpinioideae). In: Herendeen P, Bruneau A, eds. Advances in legume systematics, Vol. 9 Kew: Royal Botanic Gardens, 79–106. [Google Scholar]
- BanksH, Klitgaard BB, Lewis G, Crane P.2003. Pollen and the systematics of the tribes Caesalpinieae and Cassieae (Caes alpinioideae: Leguminosae). In: Klitgaard BB, Bruneau A, eds. Advances in legume systematics, Vol. 10. Kew: Royal Botanic Gardens, in press. [Google Scholar]
- BeerR.1906. On the development of the pollen grain and anther of some Onagraceae. Botanisches Centralblatt 19: 286–313. [Google Scholar]
- BlackmoreS, Barnes SH.1986. Harmomegathic mechanisms in pollen grains. In: Blackmore S, Ferguson IK, eds. Pollen and spores: form and function London: Academic Press, 137–149. [Google Scholar]
- BlackmoreS, Crane PR.1988. The systematic implications of pollen and spore ontogeny. In: Humphries CJ, ed. Ontogeny and systematics London: British Museum (Natural History), 83–115. [Google Scholar]
- BlackmoreS, Crane PR.1998. The evolution of apertures in the spores and pollen grains of embryophytes. In: Owens SJ, Rudall PJ, eds. Reproductive biology in systematics, conservation and economic botany Kew: Royal Botanic Gardens, 159–182. [Google Scholar]
- BretelerFJ.1995. The boundary between Amherstieae and Detarieae (Caesalpinioideae). In: Crisp MD, Doyle JJ, eds. Advances in legume systematics, Vol. 7 Kew: Royal Botanic Gardens, 53–61. [Google Scholar]
- BruneauA, Breteler FJ, Wieringa JJ, Gervais GYF, Forest F.2000. Phylogenetic relationships in tribes Macrolobieae and Detarieae as inferred from chloroplast trnL intron sequences. In: Herendeen P, Bruneau A, eds. Advances in legume systematics, Vol. 9 Kew: Royal Botanic Gardens, 121–149. [Google Scholar]
- BruneauA, Forest F, Herendeen PS, Klitgaard BB, Lewis GP.2001. Phylogenetic relationships in the Caesalpinioideae (Leguminosae) as inferred from chloroplast trnL intron sequences. Systematic Botany 26: 487–514. [Google Scholar]
- CowanRS, Polhill RM.1981. Detarieae. In: Polhill RM, Raven PH, eds. Advances in legume systematics, Vol. 1. Kew: Royal Botanic Gardens, 135–142. [Google Scholar]
- CranePR.1986. Form and function in wind dispersed pollen. In: Blackmore S, Ferguson IK, eds. Pollen and spores: form and function London: Academic Press, 179–202. [Google Scholar]
- CrestiM, Tiezzi A.1990. Germination and pollen tube formation. In: Blackmore S, Knox RB, eds. Microspores: evolution and ontogeny London: Academic Press, 239–263. [Google Scholar]
- DoyleJJ, Doyle JL, Ballenger JA, Dickson EE, Kajita T, Ohashi H.1997. A phylogeny of the chloroplast gene rbcL in the Leguminosae: taxonomic correlations and insights into the evolution of nodulation. American Journal of Botany 84: 541–554. [PubMed] [Google Scholar]
- DoyleJJ, Chappill JA, Bailey CD, Kajita T.2000. Towards a comprehensive phylogeny of legumes: evidence from rbcL sequences and non-molecular data. In: Herendeen P, Bruneau A, eds. Advances in legume systematics, Vol. 9 Kew: Royal Botanic Gardens, 1–20. [Google Scholar]
- El-GhazalyG.1999a. Tapetum and orbicules (Ubisch Bodies): development, morphology and role of pollen grains and tapetal orbicules in allergenicity. In: Cresti M. Cai G, Moscatelli A, eds. Fertilization in higher plants Berlin: Springer-Verlag, 157–171. [Google Scholar]
- El-GhazalyG.1999b. Development and substructures of pollen grains wall. In: Cresti M, Cai G, Moscatelli A, eds. Fertilization in higher plants Berlin: Springer-Verlag, 175–196. [Google Scholar]
- El-GhazalyG.2000. New and improved methods in palynology. In: Nordenstam B, El-Ghazaly G, Kassas M, eds. Plant systematics for the 21st century London: Portland Press, 143–160. [Google Scholar]
- El-GhazalyG, Jensen WA.1986a. Studies on the development of wheat (Triticum aestivum) pollen. I. Formation of the pollen wall and Ubisch bodies. Grana 25: 1–29. [Google Scholar]
- El-GhazalyG, Jensen WA.1986b. Studies of the development of wheat (Triticum aestivum) pollen: formation of the pollen aperture. Canadian Journal of Botany 64: 3141–3154. [Google Scholar]
- El-GhazalyG, Jensen WA.1987. Development of wheat (Triticum aestivum) pollen. II. Histochemical differentiation of wall and ubisch bodies during development. American Journal of Botany 74: 1396–1418. [Google Scholar]
- ErdtmanG.1960. The acetolysis method. A revised description. Svensk Botanisk Tidskrift 54: 561–564. [Google Scholar]
- FasbenderMV.1959. Pollen grain morphology and its taxonomic significance in the Amherstieae, Cynometreae and Sclerolobieae (Caesalpiniaceae) with special reference to American genera. Lloydia 22: 107–162. [Google Scholar]
- FergusonIK, Skvarla JJ.1981. The pollen morphology of the Papilionoideae (Leguminosae). In: Polhill RM, Raven PH, eds. Advances in legume systematics, Vol. 1 Kew: Royal Botanic Gardens, 858–896. [Google Scholar]
- FergusonIK.1987. A preliminary survey of the pollen exine stratification in the Caesalpinieae. In: Stirton CH, ed. Advances in legume systematics, Vol. 3 Kew: Royal Botanic Gardens, 355–385. [Google Scholar]
- FrenguelliG, Ferranti F, Tedeschini E, Andreutti R.1997. Volume changes in the pollen grain of Corylus avellana (Corylaceae) during development. Grana 36: 289–292. [Google Scholar]
- FritzcheCJ.1837. Über der pollen. In: Mémoírs de l’Academie Impériale des Sciences de St. Petersburg savans Étrangeres Vol. 3 St Petersburg: Imperial Science Academy, 649–672. [Google Scholar]
- GuinetP, Ferguson IK.1989. Structure, evolution and biology of pollen in Leguminosae. In: Stirton CH, Zarucchi JL, eds. Advances in legume biology. Monographs in Systematic Botany, No. 29 St Louis: Missouri Botanical Garden, 77–103. [Google Scholar]
- GrahamA, Barker G.1981. Palynology and tribal classification in the Caesalpinioideae. In: Polhill RM, Raven PH, eds. Advances in legume systematics, Vol. 2 Kew: Royal Botanic Gardens, 801–834. [Google Scholar]
- HerendeenPS, Crepet WL, Dilcher DL.1992. The fossil history of the Leguminosae: phylogenetic and biogeographical implications. In: Herendeen PS, Dilcher DL, eds. Advances in legume systematics. Vol. 4. The fossil record Kew: Royal Botanic Gardens, 801–834. [Google Scholar]
- Heslop-HarrisonJ.1976. The adaptive significance of the exine. In: Ferguson IK, Muller J, eds. The evolutionary significance of the exine London: Academic Press, 27–37. [Google Scholar]
- Heslop-HarrisonJ, Heslop-Harrison Y.1980. Cytochemistry and function of the Zwischenkörper in grass pollens. Pollen et Spores 23: 5–10 [Google Scholar]
- Heslop-HarrisonJ, Heslop-Harrison Y.1981. The pollen-stigma interaction in the grasses. 2. Pollen-tube penetration and the stigma response in Secale Acta Botanica Neerlandica 30: 289–307. [Google Scholar]
- Heslop-HarrisonJ, Heslop-Harrison Y.1991. Structural and functional variation in pollen intines. In: Blackmore S, Barnes SH, eds. Pollen and spores: patterns of diversification Systematics Association Special Volume 44 Oxford: Oxford Scientific Publishing, 331–343. [Google Scholar]
- Heslop-HarrisonY, Heslop-Harrison JS, Heslop-Harrison J.1986. Germination of Corylus avellana L. (Hazel) pollen: hydration and the function of the oncus. Acta Botanica Neerlandica 35: 265–284. [Google Scholar]
- LewisG, Schrire B, Mackinder B, Lock M.2003.Legumes of the world. Kew: Royal Botanic Gardens, in press. [Google Scholar]
- MullerJ.1981. Fossil pollen records of extant angiosperms. Botanical Review 47: 1–142. [Google Scholar]
- MullerJ.1984. Significance of fossil pollen for angiosperm history. Annals of the Missouri Botanical Garden 71: 419–443. [Google Scholar]
- PuntW, Blackmore S, Nilsson S, Le Thomas A.1994.Glossary of pollen and spore terminology. Utrecht: LPP Foundation. [Google Scholar]
- Rodríguez-GarcíaMI, Fernández MC.1988. A review of the terminology applied to apertural thickenings of the pollen grain: Zwischenkörper or oncus? Review of Palaeobotany and Palynology 54: 159–163. [Google Scholar]
- RowleyJR.1964. Formation of the pore in pollen of Poa annua In: Linskens HF, ed. Pollen physiology and fertilisation Amsterdam: North-Holland Publishing Co., 59–69. [Google Scholar]
- RowleyJR.1975. Germinal apertural formation in pollen. Taxon 24: 17–25. [Google Scholar]
- SkvarlaJJ.1966. Techniques of pollen and spore electron microscopy. I. Staining, dehydration, and embedding. Oklahoma Geological Notes 26: 179–186. [Google Scholar]


