Abstract
The Balanopaceae, whose flowers were poorly known, have, in the past, been variously allocated to the Fagales, Euphorbiaceae, Salicales or other hamamelids and rosids (these groups being in Fagales, Malpighiales and Saxifragales, according to the Angiosperm Phylogeny Group). This paper attempts a clarification based on flower morphology. Female flowers and cupules were studied in Balanops vieillardii, young fruits in B. australiana. The cupules are simple involucres of bracts which are spirally arranged (according to a Fibonacci pattern) on the floral axis preceding the flower. They contrast with the complicated cupules of Fagaceae which consist of a condensed cymose ramification system of axes of several orders around the flower. Flowers appear later than most of the cupular bracts, in contrast to Fagaceae. In addition to a terminal flower there may be several smaller lateral flowers in the axil of cupular bracts, each surrounded by its own small cupule. The female flowers do not have a perianth. They consist of two to three large carpels. At anthesis, the ovary is completely septate; the syncarpous part (ovary and lower style) is completely symplicate. The carpels are free for most of their length, with the free parts once, twice or three times bifurcate, in contrast to simple in Fagales. The stigmatic surface covers the ventral side of each stigmatic branch and at the margins also spreads to the dorsal side. The stigma is wet and secretion appears holocrine. The two ovules per carpel are collateral and axile in early development. However, at anthesis they appear one above the other, because in one ovule the funicle greatly elongates. As the ovary elongates only above the placenta, the ovules appear basal at anthesis. The ovules are (weakly) crassinucellar, bitegmic (not unitegmic), anatropous, and intermediate between apotropous and epitropous (not apotropous). The ovules are mature at anthesis, in contrast to Fagales. In mature ovules the upper part of the nucellus disintegrates, and a weakly differentiated endothelium is present in the inner integument. The morphological results of this study support a position of Balanopaceae in Malpighiales, and not Fagales or other orders, and are thus in accordance with recent molecular results based on chloroplast rbcL sequences data. However, within Malpighiales, as opposed to molecular results, Balanopaceae agree more with Euphorbiaceae s.l. than with Dichapetalaceae/Trigoniaceae and Chrysobalanaceae/Euphroniaceae.
Keywords: Balanopaceae, Balanops, cupule, Euphorbiaceae, Fagaceae, Fagales, floral structure, involucre, Malpighiales, ovule, rosids
INTRODUCTION
Balanops, the only genus in the Balanopaceae, contains nine species in New Caledonia, Fiji and Northern Queensland (Carlquist, 1980). Balanopaceae have a chequered systematic history. Balanops was first described by Baillon (1871), and a second genus, Trilocularia, by Schlechter (1906). Trilocularia was later incoporated into Balanops (Hjelmqvist, 1948, 1960; Smith, 1950). The phylogenetic position was unclear for a long time, and the genus and family were moved back and forth among the vicinity of Fagales (Baillon, 1877; van Tieghem, 1906; Takhtajan, 1959, 1969; Cronquist, 1968, 1981, 1988; Dahlgren, 1975, 1980), Euphorbiaceae (Bentham, 1880; Däniker, 1946), Salicales (van Tieghem, 1884; Guillaumin, 1925), Myricales–Leitneriales–Juglandales (Engler, 1897; Hjelm qvist, 1948), very broadly circumscribed Hamamelid aceae (Hallier, 1908, 1912), Daphniphyllineae, i.e. with Daphniphyllaceae (Thorne, 1976, 1992, 2000), close to Hamamelidales (Takhtajan, 1980), and Hamamelididae–Daphniphyllanae (Takhtajan, 1997), and several authors were undecided about the closest relatives (Baillon, 1871; Carlquist, 1980; Kubitzki, 1993).
Phylogenetic studies based on rbcL place Balanops in Malpighiales (sensu APG, 1998), as sister of a clade consisting of Chrysobalanaceae/Euphroniaceae and Dicha petalaceae/Trigoniaceae, the entire clade being supported with a bootstrap value of 79 % (Litt and Chase, 1998) or 68 %, respectively (Savolainen et al., 2000). This position is nested among constituents of Euphorbiaceae in the classical sense (Chase et al., 2002; without indication of bootstrap support value for this clade). Thus, the opinion of Bentham (1880) is currently supported again.
One of the reasons for the uncertainty of the systematic position was that the flowers are simple and had scarcely been studied. The plants are tall trees, which often grow on mountain ridges. They have unisexual flowers, are wind-pollinated, and apparently have a very short and inconspicuous flowering time so that the presence of flowers is easily overlooked. The wood is tough, and branches are difficult to collect. All this may account for the scarcity of collections of flowering material in herbaria. Even some of the species descriptions are based on fruiting herbarium material alone. Although Carlquist (1980) published photographs of fresh male inflorescences of B. sparsifolia and B. pancheri, anatomical studies were, apparently, never carried out on freshly fixed flowers of either gender. Therefore, reports on floral structure in the literature are only superficial and contain erroneous interpretations of structures. It should also be noted that the original line drawing of a longitudinal section of a female flower by Baillon (1877) was redrawn with unusual proportions in ovary and ovules exaggerated. In addition, the septum was mistakenly omitted in Hutchinson (1926), which may have caused misinterpretations of ovary and placenta structure by later authors. Here we present the first detailed study on the female flowers and cupules in representatives of the family Balanopaceae.
MATERIALS AND METHODS
Material of Balanops vieillardii Baillon, H.S. MacKee 38214, 5 Nov. 1980, New Caledonia (fruits), was studied. Plants were raised from seeds in this collection in a glasshouse in the Botanic Garden of the University of Zurich. One individual produced female flowers, which were used in this study. Fruits of Balanops australiana F. Muell. were observed for comparison (young fruits: B.P.M. Hyland 9278, northern Queensland; older fruits: P.K. Endress 4211, northern Queensland). Cupules with flowers of different stages were fixed in FAA and used for SEM and serial microtomy. Material for microtomy was embedded in Kulzer’s Technovit (2-hydroxyethyl methacrylate), sectioned at 8–10 µm, and stained with ruthenium red and toluidine blue; the sections were enclosed in Histomount. For SEM studies the specimens were critical-point dried and sputter-coated with gold.
RESULTS
Structure and development of the cupule
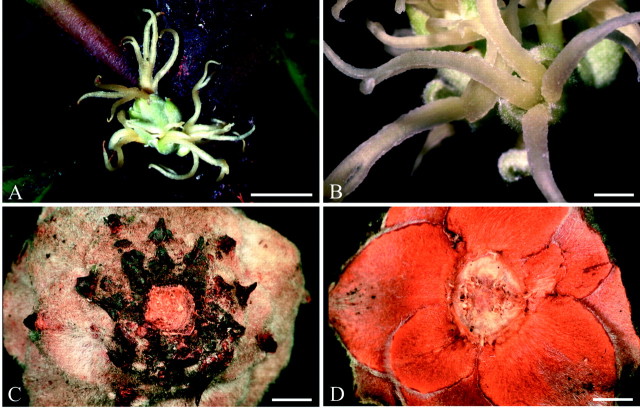
Each female flower is surrounded by a group of tightly arranged bracts, which form an involucre for the flower (Fig. 1A and B). This involucre is superficially similar to an oak cupule and may have caused, at least partly, the systematic association of Balanopaceae with Fagaceae by some earlier authors (Fig. 1C and D). However, this involucre or cupule in Balanops has neither been analysed morphologically before nor compared with the cupule in Fagaceae. Each cupule has either a single terminal flower or, in addition, several lateral flowers, which are each situated in the axil of a bract (Fig. 1A and B). Each lateral flower is surrounded by its own set of cupular bracts. In young cupules, all or most bracts seem to have an axillary bud, which may or may not produce a lateral flower (Fig. 2A and B).
Fig. 1.Balanopsvieillardii. Inflorescence and cupule of fruit: A, inflorescence, terminal flower above, lateral flowers below; B, lateral flower; C, fruit cupule from below; D, fruit cupule from above. Bars: A = 1 cm; B–D = 2 mm.
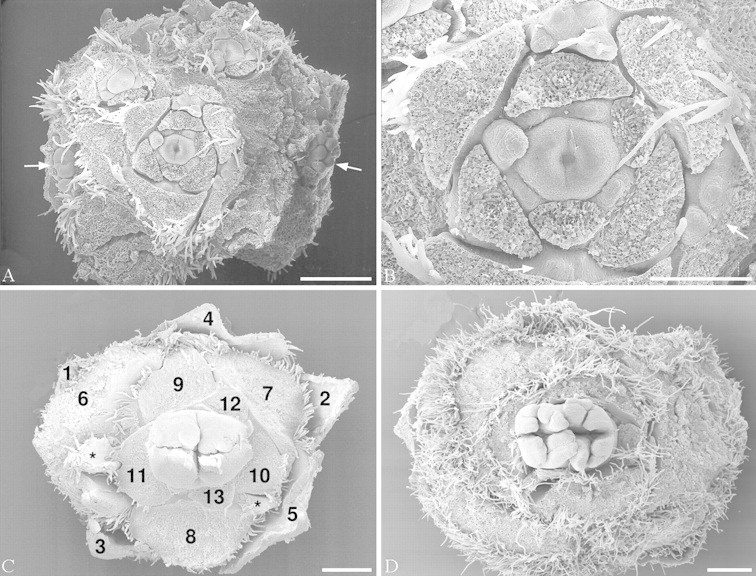
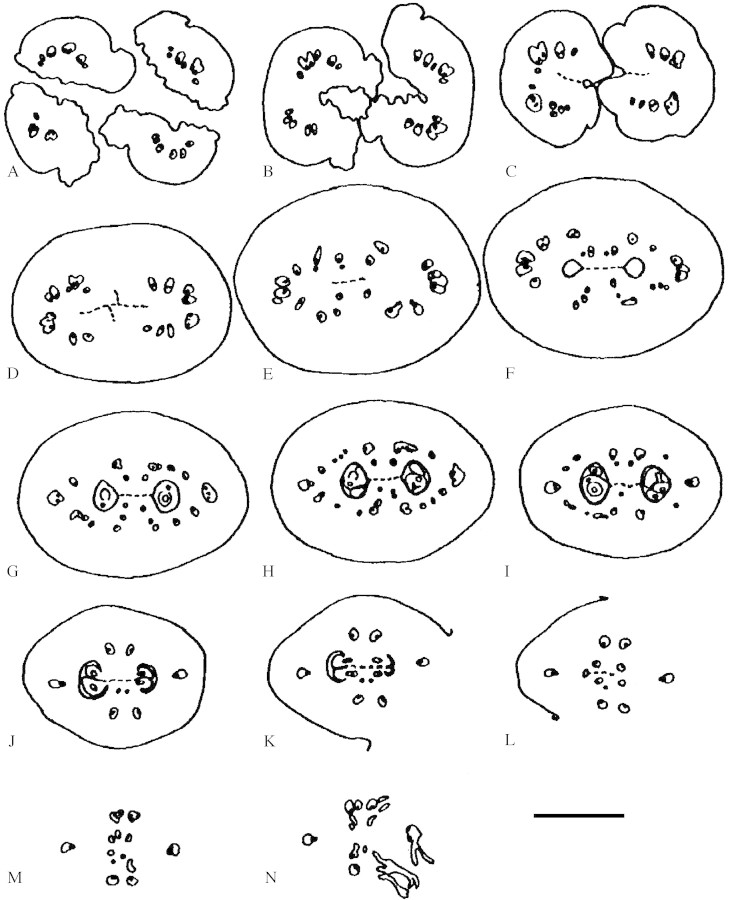
Fig. 2.Balanopsvieillardii. Young inflorescence and terminal flowers. Bracts removed to show flowers. A, Overview of inflorescence with terminal and lateral flowers. B, Terminal flower of the same inflorescence, at higher magnification. C, Terminal flower, older, showing first and second bifurcation of carpels. Subsequent bracts of cupule numbered to show spiral arrangement. D, Terminal flower, showing first, second and third bifurcation of carpels. White arrows: reduced lateral flowers; stars: lateral buds. Bars: A, C and D = 0·5 mm; B = 0·25 mm.
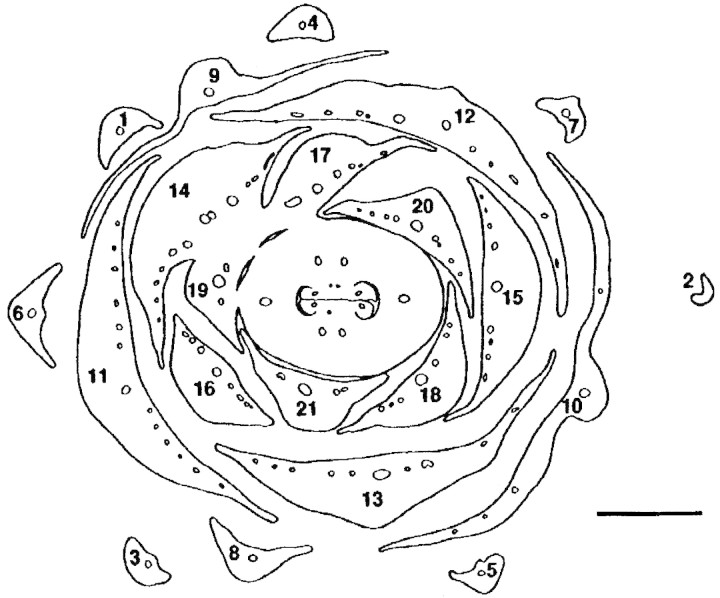
The bracts that form the cupule arise acropetally. They are spirally arranged with a divergence angle of approx. 137°, i.e. in a normal Fibonacci pattern (Figs 2C and 3). The innermost organs are quite retarded and take the last available spaces around the seemingly somewhat precocious gynoecium (Fig. 2A and B). They may then deviate from a Fibonacci pattern and tend to form approximate whorls of three organs or have a somewhat irregular arrangement (Fig. 2A and B). In addition, the innermost organs lack axillary buds (or they may be so retarded that the axial buds are also retarded) (Fig. 2B). Although the flower seems perianthless, it is not completely impossible that the innermost retarded organs, which are devoid of an axillary bud may represent a reduced perianth. However, it would then seem unusual that the flower does not build up its own phyllotaxis but is so dependent on the position of the uppermost bracts. Lateral flowers have fewer cupular bracts, all spirally arranged (also with a Fibonacci pattern), and are without axillary buds. In any case, compared with Fagaceae, the terminal flower is formed later than most of the bracts of the cupule. The number of cupular bracts may differ between species: Balanops vieillardii has 19–29 bracts, B. australiana 7–11; according to Smith (1942) B. pedicellata (as Trilocularia vitiensis) has only 6–8.
Fig. 3.Balanopsvieillardii. Transverse section of cupule with terminal female flower at the level at which the flower and the uppermost bracts of the cupule fuse. Subsequent bracts of cupule numbered to show spiral arrangement. Bar = 1 mm.
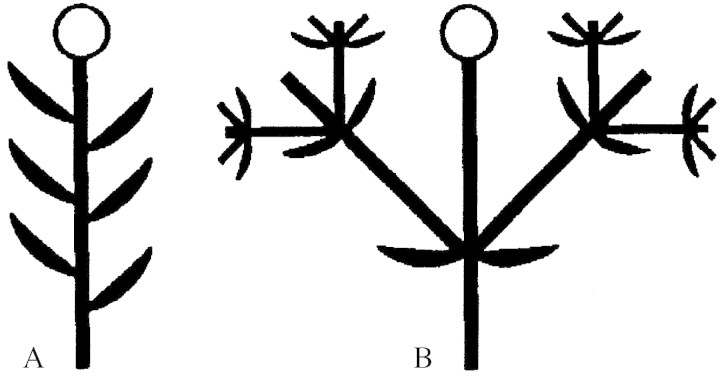
Thus, the cupule is a simple uniaxial structure (Fig. 4A). At anthesis, the cupular bracts are thick. Their tissue is tanniferous, and they are densely covered with lignified unicellular hairs on the dorsal side. The bracts tightly surround the female flower. Only shortly before anthesis, the stigmatic part of the gynoecium greatly elongates and is exposed above the cupule. In sharp contrast to the cupule, the tissues of the flower largely lack tanniferous cells. Each bract has a median and several lateral vascular bundles and three or more vascular traces in the base, some of the innermost ones may have only one trace (Fig. 3).
Fig. 4. Diagrams of cupules: A, Balanopaceae; B, Fagaceae. In the diagrams the axes are much longer than they are in reality to illustrate the ramification pattern. Flowers are represented by circles. Axes and bracts that are constituents of the cupule are represented by thick black symbols.
Structure and development of the female flower
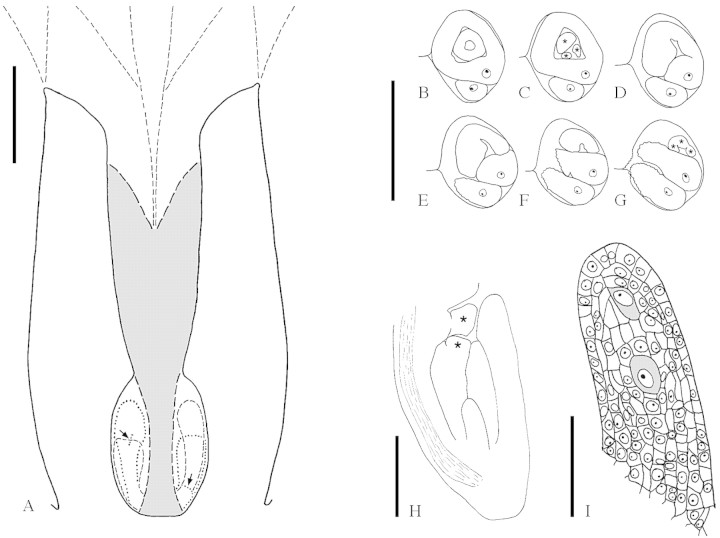
The terminal female flowers consist of two or three carpels and no other organs (see above). At anthesis, the main portion of anthetic flowers (approx. 1·5 cm long) is taken up by the extensive stigmas, whereas the ovary is short and narrow (Figs 1A and B and 7A). The stigmatic part of each carpel commonly consists of four (to eight) long and relatively broad, ribbon-like lobes, which arise by double (or triple) bifurcation of the free part of the carpel (Figs 2C and D, 3, 5A–D and 6). The ventral side of the lobes is stigmatic. Because it is somewhat revolute, the stigmatic area is peripherally also exposed towards the dorsal side (Fig. 5C and E). In addition, each lobe is somewhat twisted so that it gives the superficial impression of a terete structure that is stigmatic everywhere (Fig. 5D). The ventral surface of the carpels is completely expanded and does not form a ventral slit at the stigmatic level. Below the stigmatic area the carpels are congenitally united. The first bifurcation is shortly above the transition from the syncarpous to the apocarpous zone, the second bifurcation is somewhat higher up (Figs 5A and C and 6A). Coming from above towards this transition zone, the carpels become close to each other, and a ventral slit is formed in each carpel (Fig. 6B and C). If two carpels are present, their connection is asymmetrical, in that each carpel directs one flank towards the ventral slit of the other. This kind of asymmetry is also known from similar bicarpellate gynoecia in other groups (e.g. Hamamelidaceae, Endress, 1967; Buxaceae, von Balthazar and Endress, 2002). It results in a zig-zag-shaped common ventral slit for both carpels (Fig. 6B and C). Lower down, the common ventral slit becomes straight and more narrow as seen in the median plane of the gynoecium (Figs 6D and E and 7A).
Fig. 7.Balanops vieillardii. A, Schematic median LS of gynoecium at anthesis (upper stigmatic region not drawn). Post-genitally fused area is shaded. Outline of parts that are not exactly in the median plane interrupted. Micropyles are marked with arrows (longitudinal in longer ovule, transverse in shorter ovule). B–G, TS series of the two ovules in a locule, shorter ovule showing micropylar region, longer ovule showing funicle. Vascular bundle in raphe indicated (xylem black). Lobes of inner (in C) and outer (in G) integument marked with stars. B, Level of both integuments; C, lobes of inner integument; D–G, lobes of outer integument. H, Median LS ovule at megaspore mother cell stage. Vascular bundle in raphe indicated. Lobes of inner and outer integument marked with stars. I, Nucellus of the same ovule, showing two potential megaspore mother cells (shaded). Bars: A = 1 mm; B–G = 0·5 mm; H = 0·2 mm; I = 0·05 mm.
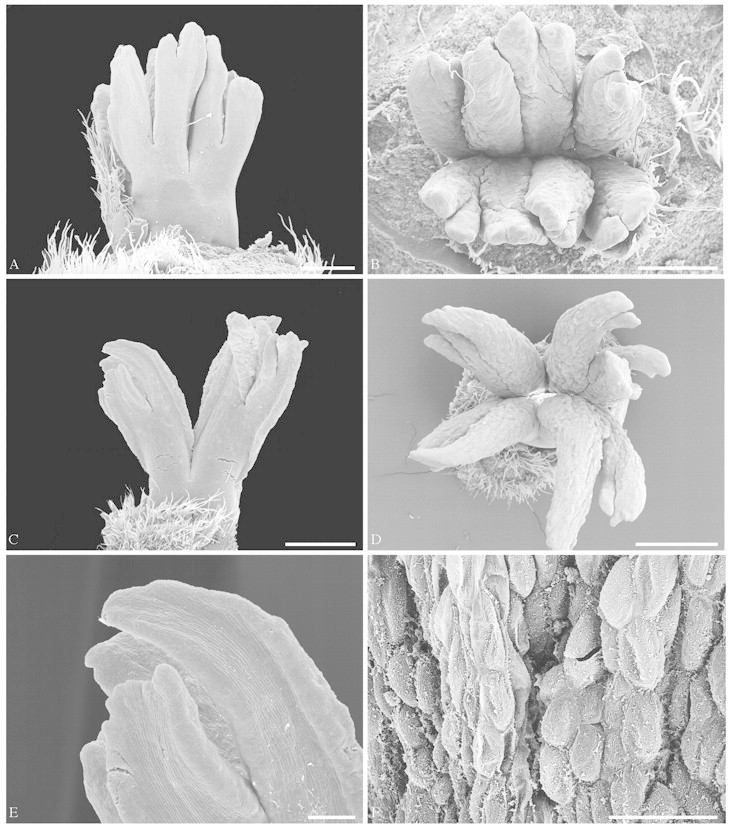
Fig. 5.Balanops vieillardii. Female flowers at different developmental stages. A and B, Younger preanthetic flower: A, from the side; B, from above. C–E, More advanced preanthetic flower: C, from the side; D, from above; E, close-up of C, showing dorsal side of stigmatic lobes with bulging stigmatic tissue at the margins. F, Part of stigma of anthetic flower, epidermal cells secretory and collapsing. Bars: A and B = 0·5 mm; C and D = 1 mm; E = 0·2 mm; F = 0·1 mm.
Fig. 6.Balanops vieillardii. TS series of somewhat preanthetic flower. A–C, Apocarpous zone: A, level of first bifurcation of free carpels; B, below first bifurcation, ventral slits open; C, ventral slits closed. D–L, Symplicate zone: D and E, style; F–L, ovary, F, top of locule without ovules; G, upper level of longer ovules; H, micropylar level of longer ovules; I, micropylar level of one of the shorter ovules; J, funicular level of all ovules; K, placenta; L, lowermost section with one locule. M and N, Level below locules. Post-genitally fused areas are marked with interrupted lines. Vascular bundles (xylem black) marked with thinner lines. Bar = 1 mm.
The syncarpous part of the gynoecium is symplicate. A synascidiate base is not formed (Fig. 6D–L). The ovary is completely bi- or trilocular. Each carpel has two ovules on an axile placenta at the base of the locule. The two ovules of a carpel are inserted at the same level on both sides of the carpel but are unequal in size and shape (Fig. 6J and K). One of them has a long funicle which brings this ovule in a position above the other (Fig. 7A). In bicarpellate ovaries the longer and shorter ovules alternate with each other, thus ovules of the same kind appear diagonal to each other. The ovules are clearly bitegmic, although they were described as unitegmic (see Discussion) (Figs 7B–H and 8A, B, F and G). In mature ovules the outer integument is five to seven cell layers thick, the inner five or six cell layers. The nucellus is relatively thin compared with the thickness of the integuments. However, there is more than one cell layer above the megaspore mother cells; thus, the ovules are (weakly) crassinucellar (Fig. 7I). At maturity the embryo sac expands and the upper part of the nucellus disintegrates; a weakly differentiated endothelium is formed in the inner integument (Fig. 8G). The two ovules of a carpel are curved away from each other. The micropyle faces the lateral region of the carpel. It is formed by both integuments. In the longer ovule, the micropyle is directed more or less to the side and forms a vertical (longitudinal) slit (Fig. 8C). In contrast, in the shorter ovule the micropyle faces more downwards and forms a more or less horizontal (transverse) slit (Fig. 8E). The micropyle of the shorter ovule is appressed to its funicle so that the micropyle is somewhat hidden and the outer integument forms an edge at the periphery of this appressed area. The ovules are asymmetrical and cannot be sectioned in a median longitudinal plane, because their anatropous curvature is combined with an upward bending at the placenta, which gives them a twisted appearance. Both integuments are lobed, especially prominent in the shorter ovule (Fig. 7C, G and H). The path of pollen tubes has not been investigated, as male flowers were not available. It may be expected that pollen tubes grow through the post-genitally fused common ventral slit of both carpels down to the placenta and from there upwards along the funicle towards the micropyle. In Balanops australiana the position of the ovules and the direction of the micropyles is the same as in B. vieillardii.
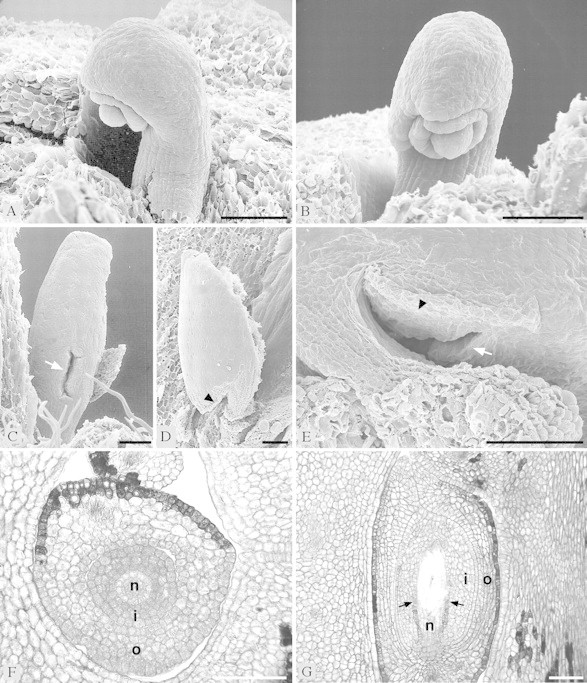
Fig. 8.Balanops vieillardii. Ovules. A and B, From preanthetic flower, showing two integuments and nucellus: A, from the side; B, from the front. C–E, From anthetic flower. Micropyle marked with white arrow. Edge around micropylar region where ovule is appressed to funicle marked with black arrowhead. C, Longer ovule from the front, showing micropyle; D–E, shorter ovule; D, from the side, with microyple appressed to funicle; E, obliquely from below, showing micropyle. F–G, Microtome sections of ovules (n, nucellus; i, inner integument; o, outer integument): F, TS of preanthetic ovule; G, LS of anthetic ovule, with nucellus almost disintegrated by growing embryo sac. Weakly differentiated endothelium marked with arrows. Bar = 0·1 mm.
Each of the massive stigmatic lobes of a carpel contains several strong vascular bundles (Fig. 6A). In the upper syncarpous part of the gynoecium the two bands of vascular bundles of the next higher bifurcation join to form a semicircular to almost circular complex of bundles (Fig. 6B–F). At mid-level of the ovary these two bundle complexes are rearranged into a large dorsal and two main lateral bundles for each carpel (Fig. 6G–J). Between the dorsal and the lateral bundles, there are several smaller laterals, which irregularly connect the dorsal and main lateral bundles (Fig. 6H and I). Toward the base of the ovary, the lesser lateral bundles disappear (Fig. 6J–L). The two ovules of each carpel are each served by a basal branch of the two main lateral bundles (Fig. 6K–N). In the floral base the three main bundles of each carpel irregularly join the bundles of the adjacent bracts (Fig. 6N).
The ventral part of the stigmatic lobes has large cells and is strongly vacuolized. This increase in cellular size probably causes the revolute shape mentioned above. The stigmatic surface is secretory. At anthesis, stigmatic epidermal cells collapse and give the impression of holocrine secretion (for terminology, see Fahn, 1979) (Fig. 5F). However, this has not been studied in detail. The dorsal epidermis has small cells and is tanniferous; however, there is a conspicuous contrast between the highly tanniferous bracts and the only peripherally tanniferous flower. The entire flower is glabrous.
Lateral flowers are retarded in development and reduced in morphology. The stigmatic branches are less bifurcate (only once or twice). The carpels often have only one ovule or none at all. These flowers are probably sterile.
DISCUSSION
Structural aspects
The only published figures of female flowers of Balanops are line drawings made from entire flowers or hand-sectioned flowers in Baillon (1877), Schlechter (1906), Hjelmqvist (1948) and Carlquist (1989). It is not clear from the literature whether the involucre or cupule of numerous imbricate scales that surrounds the gynoecium is made up of sepals (Baillon, 1871) or bracts (Engler, 1897; Schlechter, 1906; Guillaumin, 1925; Carlquist, 1980; Smith, 1981) or both. Baillon (1877) is undecided in this question. The suggested similarity of this assemblage of scales with a scaly cupule of, for example, Quercus (Fagaceae) seemed only superficial to some authors (Engler, 1897; Hjelmqvist, 1948; Cronquist, 1981), although a study of the cupule of Balanopaceae and, therefore, also a comparative study between the two families is lacking. This tentative interpretation of non-homology is supported by the present study. In Fagaceae and Nothofagaceae, the cupule consists of a robust circular rim or of several valves, associated with bracts, whereas in Balanops such a rim or valves are lacking. Still more importantly, from the point of view of comparative morphology, in Fagaceae and Nothofagaceae the cupule is formed by the peripheral parts of a compacted complex cymose sterile branching system (Fey and Endress, 1983; Rozefelds and Drinnan 2002), whereas in Balanops, it consists simply of a number of spirally arranged bracts on the floral axis (this study) (Fig. 4A and B). In male flowers of Balanops a perianth was said to be absent by earlier authors (Baillon, 1871, 1877; Guillaumin, 1925; Hjelm qvist, 1948). Baillon (1872) and Bentham (1880) mention the presence of just one bract immediately below each male flower (Bentham calls it a perianth); Hallier (1908: 243) also presumes it is a perianth, and Carlquist (1980) states that all male flowers have a perianth. However, Carlquist does not specify number and position of these organs, and this is not clear from his photographs. It is unknown whether the small scales forming this perianth are homologous to the scales surrounding the female flowers. Stamen number is indicated as 1–12 per flower (Smith, 1981), and as greatly variable even within an inflorescence (Carlquist, 1980).
In general, the term cupule has been used for cup-like structures that surround flowers and provide protection for floral buds and sometimes also for young fruits, but which are formed by non-floral organs. The homology was originally not assessed and thus they may represent quite different structures in different families for which the term has been applied (cf. also Podostemaceae; Jäger-Zürn and Mathew, 2002). In addition, the term has been used outside of the angiosperms for envelopes of ovules in basal spermatophytes (cf. e.g. Doyle, 1994). Thus, an uncritical use of the term may lead to wrong conclusions if non-homologous structures are compared. The present study clearly shows, as presumed by some earlier authors, that the cupules in Balanopaceae are not homologous with those in Fagaceae and Nothofagaceae (Fey and Endress, 1983; Rozefelds and Drinnan, 2002). Thus, one of the most striking similarities between these families used for systematic conclusions in earlier works has now shown to have no basis. In addition, the presence of lateral flowers below the terminal flower seems not to have been recorded before. Only Hjelmqvist (1948) mentions lateral buds, but not flowers.
There has been confusion about ovary structure ever since the first description of Balanops. According to Baillon (1871, 1877) and Engler (1897) the ovary is incompletely septate in B. vieillardii. This view of incomplete septation has even been reinforced by the incorrect re-drawing of Baillon’s (1877) figure in Hutchinson (1926), in which the septum was completely omitted. Hutchinson’s figure wrongly implies a free basal-central placenta. According to Carlquist (1980, 1989) the locules of Balanops in general are imperfectly septate at anthesis, but fully septate in fruit. However, Schlechter (1906) and Hjelmqvist (1948) mentioned that B. sparsifolia shows complete septation at anthesis. In the present study it is shown that the locule is completely septate at anthesis even in B. vieillardii. Septation is achieved by the complete closure of the ventral slits of the carpels in the centre of the ovary. Thus, at anthesis the placenta is axile, at the base of each locule. Simply to state that it is basal (or near basal) (Baillon, 1871, 1877; and subsequent authors) is therefore not sufficient.
Each carpel has two ovules, more or less superimposed, the lower one of them shorter than the upper one (Baillon, 1877; Hjelmqvist, 1948; Carlquist, 1980). However, the attachment of both ovules is at the same level, thus they are collateral. Only one of the two ovules in each carpel commonly develops into a seed, and sometimes only one per fruit. In anthetic flowers studied, the longer ovule of each locule tends to be somewhat more advanced than the shorter. This may indicate that the longer ovules tend to develop into seeds, while the shorter ones will abort. However, post-anthetic development was not studied.
The ovules have been described as apotropous (Hjelmqvist, 1948; Carlquist, 1980; Smith, 1981), and sometimes the same has been expressed with other words: ‘micropyle extrorsum infera’ (Baillon, 1871) or ‘funiculus directed toward the septum’ (Engler, 1897; van Tieghem, 1906). Such an apotropous direction was also illustrated by Baillon (1877). However, this illustration and the descriptions are not completely accurate. The micropyles of both ovules of a carpel are directed toward the side, away from each other, thus the ovules are intermediate between apotropous and epitropous. The terms apotropous and epitropous are more conveniently applied for carpels with a single ovule in median position; if more than one ovule is present, such as in Balanops, the direction is often intermediate (see also Endress, 1967, 1994; Wagenitz, 1996)
The early publications on Balanops, such as Baillon (1871, 1872, 1877), Mueller (1877), Bentham (1880), Engler (1897), Wettstein (1901), Schlechter (1906) and van Tieghem (1906), did not address the number of integuments. The first mention of unitegmic ovules was found in Engler (1903), followed by Hallier (1908: 89), Wettstein (1924), Guillaumin (1925), Hjelmqvist, 1948), Melchior (1964), Dahlgren (1975), Corner (1976), Thorne (1977), Carlquist (1980), Cronquist (1981), Mametyeva (1981), Nemirovich-Danchenko (1991) and Takhtajan (1997). But it seems that during the past 100 years nobody had really studied the ovules [there are only simple outline drawings from hand-dissected flowers in Baillon (1877), Hjelmqvist, 1948) and Carlquist (1989)]. Thus the presence of two integuments as reported here for the first time, is a surprising result.
An obturator (i.e. a peg-like extension of the placenta towards the micropyle) as in Euphorbiaceae and other Malpighiales (Sutter and Endress, 1995) is lacking. Baillon (1871, 1877), and other authors quoting him (Guillaumin, 1925; Däniker, 1946), mention a funicular obturator in Balanops. Baillon (1877: Fig. 211) also figures a thin transversal lid below the micropyle in both ovules. This structure was not found in the material described here. Moreover, in the longer ovule the micropyle is vertically and not horizontally directed. In the shorter ovule the micropyle is just appressed to the funicle. For pollen tube guidance and transmission, an obturator only makes sense if it connects the placenta with the micropyle, which is not the case in Baillon’s figure. It is common for pollen tubes to grow along the funicle and, for this, an obturator is not necessary.
Systematic aspects
The following features of Balanopaceae support a position in Malpighiales and not Fagales. (a) Cupule organization is fundamentally different from that in Fagales and more in line with the simple involucre of bracts of some Euphorbiaceae with reduced flowers. (b) A perianth is absent as in some Euphorbiaceae, but not in comparable Fagales (Fagaceae). (c) Stigmatic lobes are once or twice (or even more) bifurcate as in many Euphorbiaceae s.l. (e.g. Endress, 1994; Sutter and Endress, 1995) but unlike Fagaceae, which have simple stigmatic branches. (d) Ovules are always bitegmic in Malpighiales (cf. Endress, 2003), whereas they are unitegmic in most families of Fagales (Nothofagaceae, Betulaceae, Juglandaceae, Myricaceae), although bitegmic in Fagaceae and Casuarinaceae, and unknown in Ticodendraceae; thus, with the new finding of two, and not one integument, Balanops is no longer a stranger in Malpighiales. (e) The inner integuments are thick (multilayered) as in some Malpighiales (Chrysobalanaceae, Tobe and Raven, 1984; Dichapetalaceae, Boesewinkel and Bouman, 1980; Erythroxylaceae, Boesewinkel and Geenen, 1980; many Euphorbiaceae s.l., Sutter and Endress, 1995; Linaceae, Boesewinkel, 1980; Rhizo phoraceae, Dahlgren, 1988; Trigoniaceae, Boesewinkel, 1987) but less so in the bitegmic taxa of Fagales. (f) Ovules, although crassinucellar, have relatively thin nucelli; thus, they fit better with the relatively thin nucelli (but crassinucellar ovules) of Euphorbiaceae (Sutter and Endress, 1995) or even tenuinucellar (or incompletely tenuinucellar) ovules of Chrysobalanaceae, Clusiaceae, Dichapetalaceae, Linaceae p.p., Ochnaceae and Trigon iaceae (cf. Endress, 2003) than with the thicker nucelli of Fagales [although in some Fagaceae the nucelli are also relatively thin (Hjelmqvist, 1953; Fey, 1981)]. (g) In mature ovules the upper part of the nucellus disintegrates, and a weakly developed endothelium on the inner integument is present as in some Malpighiales (e.g. Chrysobalanaceae, Tobe and Raven, 1984; Erythroxylaceae, Boesewinkel and Geenen, 1980; Linaceae, Boesewinkel, 1980; Ochnaceae, Narayana, 1975; Putranjivaceae of Euphorbiaceae s.l., Thathachar, 1952; Tokuoka and Tobe, 1999; Rhizo phoraceae, Dahlgren, 1988; Trigoniaceae, Boesewinkel, 1987) but not in Fagales. (h) Ovules are mature at anthesis, in contrast to Fagales, in which they are often retarded in development (Endress, 1977; Fey, 1981).
Balanopaceae are unusual in the position of the ovules at anthesis, which differs from both Fagales and Malpighiales. Although they agree with both Nothofagaceae/Fagaceae and many Euphorbiaceae s.l. in that collateral ovule pairs begin development in axile position, intercalary elongation of the ovary is above the ovules in Balanopaceae but below in Nothofagaceae/Fagaceae and Euphorbiaceae s.l. (and other Malpighiales) so that the ovules are at the base of the locules in Balanopaceae and on top in Euphorbiaceae s.l. (Sutter and Endress, 1995), Nothofagaceae (Langdon, 1947; Poole, 1952; Kummerow and Labarca, 1961; Fey, 1981) and Fagaceae (Endress, 1977; Fey, 1981) [except for some Quercus species in which they become secondarily ‘basal’ during fruit development (Borgardt and Nixon, 2002)]. The allegedly basal placenta in Chrysobalanaceae and some Ochnaceae among Malpighiales is not directly comparable with that in Balanopaceae, because in those families the ventral part of the locule is extremely shortened so that it becomes impossible to distinguish an apical from a basal position.
As mentioned in the Introduction, within the large order Malpighiales (sensu APG, 1998), Balanopaceae appear as sister to a moderately supported clade comprising Dichapetalaceae/Trigoniaceae and Chrysobalanaceae/Euphroniaceae, based on rbcL analysis (Litt and Chase, 1998; Savolainen et al. 2000; Chase et al., 2002). How do the flowers of Balanopaceae compare with those in these four families? Their flowers have a double perianth, are bisexual and largely monosymmetric, thus very different from Balanopaceae. However, within Malpighiales, Balano paceae share unisexual flowers, multiple bifurcate stylar branches and lack of a perianth with some Euphorbiaceae s.l. Also in ovule structure Balanopaceae are more like Euphorbiaceae s.l. than Dichapetalaceae/Trigoniaceae and Chrysobalanaceae/Euphroniaceae. Chrysobalanaceae (Tobe and Raven, 1984), Dichapetalaceae (Boesewinkel and Bouman, 1980) and Trigoniaceae (Boesewinkel, 1987), have incompletely tenuinucellar ovules; Euphroniaceae are unknown (review in Endress, 2003). In contrast, in Balanops vieillardii, the ovules are (weakly) crassinucellar, although the nucellus is thin in comparison with the integuments. This is also the case in Euphorbiaceae s.l. (Euphorbiaceae, Phyllanthaceae and Picrodendraceae, and Putranjivaceae, according to Chase et al., 2002), which have crassinucellar or weakly crassinucellar ovules (Kapil and Bhatnagar, 1994; Sutter and Endress, 1995; Tokuoka and Tobe, 1995, 1998, 1999, 2001, 2002; Carmichael and Selbo, 1999). Crassinucellar ovules are also present in many other families of Malpighiales (Achariaceae, Bernhard, 1998; Erythroxylaceae, Boesewinkel and Geenen, 1980; Humiriaceae, Boesewinkel, 1985; Linaceae p.p., Boese winkel, 1980; Malesherbiaceae, Ricardi, 1967; Malpig hiaceae, Stenar, 1937; Medusagynaceae, Dickison, 1990; Passifloraceae, Kloos and Bouman, 1980; Rhizophoraceae, Dahlgren, 1988; Salicaceae, Narayanaswami and Sawhney, 1959; Turneraceae, Vijayaraghavan and Kaur, 1966; Violaceae, Singh, 1963). Many of these families, although weakly to strongly supported as families by rbcL, are still unresolved in their interrelationships within Malpighiales (Chase et al., 2002). Thus it remains open whether the floral morphological similarities between Balanopaceae and Euphorbiaceae s.l. reflect a cladistic relationship or are the result of parallel evolution.
ACKNOWLEDGEMENTS
We thank H. S. MacKee for providing live fruits of Balanops vieillardii, and B. P. M. Hyland for supporting P.K.E. in the field and for providing fixed fruits of B. australiana. We also thank R. Siegrist for microtomy, U. Jauch for help with the SEM and A. Bernhard for help with the illustrations.
Received: 9 April 2003; Returned for revision: 2 June 2003; Accepted: 10 June 2003
References
- APG(The Angiosperm Phylogeny Group).1998. An ordinal classification for the families of flowering plants. Annals of the Missouri Botanical Garden 85: 531–553. [Google Scholar]
- BaillonH.1871. Sur deux nouveaux genres apétales. Adansonia 10: 112–119. [Google Scholar]
- BaillonH.1872. Stirpes exoticae novae (continué). Adansonia 10: 334–345. [Google Scholar]
- BaillonH.1877.Histoire des plantes VI. Paris: Hachette. [Google Scholar]
- BenthamG.1880. Balanopseae. In: Bentham G, Hooker JD, eds. Genera plantarum, Vol. 3. London: Reeve, 341. [Google Scholar]
- BernhardA.1998. Floral structure and development of Ceratiosicyos laevis (Achariaceae) and its systematic position. Botanical Journal of the Linnean Society 131: 103–113. [Google Scholar]
- BoesewinkelFD.1980. Development of ovule and testa of Linum usitatissimum L. Acta Botanica Neerlandica 29: 17–32. [Google Scholar]
- BoesewinkelFD.1985. The ovule and seed of Humiria balsamifera (Aubl.) St. Hil. Acta Botanica Neerlandica 34: 183–191. [Google Scholar]
- BoesewinkelFD.1987. Ovules and seeds of Trigoniaceae. Acta Botanica Neerlandica 36: 81–91. [Google Scholar]
- BoesewinkelFD, Bouman F.1980. Development of ovule and seed-coat of Dichapetalum mombuttense Engl. with notes on other species. Acta Botanica Neerlandica 29: 103–115. [Google Scholar]
- BoesewinkelFD, Geenen J.1980. Development of ovule and seed-coat of Erythroxylum coca Lamk. Acta Botanica Neerlandica 29: 231–241. [Google Scholar]
- BorgardtSJ, Nixon KC.2002. A phylogenetic based ovule, seed and fruit anatomical/developmental study within the genus Quercus (Fagaceae). Congress Botany 2002 – Botany in the Curriculum: Integrating Research and Teaching, Madison, Wisconsin. Abstract. [Google Scholar]
- CarlquistS.1980. Anatomy and systematics of Balanopaceae. Allertonia 2: 191–246. [Google Scholar]
- CarlquistS.1989. Balanopaceae. In: George AS, ed. Flora of Australia, Vol. 3 Canberra: Australian Government Publishing Service, 93–95. [Google Scholar]
- CarmichaelJS, Selbo SM.1999. Ovule, embryo sac, embryo, and endosperm development in leafy spurge (Euphorbia esula). Canadian Journal of Botany 77: 599–610. [Google Scholar]
- ChaseMW, Zmarzty S, Lledó MD, Wurdack KJ, Swensen SM, Fay MF.2002. When in doubt, put it in Flacourtiaceae: a molecular phylogenetic analysis based on plastid rbcL DNA sequences. Kew Bulletin 57: 141–181. [Google Scholar]
- CornerEJH.1976.The seeds of dicotyledons, Vol. 1. Cambridge: Cambridge University Press. [Google Scholar]
- CronquistA.1968.The evolution and classification of flowering plants. Bronx: A. Cronquist. [Google Scholar]
- CronquistA.1981.An integrated system of classification of flowering plants. New York: Columbia University Press. [Google Scholar]
- CronquistA.1988.The evolution and classification of flowering plants, 2nd edn. Bronx: New York Botanical Garden. [Google Scholar]
- DahlgrenRMT.1975. A system of classification of the angiosperms to be used to demonstrate the distribution of characters. Botanical Notiser 128: 119–147. [Google Scholar]
- DahlgrenRMT.1980. A revised system of classification of the angiosperms. Botanical Journal of the Linnean Society 80: 91–124. [Google Scholar]
- DahlgrenRMT.1988. Rhizophoraceae and Anisophylleaceae: summary statement, relationships. Annals of the Missouri Botanical Garden 75: 1259–1277. [Google Scholar]
- DänikerAU.1946. Über die Euphorbiaceen und die Entwicklung der Monochlamydeae. Archiv der Julius-Klaus-Stiftung 21: 465–469. [PubMed] [Google Scholar]
- DickisonWC.1990. The morphology and relationships of Medusagyne (Medusagynaceae). Plant Systematics and Evolution 171: 27–55. [Google Scholar]
- DoyleJA.1994. Origin of the angiosperm flower: a phylogenetic perspective. Plant Systematics and Evolution, Suppl. 8: 7–29. [Google Scholar]
- EndressPK.1967. Systematische Studie über die verwandtschaftlichen Beziehungen zwischen den Hamamelidaceen und Betulaceen. Botanische Jahrbücher für Systematik 87: 431–525. [Google Scholar]
- EndressPK.1977. Evolutionary trends in the Hamamelidales–Fagales group. Plant Systematics and Evolution, Suppl. 1: 321–347. [Google Scholar]
- EndressPK.1994.Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press. [Google Scholar]
- EndressPK.2003. What should a ‘complete’ morphological phylogenetic analysis entail? In: Stuessy TF, Hörandl E, Mayer V, eds. Deep morphology: toward a renaissance of morphology in plant systematics. Königstein: Koeltz, in press. [Google Scholar]
- EnglerA.1897. Balanopsidaceae. In: Engler A, Prantl K, eds. Die natürlichen Pflanzenfamilien, Nachträge zum II–IV. Teil Leipzig: Engelmann, 114–116. [Google Scholar]
- EnglerA.1903.Syllabus der Pflanzenfamilien, 3rd edn. Berlin: Borntraeger. [Google Scholar]
- FahnA.1979.Secretory tissues in plants. London: Academic Press. [Google Scholar]
- FeyBS.1981.Untersuchungen über Bau und Ontogenese der Cupula, Infloreszenzen und Blüten sowie zur Embryologie bei Vertretern der Fagaceae und ihre Bedeutung für die Systematik. Doctoral dissertation, University of Zurich. [Google Scholar]
- FeyBS, Endress PK.1983. Development and morphological inter pretation of the cupule in Fagaceae. Flora 173: 451–468. [Google Scholar]
- GuillauminA.1925. Recherches sur l’anatomie et la classification des Balanopsidacées. Revue Générale de Botanique 37: 433–449. [Google Scholar]
- HallierH.1908. Über Juliania, eine Terebinthaceen-Gattung mit Cupula, und die wahren Stammeltern der Kätzchenblütler. Neue Beiträge zur Stammesgeschichte der Dicotyledonen. Beihefte zum Botanischen Centralblatt II 23: 81–265. [Google Scholar]
- HallierH.1912. L’origine et le système phylétique des angiospermes exposés à l’aide de leur arbre généalogique. Archives Néerlandais des Sciences Exactes et Naturelles, IIIB, 1: 146–234. [Google Scholar]
- HjelmqvistH.1948. Studies on the floral morphology and phylogeny of the Amentiferae. Botaniska Notiser Suppl. 2: 1–171. [Google Scholar]
- HjelmqvistH.1953. The embryo sac development of Quercus robur L. Phytomorphology 3: 377–384. [Google Scholar]
- HjelmqvistH.1960. Notes on names and combinations within the Amentiferae. Botaniska Notiser 113: 373–380. [Google Scholar]
- HutchinsonJ.1926.The families of flowering plants, 1st edn. Oxford: Clarendon Press. [Google Scholar]
- Jäger-ZürnI, Mathew CJ.2002. Cupule structure of Dalzellia ceylanica and Indotristicha ramosissima (Podostemaceae). Aquatic Botany 72: 79–91. [Google Scholar]
- KapilRN, Bhatnagar AK.1994. The contribution of embryology to the systematics of the Euphorbiaceae. Annals of the Missouri Botanical Garden 81: 145–159. [Google Scholar]
- KloosA, Bouman F.1980. Case studies in aril development: Passiflora suberosa L. and Turnera ulmifolia L. Beiträge zur Biologie der Pflanzen 55: 49–66. [Google Scholar]
- KubitzkiK.1993. Introduction. In: Kubitzki K, Rohwer J, Bittrich V, eds. Families and genera of vascular plants, Vol. III Berlin: Springer, 1–12. [Google Scholar]
- KummerowJ, Labarca C.1961. Estudios sobre el fruto y la semilla de Nothofagus alpina (Poepp. y Endl.) Krasser. Phyton 17: 205–210. [Google Scholar]
- LangdonLM.1947. The comparative morphology of the Fagaceae. I. The genus Nothofagus Botanical Gazette 108: 350–371. [Google Scholar]
- LittA, Chase MW.1998. The systematic position of Euphronia, with comments on the position of Balanops: an analysis based on rbcL sequence data. Systematic Botany 23: 401–409. [Google Scholar]
- MametyevaTB.1981. Balanopales. In: Yakovlev MS, ed. Comparative embryology of flowering plants: Winteraceae–Juglandaceae. Leningrad: Nauka, 211. [Google Scholar]
- MelchiorH.1964. Balanopales. In: Melchior H, ed. Engler’s Syllabus der Pflanzenfamilien, Vol. 2, 12th edn Berlin-Nikolassee: Borntraeger, 43–44. [Google Scholar]
- MuellerF.1877.Fragmenta phytographiae Australiae, Vol. 10. Melbourne: J. Ferres. [Google Scholar]
- NarayanaLL.1975. A contribution to the floral anatomy and embryology of Ochnaceae. Journal of Japanese Botany 50: 329–336. [Google Scholar]
- NarayanaswamiS, Sawhney S.1959. Microsporogenesis and embryo sac development in Casearia tomentosa Roxb. Phyton 13: 133–144. [Google Scholar]
- Nemirovich-DanchenkoEN.1991. Balanopaceae. In: A. Takhtajan, ed. Comparative seed anatomy, Vol. 3 Leningrad: Nauka, 115–116. [Google Scholar]
- PooleAL.1952. The development of Nothofagus seed. Transactions of the Royal Society of New Zealand 80: 207–212. [Google Scholar]
- RicardiSM.1967. Revisión taxonómica des las Malesherbiáceas. Gayana Botanica 16: 1–139. [Google Scholar]
- RozefeldsA, Drinnan AN.2002. Ontogeny of pistillate flowers and inflorescences in Nothofagus subgenus Lophozonia (Nothofagaceae). Plant Systematics and Evolution 233: 105–126. [Google Scholar]
- SavolainenV, Fay MF, Albach DC, Backlund A, van der Bank M, Cameron KM, Johnson SA, Lledó MD, Pintaud J-C, Powell Met al.2000. Phylogeny of the eudicots: a nearly complete familial analysis based on rbcL gene sequences. Kew Bulletin 55: 257–309. [Google Scholar]
- SchlechterR.1906. Beiträge zur Kenntnis der Flora von Neu-Kaledonien. Botanische Jahrbücher für Systematik 39: 1–274. [Google Scholar]
- SinghD.1963. Structure and development of ovule and seed of Viola tricolor L. and Ionidium suffruticosum Ging. Journal of the Indian Botanical Society 42: 448–462. [Google Scholar]
- SmithAC.1942. Fijian plant studies. II. Botanical results of the 1940–41 cruise of the ‘Cheng Ho’. Sargentia 1: 1–148. [Google Scholar]
- SmithAC.1950. Studies on Pacific Island plants. VI. New and noteworthy plants from Fiji. Journal of the Arnold Arboretum 31: 137–171. [Google Scholar]
- SmithAC.1981.Flora Vitiensis nova, Vol. 2. Lawai, Kauai, Hawaii. [Google Scholar]
- StenarH.1937. Zur Embryoentwicklung einiger Malpighiazeen. Botaniska Notiser 1937: 110–118. [Google Scholar]
- SutterD, Endress PK.1995. Aspects of gynoecium structure and macrosystematics in Euphorbiaceae. Botanische Jahrbücher für Systematik 116: 517–536. [Google Scholar]
- TakhtajanA.1959.Die Evolution der Angiospermen. Jena: Fischer. [Google Scholar]
- TakhtajanA.1969.Flowering plants: origin and dispersal. Edinburgh: Oliver & Boyd. [Google Scholar]
- TakhtajanA.1980. Outline of the classification of flowering plants (Magnoliophyta). Botanical Review 46: 225–359. [Google Scholar]
- TakhtajanA.1997.Diversity and classification of flowering plants. New York: Columbia University Press. [Google Scholar]
- ThathacharT.1952. Morphological studies in the Euphorbiaceae. Journal of the Mysore University B 13: 363–388. [Google Scholar]
- ThorneRF.1976. A phylogenetic classification of the Angiospermae. Evolutionary Biology 9: 35–106. [Google Scholar]
- ThorneRF.1977. Some realignments in the Angiospermae. Plant Systematics and Evolution, Suppl. 1: 299–319. [Google Scholar]
- ThorneRF.1992. An updated phylogenetic classification of the flowering plants. Aliso 13: 365–389. [Google Scholar]
- ThorneRF.2000. The classification and geography of the flowering plants: dicotyledons of the class Angiospermae (subclasses Magnoliidae, Ranunculidae, Caryophyllidae, Dilleniidae, Rosidae, Asteridae, and Lamiidae). Botanical Review 66: 441–647. [Google Scholar]
- TobeH, Raven PH.1984. An embryological contribution to systematics of the Chrysobalanaceae I. Tribe Chrysobalaneae. Botanical Magazine – Tokyo 97: 397–411. [Google Scholar]
- TokuokaT, Tobe H.1995. Embryology and systematics of Euphorbiaceae sens lat: a review and perspective. Journal of Plant Research 108: 97–106. [Google Scholar]
- TokuokaT, Tobe H.1998. Ovules and seeds in Crotonoideae (Euphorbiaceae): structure and systematic implications. Botanische Jahrbücher für Systematik 120: 165–186. [Google Scholar]
- TokuokaT, Tobe H.1999. Embryology of tribe Drypeteae, an enigmatic taxon of Euphorbiaceae. Plant Systematics and Evolution 215: 189–208. [Google Scholar]
- TokuokaT, Tobe H.2001. Ovules and seeds in subfamily Phyllanthoideae (Euphorbiaceae): structure and systematic impli cations. Journal of Plant Research 114: 75–92. [Google Scholar]
- TokuokaT, Tobe H.2002. Ovules and seeds in Euphorbioideae (Euphorbiaceae): structure and systematic implications. Journal of Plant Research 115: 361–374. [DOI] [PubMed] [Google Scholar]
- van TieghemP.1884.Traité de Botanique, 2nd edn. Paris: Savy. [Google Scholar]
- van TieghemP.1906.Eléments de Botanique, Vol. 2, 4th edn. Paris: Masson. [Google Scholar]
- VijayaraghavanMR, Kaur D.1966. Morphology and embryology of Turnera ulmifolia L. and affinities of the family Turneraceae. Phytomorphology 16: 539–553. [Google Scholar]
- von BalthazarM, Endress PK.2002. Reproductive structures and systematics of Buxaceae. Botanical Journal of the Linnean Society 140: 193–228. [Google Scholar]
- WagenitzG.1996.Wörterbuch der Botanik. Jena: Fischer. [Google Scholar]
- WettsteinR.1901.Handbuch der Systematischen Botanik, 1st edn. Leipzig: Deuticke. [Google Scholar]
- WettsteinR.1924.Handbuch der Systematischen Botanik, 3rd edn. Leipzig: Deuticke. [Google Scholar]