Abstract
The National Forest Soil Inventory (NFSI) provides the Greenhouse Gas Reporting in Germany with a quantitative assessment of organic carbon (C) stocks and changes in forest soils. Carbon stocks of the organic layer and the mineral topsoil (30 cm) were estimated on the basis of ca. 1.800 plots sampled from 1987 to 1992 and resampled from 2006 to 2008 on a nationwide grid of 8 × 8 km. Organic layer C stock estimates were attributed to surveyed forest stands and CORINE land cover data. Mineral soil C stock estimates were linked with the distribution of dominant soil types according to the Soil Map of Germany (1 : 1 000 000) and subsequently related to the forest area. It appears that the C pool of the organic layer was largely depending on tree species and parent material, whereas the C pool of the mineral soil varied among soil groups. We identified the organic layer C pool as stable although C was significantly sequestered under coniferous forest at lowland sites. The mineral soils, however, sequestered 0.41 Mg C ha−1 yr−1. Carbon pool changes were supposed to depend on stand age and forest transformation as well as an enhanced biomass input. Carbon stock changes were clearly attributed to parent material and soil groups as sandy soils sequestered higher amounts of C, whereas clayey and calcareous soils showed small gains and in some cases even losses of soil C. We further showed that the largest part of the overall sample variance was not explained by fine-earth stock variances, rather by the C concentrations variance. The applied uncertainty analyses in this study link the variability of strata with measurement errors. In accordance to other studies for Central Europe, the results showed that the applied method enabled a reliable nationwide quantification of the soil C pool development for a certain period.
Keywords: C/N ratio, carbon sequestration, carbon stocks, forest stand type, mineral soil, Nation Forest Soil Inventory, organic layer, soil groups, soil organic matter, soil survey, tree species, uncertainties
Introduction
To fulfill commitments under Article 3.4 of the Kyoto Protocol, Germany has provided for the first-time verifiable estimates and an uncertainty analysis for soil carbon (C) stocks as well as for C sequestration rates over a certain period of time. Forests play an important role in the global C cycle by capturing C from the atmosphere through photosynthesis, converting it into biomass, and emitting it back into the atmosphere or fixing it into stable soil organic matter (SOM) pools (Post & Kwon, 2000). The amount of soil C is determined by the net balance between the rate of organic C input in leaf and root biomass and its mineralization (Golchin et al., 1994a; Gregorich & Janzen, 1996). The magnitude of the C pool in forest depends on soil properties, climate, and anthropogenic activities. The ability of soil to stabilize SOM and the relationship between soil structures are key elements in soil C dynamics (Six et al., 2002). Physical properties as in silt and clay content or the microaggregation of soil are considered to protect organic matter from decomposing organisms (Oades, 1988; Torn et al., 1997; Kaiser & Guggenberger, 2003). The influence of tree species on the C pool is widely studied, however, a comprehensive understanding of the magnitude of tree species influence across site types has not yet been reached (Vesterdal, 1999; Ladegaard-Pedersen et al., 2005; Prietzel & Bachmann, 2012). The C storage in ecosystems can also be affected by stand management practices.
Modeling studies suggest that European forest soils are currently sequestering 26 Tg C yr−1, which is 30–50% of the estimated C sink in the forest biomass (Liski et al., 2002). Dijkstra et al. (2009) calculated with three different modeling approaches that C sequestration rates at intensively monitored European forest plots ranged from 0 to 978 kg C ha−1 yr−1. Liski et al. (2002) estimated for forest soils in central Europe a sink of 0.6 Mg C ha−1 for the year 2000. The large C sink was mainly attributed to an increase of forest biomass, which is the result of less intensive harvesting regimes. A study of Schils et al. (2008) has shown that C sequestration rates ranged from 0.01 to 0.8 Mg ha−1yr−1 for managed forest land. European C pool and fluxes modeling tend to reveal a CO2 sink rather than a release; however, the behavior of forest soils still remains uncertain (Liski et al., 2002; Nabuurs et al., 2003; Akselsson et al., 2005). Moreover, modeling or input–output balances based on large-scaled studies on C stocks are afflicted with high uncertainties. Consequently, direct measurements by repeated soil inventories are urgently needed to further improve these estimates (Schulze et al., 2009). A review conducted for this study has shown that nationwide C stocks and C stock changes cannot be accurately determined without comparable and harmonized inventory data. Countries with an accurate single database on the national level including sufficient information to estimate national C stocks, obtained harmoniously to sufficient soil depth (i.e., C concentrations, bulk density, and stone content) are scarce. Furthermore, a representative dataset covering the whole area of the country is essential (Prechtel et al., 2009).
Reported changes in C stocks in the soils of Denmark were assessed by two forest soil inventories conducted during 1990 and 2005 (Nielsen et al., 2012). The Swedish National Forest Soil Inventory is sampling ca. 6000 permanent survey sample plots in 10 year intervals (SEPA, 2011). Most existing national monitoring systems have only undertaken one single sampling (Saby et al., 2008). Estimates of national-wide C pools and trends from repeated soil inventories or monitoring are, however, still the exception (Bellamy et al., 2005). Most studies compiled data from unrelated soil surveys to reflect C pool changes for a certain time period. Based on comprehensive soil surveys, e.g., Lettens et al. (2005) presented changes in soil C stocks in Belgium between 1960, 1990, and 2000. Bellamy et al. (2005) estimated changes in the C concentrations based on data from the National Soil Inventory of England and Wales obtained between 1978 and 2003. Nevertheless, only 40% of the original sites were resampled. In a study of Yang et al. (2010) changes of C stock were examined by comparing current measurements with historical records derived from China's National Soil Inventory during the 1980s. In the following study, direct measurements by repeated soil inventories have been applied to estimate national C pools and trends.
Many estimates of regional C sequestration potential have made use of linear regressions based on long-term experimental data (Dijkstra et al., 2009; de Vries & Posch, 2011), whereas some studies have used dynamic SOM models linked to spatial databases (Falloon et al., 2002; Stevens & van Wesemael, 2008). The reliability of these modeling results depends on data available for calibration and validation. Most regional estimates of C stocks are based on extrapolations of the mean soil C content for broad categories of soil or vegetation types (Kern, 1994; Morisada et al., 2004; Lettens et al., 2005). Kern (1994) recommended a soil taxonomic approach. Morisada et al. (2004) estimated the extent of C stored in Japanese forests by soil units, which were determined using digital soil and land use data. A study of Lettens et al. (2005) quantified changes in C stocks in Belgium between certain spatially explicit land units with unique soil association and land use type. To our knowledge, there is no existing study which has applied data of site-specific forest stands and repeated soil inventory information to estimate C pools and trends on forest sites on the national level.
Accurately estimating the organic C stocks in soils is difficult because C stocks vary over multiple spatial scales due to the complexity of physical, chemical, and biological processes that influence C cycling in the soil (Trumbore et al., 1995). Variations in soil C stocks are related to a number of environmental factors (e.g., climate, parent material, landscape position) as well as human-induced factors (e.g., land use type, management intensity) (Tan et al., 2004; Mou et al., 2005; von Lützow et al., 2006). The relative importance of environmental and human-induced factors varies at different spatial scales; however, the resulting spatial pattern of soil C stocks remains poorly understood. Calculation of soil C stocks requires the determination of soil C concentrations, bulk densities, stone contents, and soil depth, which all vary depending on site and have different measurement errors associated (Schrumpf et al., 2011). It has been shown that these variables did not contribute similar to the variability of soil C stocks. A study of Don et al. (2007) observed at two grassland sites a higher relative variability of C concentrations than that of bulk densities. This was also shown by Goidts et al. (2009) who found across different spatial scales that C concentrations and stone contents were predominant at Belgian agriculture sites. Nevertheless, it is known that soil C stock determining variables are not independent of each other and that the covariance between them needs to be considered when analyzing the variability of soil C stocks (Goidts et al., 2009; Schrumpf et al., 2011). So far, only a few studies have considered uncertainties of various variables in the soil C stocks in an integrated approach to quantify the relative contribution of each variable and their interactions involved on the soil C stock variability on forest sites on the national level.
With the repetition of the National Forest Soil Inventories (NFSI), Germany obtained a capacious basis to evaluate the status quo of SOM and the C sequestration rate of forested soils throughout a certain time period. Soil sampling, preparation and analyses were exclusively conducted by the Federal States authorities according to the predefined soil survey manual of a German Federation/Federal States working group (BMELV, 1990). Results of the first NFSI (NFSI I) were presented by Wolff & Riek (1996), who showed spatial patterns of C stocks and morphological humus forms and displayed correlations to bedrock, soil texture, and main soil type. The objective of this study was to evaluate the development of the C pool in forest soils in Germany with respect to the NFSI II. Datasets from both NFSIs were combined to assess: (i) the annual C accumulation with respect to C stock changes between 1990 and 2006, (ii) the C status of different soils and forest stands at the two inventories, as well as (iii) the relative contribution of each variable and interaction involved to the final soil C stock variability of German forest soils.
Material and methods
Soil sampling schema
The NFSI I was carried out according to a issued soil survey manual from BMELV (1990). A newly developed detailed manual for soil sampling was issued by Wellbrock et al. (2006) to conduct the NFSI II. The new manual is based on the German manuals of soil mapping (Ad-hoc-Arbeitsgruppe Boden, 1982, 1994, 2005) and on the manual from the BMELV (1990) to ensure the comparability of soil sampling.
The NFSI I was conducted between 1987 and 1993 on a systematic 8 × 8 km grid. The soil inventory was repeated between 2006 and 2008 primarily at the same plots. Here, we used data from 1865 NFSI I plots and 1813 NFSI II plots. The resampling at the same plots indicates a paired sampling schema (Fig.1). In reference to the NFSI II, 624 plots were not resampled at the original NFSI I plots. On the new federal state-specific inventory grid, 577 new plots were established for sampling. The grid plots of NFSI I that were not resampled and the new established plots of the NFSI II were considered as an unpaired sampling schema. The standard method for soil sampling at the NFSI grid plots are comprised of eight satellite samples with a centralized soil profile (Fig.2). Satellites are located 10 m from the plot center (soil profile) in cardinal and intercardinal directions. Soil sampling plots of the NFSI II were shifted 10 Gon from NFSI I satellites.
Fig 1.
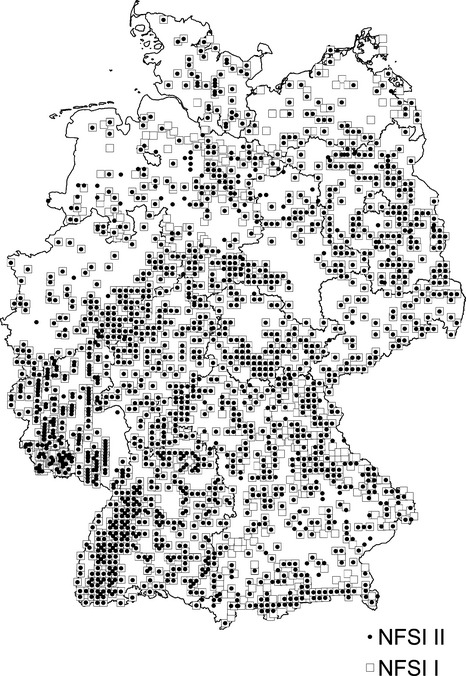
Grid plots of the first/second (I/II) National Forest Soil Inventory (NFSI).
Fig 2.
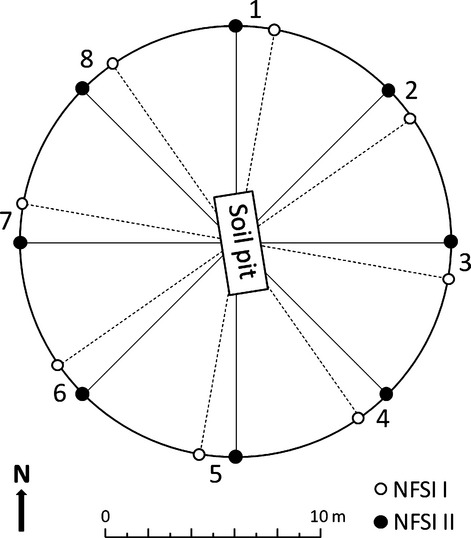
Schematic presentation of the sampling design used for the first/second (I/II) National Forest Soil Inventory (NFSI).
The soil profiles were used to designate soil horizons and to classify soil types according to the newly developed manual for soil sampling. For both inventories, the entire organic layer was collected by mixed samples from satellites with metal frames. By the first inventory branches and cones of the organic layer were not sampled, however, all fractions were sampled with the NFSI II. The subsequent partition of the coarse fraction of the organic layer was set at a diameter of >20 mm .
Sampling of the mineral soil was obligatory for both inventories and implemented at the depth increments of 0–5, 5–10, and 10–30 cm. In several federal states the soils were exclusively sampled by soil horizons which were subsequently converted to depth increments. While volume-based soil samples were taken for the NFSI I at the profile, mixed-volume-based samples were taken for the NFSI II at the satellites. Volume-based samples were crucial to estimate fine-earth stocks and bulk densities which are based on the mass and weight of the volume-based fine and coarse soil fractions. In a previous analysis of a small subset of NFSI-plots, it had been shown that fine-earth stocks based on estimated values deviate significantly from those based on measured values (Wirth et al., 2004). Therefore, estimated bulk densities and coarse-earth stocks were replaced by measured values from the other inventory. Volume-based samples were taken by volume replacement or with a cylindrical core or a cap cutter, an AMS Core sampler or a motor driven auger. If soil sampling was not practical or feasible due to a high stoniness, on-site estimates of the bulk density were permitted. To compare the depth distribution of the C pool, volume-based C stocks (kg m−3) were calculated. For both inventories, soil samples for chemical analyses were obligatory at the satellites for the 0–5, 5–10, and 10–30 cm depth increments.
Sample preparation and analyses
All laboratory analysis was provided by the Federal Forest Research Stations. The comparability of the applied laboratory analysis methods was tested by ring analysis (König & Wolff, 1993). Laboratory analysis was conducted according to the methods of the expert committee on Forest Analysis (GAFA, 2006). The organic layer samples were dried for 48 h at 60 °C, subsequently grated through a 2 mm sieve and ground. The mineral soil samples were dried for 48 h at 40 °C, subsequently sieved to <2 mm and ground. Samples of the NFSI I were either analyzed by a Woesthoff carmhograph, gas-chromatographic elemental analysis, indirect determination by the loss on ignition at 550 °C, or wet combustion with potassium dichromate and acid sulfur. Gas-chromatographic elemental analysis or Kjeldahl N digestion with a subsequent photometric or titrimetric N-determination was used to determine N of the NFSI I samples. All laboratory analysis methods were included in the subsequent analysis, however, 79% of the NFSI I-samples were exclusively analyzed by gas-chromatographic elemental analysis. The total C and N concentrations of the NFSI II were determined by gas-chromatographic elemental analysis. Inorganic C for mineral soil samples was determined according to the Scheibler method (Schlichting & Blume, 1966). The organic C concentrations of carbonated soils were calculated from the difference between total and inorganic C concentrations.
Calculation of soil organic carbon
Soil C stocks from the organic layer were calculated as a function of the size of the sampling frame, weight of the sampled material and soil C concentrations. The calculation of mineral soil C was carried out for each depth increment (D) down to a depth of 30 cm. Initially, the stocks of the fine-earth fraction (FES) were calculated as a function of dry bulk density of the fine-earth fraction (BDFE) and the volume of the coarse-soil fraction (CSV) [Eqn 1], or the stocks of the coarse-soil fraction (CSS) and the dry bulk density of the gross soil (BDg) [Eqn 2].
| 1 |
| 2 |
Carbon stocks for each depth increment were calculated by multiplying fine-earth stocks with C concentrations. Carbon stocks down to a depth 30 cm were obtained by summing up the individual depth increments. If the lowest depth level was partially included within 0–30 cm, C stocks were proportionately calculated. Horizon-wise samples have been transformed to depth increments by weighting soil horizon thickness in relation to the thickness of the overlapping segments.
Estimation of regional carbon stocks and carbon changes
Site-specific forest stand and soil information were attributed to available nationwide geo-datasets. All plots were grouped into classes, which meet the classification scheme of the geo-datasets. In reference to the estimation of mineral soil C stocks, the plots were grouped into classes of soil groups plus federal states. For the estimation of organic layer C stocks, the plots were grouped into classes of forest stand type plus federal state. The inclusion of the federal states in the classification was necessary as the sampling density deviated between states.
The distribution of forests in Germany was derived from CORINE land cover data from 1990 to 2006 that distinguished between deciduous, coniferous, and mixed forests (EEA 2010a, b). For the area-related calculation of the national C sequestration rate, we considered a forest area without organic soils of ca. 10.2 Mha−1 (FEA, 2012). The distribution of dominant soil group formations was derived from the 72 units of the national soil map 1 : 1 000 000. The soil map described soil groups and parent material according to the revised FAO Legend (FAO UNESCO ISRIC, 1990) and soil mapping units assigned to soil groups (BGR, 1998). If the soil inventory would be stratified according to all legend units of the soil map, sampling density for statistical evaluation would become insignificant for some units. Therefore, the soil types of the soil map legend were grouped according to parent material, texture, and carbonate contents as well as soil forming processes. Due to the exceptional soil properties of Histosols and the infrequency of Anthrosols under forest, these soil groups and their corresponding forest area are not considered in the analyses. Altogether, 52 different dominant soil groups were designated resulting in 16 newly assigned soil groups (Table1). In addition, a stratification exclusively based on substrates was conducted resulting in four substrate groups: dystrophic loose sediments of the lowland, eutrophic loose sediments of the lowland, solid rocks with a high base saturation of mountains and hills, and solid rocks with a low base saturation of mountains and hills.
Table 1.
List of the newly assigned soil groups (NSG)
| NSG | Soil types | Parent material | Explanation |
|---|---|---|---|
| 1 | Regosols, Arenosols, Podzols | Dystrophic sand deposits | Indifferent |
| 2 | Fluvisols, Gleysols, Podzols | Sandy to loamy deposits | Soils in broad river valleys, including terraces and lowlands |
| 3 | Fluvisols, Gleysols, Luvisols | Loamy to clayey partly calcareous deposits | Soils in broad river valleys, including terraces and lowlands |
| 4 | Cambisols, Luvisols, Regosols, Podzoluvisols | Boulder clay and till | Soils in undulating lowlands and hilly areas |
| 5 | Gleysols, Arenosols, Regosols, Cambisols | Sandy deposits overlaying boulder clay | Soils in undulating lowlands and hilly areas |
| 6 | Cambisols, Arenosols | Eutrophic sand deposits | Indifferent |
| 7 | Luvisols, Podzoluvisols, Cambisols | Sandy loess to loessic loam partly overlying various rocks | Soils in loess areas of the lowlands and hilly areas |
| 8 | Leptosols, Cambisols | Slope deposits over limestone, marlstone and dolomite | Shallow soils derived from limestone weathering |
| 9 | Cambisols, Luvisols | Redeposited material derived from limestone, marlstone, and dolomite | Deep soils derived from limestone weathering |
| 10 | Cambisols, Gleysols | Marlstone and claystone or calcareous gravels | Weathered marlstone and claystone |
| 11 | Cambisols | Basic and intermediate igneous rocks | Soils from solid rocks of mountains and hills |
| 12 | Cambisols, Gleysols | Igneous and metamorphic rocks | Soils from solid rocks of mountains and hills |
| 13 | Cambisols, Podsols | Hard argillaceous and silty slates with greywacke, sandstone, quartzite, and phyllite | Soils from solid rocks of mountains and hills |
| 14 | Cambisols, Podsols, Gleysols | Sandstones, quartzite, and conglomerates | Loss bearing sediments overlying various rocks |
| 15 | Leptosols, Cambisols, Luvisols, Gleysols | Frequently alternating soils from slate, greywacke, limestone, marlstone, sandstone, siltstone | Loss bearing sediments mixed with various rocks |
| 16 | Leptosols, Cambisols | Limestone, dolomite, and noncalcareous silicate rocks | Alpine soils |
In reference to paired samples, C stock changes of the organic layer and the mineral soil down to a depth of 30 cm of each soil group and forest stand type within a federal state were estimated by the difference of averaged C stocks. These C stocks were related to the elapsed years between inventories [Eqn 3]:
| 3 |
where rCm is the soil C stock change in the mineral soil or organic layer of the category m (soil group or forest stand type within a federal state), C Ii is the soil C stock of NFSI I, C IIi is the soil C stock of NFSI II, and Δti is the elapsed time between NFSI I and NFSI II of plot i.
Soil C stock changes in reference to the unpaired samples were derived by averaged C stocks (soil groups or forest stand type), which were related to the averaged elapsed time between the inventories [Eqn 4]:
| 4 |
To obtain nationwide averages of soil C stock changes, soil group or forest stand type C stock changes were related to the corresponding forest area [Eqn 5]:
| 5 |
where Am is the forest area of the category m (soil group or forest stand type within a federal state). A minimum sample size of n = 5 was applied. The areas were derived from a GIS-based intersection of the German soil map with the use of the CORINE land cover raster map. The approach enabled the calculation of area-weighted means, which accounted for state-specific differences in sample density.
To demonstrate the representativeness of the sample in respect to the up-scaling to soil groups, plot- and area-based percentages for both NFSI were determined. For this purpose, we related the forest area of each soil group or forest stand type to the whole forest area within the federal states. In addition, the number of inventory plots of each soil group or forest stand type was related to the total number of plots within the federal states. The comparison of the area- and the plot-based percentages, however, were not significant for both inventories.
Uncertainties analysis
Uncertainties of estimated C stocks of the mineral soil and the organic layer resulted from the single uncertainty of measured variables [Eqn 6, law of error propagation, ISO/IEC Guide 98–3: 2008].
| 6 |
with y = f (x1, …, xn); Sxi = standard deviation of the mean from xi.; cov (xi, xj) = covariance from xi and xj; 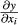 ,
, 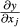 = partial derivative from y according to xi, e.g., xj.
= partial derivative from y according to xi, e.g., xj.
At first, uncertainties due to sample variance were calculated. Regarding mineral soils (soil group + federal state) and organic layers (forest stand type + federal state), the calculation of variances of the C stock estimations was performed individually for each category. In each of these categories, the standard errors and means of FESI, FESII, CorgI, CorgII, tΔ and the covariance among those variables as well as the sampling number according to Eqns 3, 4 and 6 were included. In the event of unpaired samples, covariance of variables between both inventories did not play a role. Subsequently, variances of the individual categories were combined to the area-weighted overall variance [Eqn 7]:
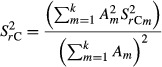 |
7 |
with 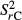 = overall variance;
= overall variance; 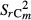 = variance of the category m; Ai = area of the category m.
= variance of the category m; Ai = area of the category m.
To assess the impact of the input variables' variance on the total sample variance, each term in Eqn 6 was separately calculated and expressed as a percentage of the sum of their absolute values (Schrumpf et al., 2011). For this analysis, only measured fine-earth stocks from plots of both inventories were selected.
In addition to the sample variance, there are uncertainties obtained by single measurement techniques. Results of interlaboratory tests served to quantify C analysis uncertainties. Interlaboratory tests were performed during both inventories. We used these results as an estimate of the uncertainty for C measurements within the inventories. Considering interlaboratory tests of NFSI II samples, the standard deviation of repeatability as pooled intra-laboratory standard deviation of C analyses within the labs, and the interlaboratory standard deviation was used to estimate the standard deviation of all means. This allows the calculation of the standard deviation of reproducibility [Eqn 8], which is suitable as an estimated value for the measurement uncertainties:
| 8 |
with 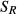 = standard deviation of reproducibility;
= standard deviation of reproducibility; 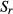 = standard deviation of repeatability;
= standard deviation of repeatability; 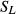 = interlaboratory standard deviation.
= interlaboratory standard deviation.
The interlaboratory ring tests of the NFSI II revealed standard deviations of reproducibility ranging from 0.9 mg g−1 for noncalcareous soils to 2.9 mg g−1 for calcareous soils. A standard deviation of reproducibility of 20.2 mg g−1 was estimated for the organic layer. The values were in the range of ca. 5% of the sample means. For calcareous soil samples, the coefficient of variation amounted to 18%. Based on analyses of laboratory ring tests of the NFSI I, Wolff & Riek (1996) presented CV's of the mineral soil (5–20%) and the organic layer (5–10%).
To assess the uncertainties which derived from methodological changes in analysis techniques between both inventories, NSFI I samples (wet combustion with potassium dichromate and acid sulfur) from 40 plots were reanalyzed with NSFI II methodology (dry combustion). The results revealed for the organic layer an average increase from 309.9 ± 11.9 mg g−1 (NSFI I, wet combustion) to 320.8 ± 12.6 mg g−1 (NSFI II, dry combustion). The root mean square error (RMSE) amounted to 54.3 mg g−1. The C concentrations of mineral soil samples decreased from 12.2 ± 1.0 mg g−1 to 11.3 ± 1.1 mg g−1. The RMSE amounted to 2.8 mg g−1. Regression analyses with both sets of C concentrations were performed. The C concentrations of the mineral soil were log transformed to obtain homoscedasticity. In both cases F-statistics revealed, that neither the intercept deviated significantly from 0 nor the regression coefficient deviated significantly from 1. Therefore, we concluded that no systematic error occurred due to changes in C estimation method.
To estimate uncertainties of fine-earth stocks, only data from plots was used where fine-earth stocks were measured during both inventories. The fine-earth stock of NFSI I was on average 193 ± 35 Mg ha−1 higher than that of NFSI II. It was assumed that fine-earth stocks are constant between both inventories. Moreover, we assumed that the mean deviation plus the deviation (standard error) of the fine-earth stocks represents a certain degree of measurement inaccuracy of fine-earth stocks.
The variance of the annual C stock changes was combined with inaccuracies of the measurement technique to obtain the uncertainty within each category m [Eqn 9]:
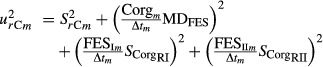 |
9 |
with 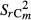 = sample variance of the C stocks in category m; MDFES = Mean deviation of fine-earth stocks (+ its standard error) between both inventories; Corgm = mean C concentrations in category m; SCorgRI, SCorgRII = uncertainties due to C concentrations estimation within both inventories (I and II); FESIm, FESIIm = fine-earth stocks in category m; Δtm = average time between both inventories in category m
= sample variance of the C stocks in category m; MDFES = Mean deviation of fine-earth stocks (+ its standard error) between both inventories; Corgm = mean C concentrations in category m; SCorgRI, SCorgRII = uncertainties due to C concentrations estimation within both inventories (I and II); FESIm, FESIIm = fine-earth stocks in category m; Δtm = average time between both inventories in category m
The area-weighted overall uncertainty was calculated according to Eqn 7.
Statistics
Statistical analysis was performed with SAS 9.2. (SAS Institute, Carry, NC, USA) to perform statistical tests. The data were treated as unpaired samples and examined for normality by the Shapiro–Wilk Test and homogeneity of variances by the Levené test. To perform statistical tests not normally distributed data were square-root or log-transformed to receive a normal distribution. Two-way repeated measures anova of Type I and Tukey–Kramer test were performed to test at a significance level of α = 0.05.
Outlier detection has been applied to identify, and where appropriate, remove anomalous observations or untypical and biased values from data. We defined outliers for each forest stand and soil group individually by twice the standard deviation (x ± 2σ). In addition, C stocks of the paired sample were fitted in a simple linear regression to carry out subsequently the outlier removal procedure. The leverage and the student residues have been used as an impartial statistical measure to evaluate outliers.
Results
C/N ratio and organic carbon concentrations in the organic layer
The C/N ratios of the organic layer of the NFSI II were the lowest under deciduous tree species, whereas coniferous tree species are characterized by high C/N ratios and a higher variability (Fig.3). We found C/N ratios under tree species that ranged between 23.4 ± 0.4 (misc. deciduous tree species) and 27.1 ± 0.2 (spruce). There were significant differences among tree species as well as an increase in C/N ratios with resampling (anova; P < 0.001). Despite the different response to tree species and inventory, indicated by a significant interaction (anova; P < 0.001), an increase in C/N ratios of the organic layer under all tree species was evident between inventories (Tukey; P < 0.05). The mean organic layer C concentrations of the NFSI II ranged between 240.4 ± 4.3 g kg−1 (pine) and 345.6 ± 7.6 g kg−1 (misc. deciduous tree species). In contrast to the C/N ratios, the variability of C concentrations among tree species was less pronounced for the NFSI I. Nevertheless, there were no differences between inventories, whereas the difference among the tree species was significant (anova; P < 0.001). This was evident by higher C concentrations under deciduous tree species compared to stands with coniferous tree species. Due to an interaction between the class variables (anova; P < 0.001), there was an increase in C concentrations under misc. deciduous forest and spruce (Tukey; P < 0.05), whereas under pine the C concentrations declined (Tukey; P < 0.05).
Fig 3.
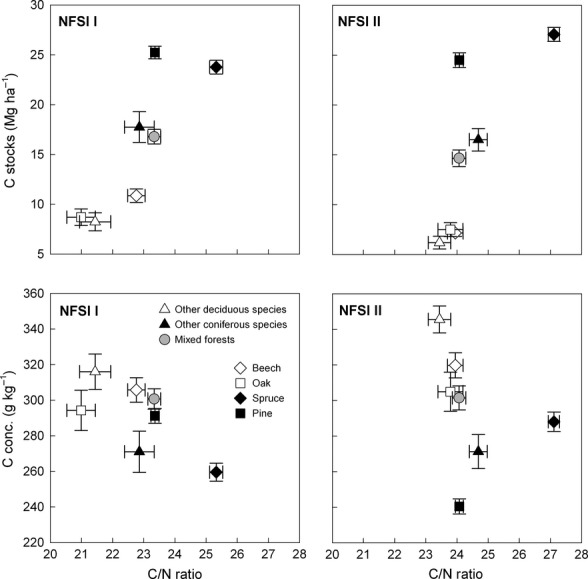
Soil organic C stocks, C concentrations, and C/N ratios of the organic layer under differing tree species of the first/second (I/II) National Forest Soil Inventory (NFSI). Circles and error bars represent means and standard errors, respectively. The number of observations is ranging from 55 (C/N ratio under oak of NFSI I) to 529 (C stocks under pine of NFSI I).
Organic carbon stocks and carbon sequestration in the organic layer
We estimated that in Germany, the C pool of the organic layer decreased from 19.0 ± 0.3 to 18.8 ± 0.3 Mg ha−1, indicating a loss of 0.02 ± 0.02 Mg C ha−1 yr−1 between 1990 and 2006. The standard error equaled the sequestration rate and the overall uncertainty was with 0.03 Mg C ha−1 somewhat higher than the C change between the inventories; hence, significant differences cannot be proved when considering all uncertainties. Taking the individual substrate groups into account, high base saturated solid rocks were identified as substrate with the lowest C storage capacity (Table2). On the other hand, dystrophic lowland substrates stored the highest amount of C. The C stocks among forest stand types within the individual substrate groups varied considerably. With the NFSI II, the lowest C stocks were found under deciduous forests on high base saturated solid rock sites. The highest C stocks, however, were observed under coniferous forests on dystrophic lowland sites. This pattern was also evident for the NFSI I. Consequently, we observed differences among the substrate groups (anova; P < 0.001). We also identified an effect of forest stand type on organic layer C stocks (anova; P < 0.001). Nevertheless, the change in the C pool was both nondirectional between the substrate groups and the inventories, as well as between the forest stand type and inventories (anova; P < 0.001). We found an increase in the C pool of dystrophic lowlands under coniferous forest, whereas the C stocks of high and low base saturated mountain sites decreased under all forest stand types (Tukey; P < 0.05).
Table 2.
Carbon stocks and changes in the organic layer C pool for the first (NFSI I) and second (NFSI II) National Forest Soil Inventory under different substrate groups and forest stand types
| Substrate | Tree species | C stocks NFSI I | C stocks NFSI II | ΔC | ΔC (UC) | ||
|---|---|---|---|---|---|---|---|
| n | [Mg ha−1] | N | [Mg ha−1] | [Mg ha−1 yr−1] | [Mg ha−1 yr−1] | ||
| 1 | CF | 148 | 26.2 ± 1.2a | 152 | 28.5 ± 1.0b | 0.15 ± 0.08 | 0.11 |
| MF | 5 | 19.6 ± 7.2a | 5 | 11.2 ± 5.7a | −0.62 ± 0.15 | 0.23 | |
| 2 | DF | 100 | 8.0 ± 0.6a | 102 | 5.9 ± 0.5a | −0.14 ± 0.04 | 0.07 |
| CF | 216 | 21.5 ± 0.8a | 183 | 24.9 ± 1.0a | 0.23 ± 0.07 | 0.09 | |
| MF | 77 | 17.1 ± 1.2a | 79 | 17.2 ± 1.6a | 0.00 ± 0.11 | 0.10 | |
| 3 | DF | 79 | 5.7 ± 0.4a | 95 | 4.5 ± 0.3b | −0.09 ± 0.03 | 0.03 |
| CF | 77 | 14.3 ± 0.6a | 50 | 9.6 ± 0.9b | −0.30 ± 0.06 | 0.08 | |
| MF | 74 | 10.4 ± 0.7a | 78 | 6.1 ± 0.5b | −0.28 ± 0.05 | 0.08 | |
| 4 | DF | 116 | 10.7 ± 0.8a | 106 | 8.3 ± 0.6b | −0.18 ± 0.06 | 0.07 |
| CF | 342 | 26.4 ± 0.7a | 334 | 25.5 ± 0.7b | −0.08 ± 0.05 | 0.07 | |
| MF | 187 | 18.9 ± 0.9a | 180 | 16.2 ± 1.0b | −0.17 ± 0.06 | 0.08 | |
n, number of observations; UC, uncertainty; 1, Dystrophic loose substrates; 2, Eutrophic loose substrates; 3, High base saturation; 4, Low base saturation; DF, Deciduous forest; CF, Coniferous forest; MF, Mixed forest.
± indicating variation by standard errors.
Different letters indicate a 0.05 significance level, tested by a two-way anova and the attended Tukey HSD test for each substrate group individually.
Due to the high influence of the forest stand type on the organic layer C pool, we additionally considered the effects of various tree species (Fig.3). Organic layer C stocks of the NFSI II ranged between 6.2 ± 0.6 Mg ha−1 (misc. deciduous tree species) and 27.1 ± 0.7 Mg ha−1 (spruce). The statistical analyses revealed that the C stocks differed significantly among tree species (anova; P < 0.001), whereas there was no significant effect between inventories. Nevertheless, an increase in organic layer C stocks under spruce and a decrease under beech, misc. deciduous forests, as well as mixed forests (Tukey; P < 0.05) was elucidated by an interaction between tree species and inventories (anova; P < 0.001).
C/N ratio and organic carbon concentrations in the mineral soil
The C/N ratios of the mineral soil of the NFSI II decreased with increasing depth (Table3). The uppermost depth increments varied from 15.1 ± 0.2 (NSG 8) to 25.2 ± 0.4 (NSG 1). In the 10–30 cm increment, the values ranged between 12.5 ± 0.2 (NSG 8) and 26.9 ± 0.4 (NSG 1). Changes in the C/N ratios between the inventories were analyzed for each depth individually. It could be shown that in all increments the C/N ratios changed significantly (anova; P < 0.001). We also found differences among soil groups for all depths (anova; P < 0.001) as well as interactions between class variables in the 5–10 cm and 10–30 cm increments (anova; P < 0.001). Increasing C/N ratios were abundant in dystrophic sandy soils of the lowland and in acidic soils of the mountainous and hilly sites, independent of all depth increments. Changes were less developed in the uppermost depth increment. Lower C/N ratios were exclusively evident in alpine soils. In contrast to the C/N ratio, the C concentrations decreased in all soil groups with increasing depth (Table3). The C concentrations in the upper depth increment ranged between 25.2 ± 2.5 g kg−1 (NSG 6) and 108.6 ± 10.2 g kg−1 (NSG 16). In the 10–30 cm increment, the C concentrations ranged from 7.0 ± 0.4 g kg−1 (NSG 1) to 34.1 ± 3.4 g kg−1 (NSG 16). The statistical analyses revealed a significant change in C concentrations between inventories (anova; P < 0.01) as well as among soil groups (anova; P < 0.01) for all depth increments. There were also significant nondirectional differences between the class variables in all depth increments (anova; P < 0.01). The comparison of individual depth increments independent of the soil groups revealed increasing C concentrations in the uppermost depth, whereas the subjacent increment showed both increasing and decreasing values and the lowermost increment almost exclusively decreasing values (Tukey; P < 0.05). Sites with increasing C concentrations in the uppermost increment are dominated by sandy lowland soils. Decreasing C concentrations were especially evident in the lowest depth increment of calcareous soils of the lowlands sites as well as of the mountainous and hilly sites.
Table 3.
Depth distribution of C/N ratios and C concentrations (C conc. in g kg−1) for the first/second (I/II) National Forest Soil Inventory (NFSI) under different newly assigned soil groups (NSG)
| NSG | Depth [cm] | NFSI I | NFSI II | ||||
|---|---|---|---|---|---|---|---|
| n | C/N ratio | C conc. | n | C/N ratio | C conc. | ||
| 1 | 0–5 | 213 | 23.5 ± 0.4a | 26.5 ± 1.0a | 205 | 25.2 ± 0.4b | 33.0 ± 1.3b |
| 5–10 | 210 | 21.2 ± 0.4a | 13.8 ± 0.7a | 208 | 26.5 ± 0.4b | 17.6 ± 0.8b | |
| 10–30 | 210 | 20.1 ± 0.5a | 8.5 ± 0.6a | 209 | 26.9 ± 0.4b | 7.0 ± 0.4b | |
| 2 | 0–5 | 71 | 16.7 ± 0.6a | 39.2 ± 2.6a | 82 | 19.1 ± 0.5b | 41.8 ± 2.7a |
| 5–10 | 70 | 15.5 ± 0.6a | 21.5 ± 1.7a | 80 | 19.0 ± 0.7b | 24.0 ± 1.7a | |
| 10–30 | 69 | 13.6 ± 0.6a | 9.6 ± 0.9a | 82 | 18.9 ± 0.8b | 10.0 ± 1.0a | |
| 3 | 0–5 | 23 | 15.1 ± 0.8a | 41.4 ± 2.7a | 32 | 16.4 ± 0.5a | 59.3 ± 4.9b |
| 5–10 | 24 | 15.0 ± 0.9a | 35.6 ± 3.0a | 33 | 15.8 ± 0.6a | 30.6 ± 2.5a | |
| 10–30 | 24 | 12.7 ± 0.6a | 13.6 ± 1.6a | 32 | 14.2 ± 0.5a | 10.8 ± 1.1a | |
| 4 | 0–5 | 114 | 14.9 ± 0.3a | 43.2 ± 1.4a | 93 | 17.4 ± 0.3b | 44.3 ± 2.0a |
| 5–10 | 116 | 14.5 ± 0.4a | 33.1 ± 1.3a | 93 | 16.6 ± 0.3b | 25.0 ± 1.2b | |
| 10–30 | 117 | 12.7 ± 0.4a | 11.6 ± 0.5a | 94 | 14.7 ± 0.4b | 7.5 ± 0.4b | |
| 5 | 0–5 | 96 | 19.5 ± 0.6a | 24.7 ± 1.5a | 91 | 22.0 ± 0.5b | 38.5 ± 2.2b |
| 5–10 | 93 | 17.9 ± 0.6a | 11.6 ± 0.9a | 91 | 23.6 ± 0.6b | 15.7 ± 0.9b | |
| 10–30 | 94 | 15.5 ± 0.6a | 5.8 ± 0.5a | 92 | 23.3 ± 0.7b | 5.6 ± 0.4a | |
| 6 | 0–5 | 42 | 21.8 ± 0.8a | 15.3 ± 1.1a | 48 | 22.9 ± 0.7a | 25.2 ± 2.5b |
| 5–10 | 41 | 19.6 ± 0.7a | 6.0 ± 0.5a | 47 | 23.5 ± 0.7b | 13.0 ± 0.8b | |
| 10–30 | 41 | 16.4 ± 0.6a | 2.8 ± 0.3a | 47 | 26.0 ± 1.2b | 3.9 ± 0.3b | |
| 7 | 0–5 | 123 | 18.1 ± 0.5a | 35.7 ± 1.3a | 119 | 18.8 ± 0.4a | 41.6 ± 2.0b |
| 5–10 | 124 | 17.4 ± 0.5a | 21.5 ± 0.8a | 116 | 18.4 ± 0.4a | 20.8 ± 1.0a | |
| 10–30 | 122 | 14.6 ± 0.6a | 8.9 ± 0.4a | 118 | 15.8 ± 0.5a | 7.4 ± 0.4b | |
| 8 | 0–5 | 109 | 14.4 ± 0.3a | 58.4 ± 2.7a | 108 | 15.1 ± 0.2a | 62.6 ± 2.6a |
| 5–10 | 108 | 13.5 ± 0.3a | 42.0 ± 2.1a | 110 | 13.9 ± 0.2a | 39.1 ± 2.0a | |
| 10–30 | 108 | 12.4 ± 0.4a | 20.3 ± 1.3a | 112 | 12.5 ± 0.2a | 14.5 ± 0.9b | |
| 9 | 0–5 | 35 | 15.1 ± 0.5a | 55.9 ± 3.4a | 41 | 16.0 ± 0.4a | 52.5 ± 3.4a |
| 5–10 | 36 | 13.8 ± 0.5a | 42.7 ± 2.8a | 42 | 14.8 ± 0.3a | 34.7 ± 2.5b | |
| 10–30 | 36 | 12.5 ± 0.6a | 20.2 ± 1.9a | 42 | 12.5 ± 0.3a | 11.3 ± 1.0b | |
| 10 | 0–5 | 65 | 16.2 ± 0.5a | 34.4 ± 1.6a | 76 | 16.7 ± 0.3a | 40.3 ± 2.2b |
| 5–10 | 67 | 15.4 ± 0.6a | 23.9 ± 1.0a | 78 | 15.6 ± 0.4a | 22.3 ± 1.2a | |
| 10–30 | 64 | 12.1 ± 0.5a | 9.3 ± 0.5a | 77 | 13.3 ± 0.3b | 7.8 ± 0.4b | |
| 11 | 0–5 | 51 | 14.7 ± 0.6a | 43.1 ± 2.8a | 50 | 16.0 ± 0.4a | 48.4 ± 3.7a |
| 5–10 | 52 | 14.2 ± 0.6a | 27.8 ± 2.2a | 52 | 15.7 ± 0.4b | 32.1 ± 2.4a | |
| 10–30 | 51 | 11.7 ± 0.5a | 12.0 ± 1.2a | 52 | 12.7 ± 0.3a | 12.7 ± 1.1a | |
| 12 | 0–5 | 185 | 18.5 ± 0.4a | 50.3 ± 1.6a | 168 | 19.7 ± 0.3b | 57.8 ± 2.0b |
| 5–10 | 187 | 17.7 ± 0.4a | 37.8 ± 1.4a | 171 | 19.3 ± 0.3b | 33.9 ± 1.2b | |
| 10–30 | 185 | 17.0 ± 0.5a | 19.0 ± 0.9a | 169 | 17.8 ± 0.3a | 15.3 ± 0.7b | |
| 13 | 0–5 | 202 | 17.1 ± 0.3a | 54.5 ± 1.5a | 252 | 19.2 ± 0.2b | 58.2 ± 1.5a |
| 5–10 | 203 | 14.9 ± 0.3a | 31.5 ± 0.9a | 256 | 18.1 ± 0.3b | 32.0 ± 0.9a | |
| 10–30 | 206 | 12.7 ± 0.3a | 15.3 ± 0.5a | 257 | 15.4 ± 0.3b | 15.0 ± 0.5a | |
| 14 | 0–5 | 248 | 20.8 ± 0.4a | 36.4 ± 1.0a | 259 | 21.5 ± 0.3a | 36.4 ± 1.2a |
| 5–10 | 253 | 19.8 ± 0.4a | 21.2 ± 0.7a | 264 | 21.4 ± 0.3b | 18.6 ± 0.5b | |
| 10–30 | 243 | 16.5 ± 0.5a | 9.6 ± 0.3a | 263 | 18.5 ± 0.4b | 8.3 ± 0.3a | |
| 15 | 0–5 | 36 | 16.9 ± 0.7a | 41.7 ± 2.5a | 37 | 18.7 ± 0.7a | 39.9 ± 4.0a |
| 5–10 | 35 | 15.4 ± 0.8a | 23.5 ± 1.6a | 38 | 18.4 ± 0.8b | 23.1 ± 1.9a | |
| 10–30 | 36 | 13.7 ± 1.0a | 12.0 ± 1.4a | 38 | 17.1 ± 0.9b | 9.4 ± 1.0a | |
| 16 | 0–5 | 30 | 18.4 ± 0.6a | 98.7 ± 6.5a | 18 | 17.8 ± 0.3a | 108.6 ± 10.2a |
| 5–10 | 30 | 18.4 ± 0.6a | 98.7 ± 6.5a | 22 | 16.1 ± 0.3b | 87.2 ± 8.0a | |
| 10–30 | 29 | 16.3 ± 1.0a | 45.8 ± 4.6a | 21 | 15.0 ± 0.3a | 34.1 ± 3.4a | |
n, number of observations.
± indicating variation by standard errors.
Different letters indicate a 0.05 significance level, tested by Tukey's HSD test.
Organic carbon stocks and carbon sequestration in the mineral soil
Average C stocks of Germany's forested mineral soils (0–30 cm) were estimated at 55.6 ± 3.4 Mg ha−1 for the NFSI I and 61.8 ± 3.7 Mg ha−1 for the NFSI II, which resulted in a C accumulation of 0.41 ± 0.03 Mg ha−1 yr−1. The overall uncertainty was 0.11 Mg C ha−1, indicating that C stock changes are significant also when considering all uncertainties (anova, P < 0.001). The estimated C pools differed significantly among soil groups (anova; P < 0.001), however, interactions between the class variables (anova, P < 0.001) indicated texture depending C stocks. This is evident in low C stocks of soil groups derived from sandy substrates and in moderate C stocks of loamy soils of the lowlands as well as soils from solid rocks of mountains and hills (Table4). High C stocks could be found in calcareous soils across all landscape units. There was a strong increase in C stocks in mostly sandy soils of the lowland (Tukey, P < 0.05). These soil groups are characterized by low initial C pools. Moderate changes were evident in soils derived from solid rocks of mountains and hills as well as in soils of the Alps (Tukey, P < 0.05), whereas changes were not evident in calcareous soil groups. These results showed that a significant positive change, in particular at sites with a low initial C pool was evident, whereas sites with a large C pool did not differ significantly.
Table 4.
Carbon stocks and changes in the C pool down to a depth of 30 cm of the mineral soil for the first/second (I/II) National Forest Soil Inventory (NFSI) under different newly assigned soil groups (NSG)
| NSG | C stocks NFSI I | C stocks NFSI II | ΔC | ΔC (UC) | ||
|---|---|---|---|---|---|---|
| N | [Mg ha−1] | n | [Mg ha−1] | [Mg ha−1 yr−1] | [Mg ha−1 yr−1] | |
| 1 | 201 | 52.8 ± 1.6a | 187 | 65.5 ± 6.8b | 0.95 ± 0.12 | 0.24 |
| 2 | 56 | 60.5 ± 2.6a | 62 | 65.0 ± 4.9a | 0.02 ± 0.20 | 0.31 |
| 3 | 20 | 67.3 ± 3.2a | 25 | 68.1 ± 2.4a | 0.20 ± 0.33 | 0.88 |
| 4 | 105 | 66.4 ± 1.8a | 87 | 64.1 ± 4.5a | 0.14 ± 0.12 | 0.29 |
| 5 | 77 | 33.4 ± 1.6a | 75 | 52.8 ± 2.2b | 1.19 ± 0.14 | 0.21 |
| 6 | 34 | 24.6 ± 1.6a | 34 | 43.7 ± 1.8b | 1.35 ± 0.19 | 0.24 |
| 7 | 126 | 55.8 ± 1.5a | 109 | 63.0 ± 2.2b | 0.47 ± 0.12 | 0.20 |
| 8 | 110 | 76.3 ± 2.4a | 106 | 79.1 ± 0.8a | -0.17 ± 0.16 | 0.83 |
| 9 | 36 | 77.1 ± 4.9a | 43 | 68.3 ± 1.0a | -0.71 ± 0.28 | 1.39 |
| 10 | 55 | 56.7 ± 2.1a | 63 | 60.8 ± 0.8a | 0.19 ± 0.14 | 0.56 |
| 11 | 39 | 51.3 ± 3.2a | 39 | 54.6 ± 0.9a | 0.38 ± 0.17 | 0.36 |
| 12 | 187 | 59.5 ± 1.7a | 163 | 62.5 ± 2.2b | 0.11 ± 0.10 | 0.31 |
| 13 | 222 | 54.7 ± 1.4a | 233 | 60.1 ± 4.1b | 0.44 ± 0.08 | 0.23 |
| 14 | 245 | 50.5 ± 1.2a | 257 | 55.3 ± 3.2b | 0.35 ± 0.08 | 0.18 |
| 15 | 30 | 51.8 ± 2.9a | 30 | 49.0 ± 0.9a | -0.26 ± 0.20 | 0.64 |
| 16 | 34 | 84.4 ± 6.2a | 26 | 104.5 ± 0.5b | 0.40 ± 0.46 | 2.40 |
UC, uncertainty.
± indicating variation by standard errors.
Different letters indicate a 0.05 significance level, tested by a two-way anova, and the attended Tukey HSD test for each substrate group individually.
Carbon pool changes in forested mineral soils are considered to appear in the top soil; hence the depth distribution of volume-based C stocks was investigated (Fig.4). The highest C pool could be found in the uppermost depth increments ranging from 30.6 ± 2.3 kg m−3 (NSG 15) to 54.1 ± 2.1 kg m−3 (NSG 16). The C stocks decreased with increasing depth. The C stored in the lowest increment ranged from 9.1 ± 0.6 (NSG 6) to 30.2 ± 2.1 kg m−3 (NSG 16). The C pool of all depth increments has changed significantly with resampling (anova; P < 0.001). We also detected in all depth increments different C stocks among soil groups (anova, P < 0.001) as well as an interaction between class variables (anova, P < 0.01). This shows that the magnitude of C pool changes in each increment is affected by soil groups indicating that the considered stratification approach seems to be a suitable factor for explaining a significant part of the variance. Taking the individual soil groups into account, we observed in the upper depth increments of almost all soil groups a significant increase in C with resampling. Differences were less abundant with increasing depth. Nevertheless, sandy sites of the lowland were the most affected soils in respect to positive C pool changes in all depth increments. A C change in the 5–10 cm increment was hardly abundant apart from the sandy soils of the lowland. Outside of sandy soils, acidic soils of the mountains and hilly areas as well as alpine soils showed an increase in C stocks in the lowest increment.
Fig 4.
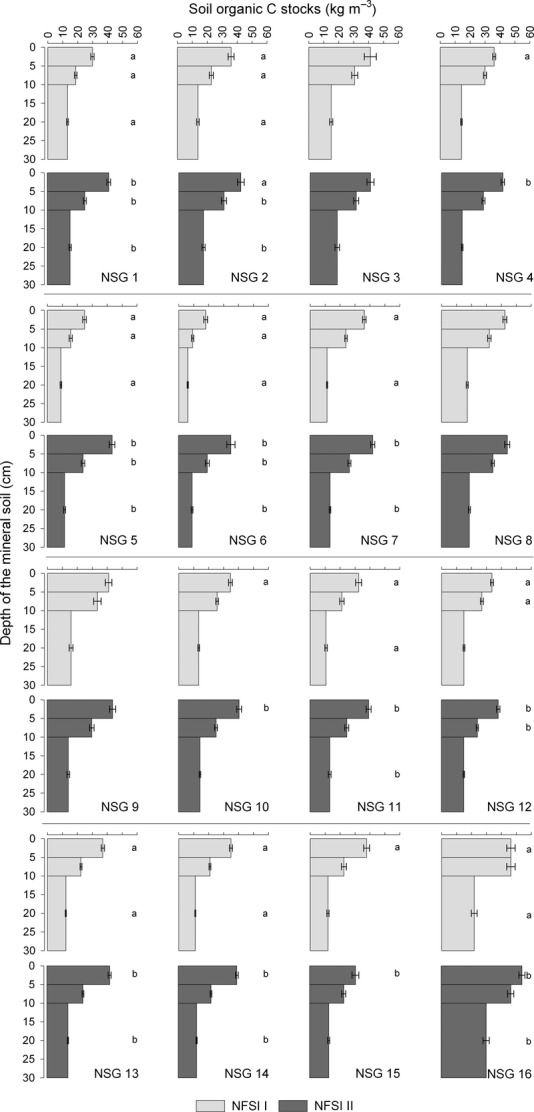
Depth distribution of soil organic C stocks under different newly assigned soil groups of the first/second (I/II) National Forest Soil Inventory (NFSI). Bars and error bars represent means and standard errors, respectively. Abbreviations in the legend represent the newly assigned soil groups (cf. Table1).
Bulk density of the mineral soil
The bulk density showed a high variability among the soil groups ranging from 0.30 ± 0.02 (NSG 16) to 1.11 ± 0.01 g cm−3 (NSG 1) in the upper depth increment (Fig.5). With increasing depth the bulk density increased, whereas the lowest increment revealed values between 0.57 ± 0.04 (NSG 16) and 1.48 ± 0.02 gcm−3 (NSG 6). We could further show that the bulk density in the 0–5 and 5–10 cm increments has changed significantly since the NFSI I (anova; P < 0.05). We further detected different bulk densities in all depth increments among soil groups (anova; P < 0.001) as well as interacting class variables (anova; P < 0.001). We showed for the uppermost increment on nearly all substrates (NSGs 1, 3, 4, 6, 7, 9, 10, 12, 14, and 16) a significantly decrease in bulk densities with resampling (Tukey; P < 0.05). Decreasing bulk densities were found mainly on calcareous sites (NSGs 4, 7, 10, 12, and 15) in the lower depth increments (Tukey; P < 0.05).
Fig 5.
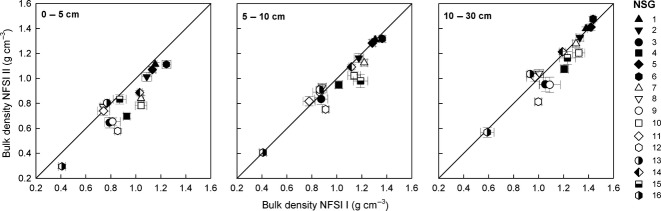
Relation between the bulk densities of the first/second (I/II) National Forest Soil Inventory (NFSI) in respect to the newly assigned soil groups (NSG). Bars and error bars represent means and standard errors, respectively. Numbers in the legend represent the newly assigned soil groups (cf. Table1).
Changes in bulk density may result in changes in the C pool. Therefore, we analyzed the relation between bulk density and C concentrations in the mineral soil. To consider that bulk densities were partly transferred from the first to the second inventory, and vice versa, the analysis was conducted exclusively with untransferred data. We showed that in each depth increment the bulk density decreased with increasing C concentrations (Fig.6). Similar results could be obtained for the NFSI I in the uppermost increment (r2 = 0.37; y = 1.3181 − 0.0082x), for the 5–10 cm increment (r2 = 0.46; y = 1.4238 − 0.0113x), as well as for the lowest depth (r2 = 0.24; y = 1.4626 − 0.0180x). The relations, however, differed in each depth increment indicated by shallower slopes in the uppermost increment compared to the lower depth increments.
Fig 6.
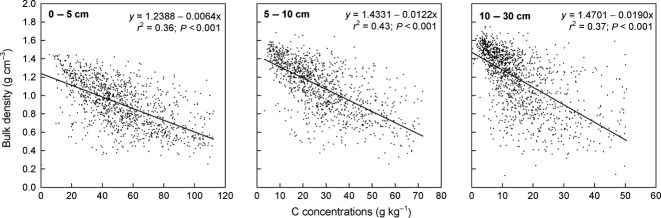
Relation between C concentrations and bulk density in different depth increments of the mineral soil.
Uncertainty
We showed that the largest part of the overall sample variance was explained by the variance of the C concentrations, which was evident for both inventories (Fig.7). In contrast, the fine-earth stock variances were less prominent for both inventories. The covariance among the variables within both inventories and in particular the covariance of the C concentrations contributed to a decrease in the overall uncertainties for both inventories. The total uncertainty of the annual C sequestration estimations of the mineral soil were composed out of differing estimated uncertainties (Fig.8). Mineral soils showed uncertainties ranging from 0.037 Mg ha−1 yr−1 (sample variance) to 0.058 Mg ha−1 yr−1 (laboratory analysis to determine C concentrations of NFSI I). The uncertainties for the organic layer ranged from 0.015 Mg ha−1 yr−1 (laboratory analysis to determine C concentrations of NFSI II) to 0.023 Mg ha−1 yr−1 (laboratory analysis to determine C concentrations of NFSI I).
Fig 7.
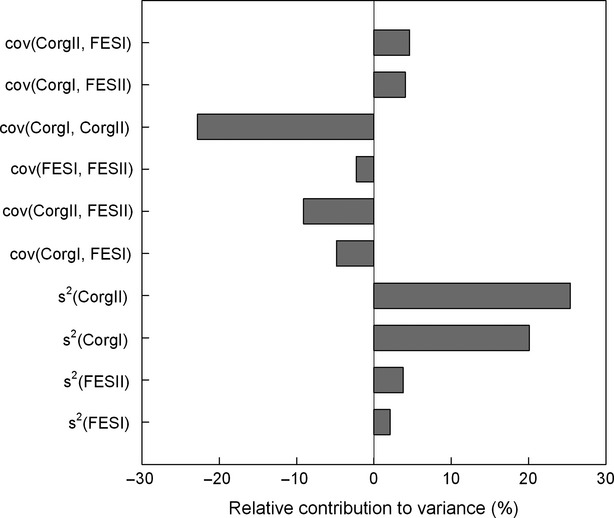
Relative contribution of differing variance components to the overall variance of the estimated C sequestration rate of the mineral soil. The relative contribution was calculated as the portion of the sum of the total contribution of the variance components consisting of covariance (cov) or variance (s2) of C concentrations/fine-earth stocks (Corg/FES) of the first/second (I/II) National Forest Soil Inventory (NFSI). The calculation considered data consisting exclusively of independent measurements of the respect parameter for both inventories.
Fig 8.
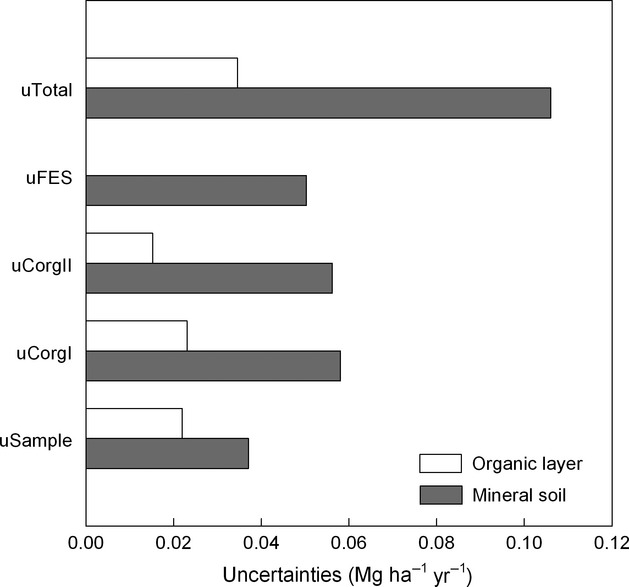
Several uncertainties for the annual C sequestration rate estimation for the mineral soil and organic layer. The total uncertainty of the annual C sequestration estimation of the mineral soil (uTotal) was composed out of uncertainties of the sample variance (uSample), uncertainties of lab C analysis from the first/second National Forest Soil Inventory (uCorgI/uCorgII), uncertainties of fine-earth stock estimations (uFES).
Discussion
Status and trends of the organic layer carbon pool
The averaged area-related amounts of C stored in the organic layer were estimated to be 18.8 Mg ha−1. A study from Wiesmeier et al. (2013) on comparable site qualities and forest stand types showed a somewhat higher C pool (24 Mg ha−1) for southern Germany. This could be explained by a higher proportion of alpine sites where thick organic layers are common. In our study, the organic layer was strongly influenced by the tree species composition as indicated by differences in the C pool among forest stand types (Table2) or among tree species (Fig.3). Tree species influence is often first detectable in forest floors and this has been shown in numerous studies (Vesterdal & Raulund-Rasmussen, 1998; Akselsson et al., 2005; Oostra et al., 2006; Schulp et al., 2008; Wiesmeier et al., 2013). Coniferous forests were favored to store higher amounts of organic layer C (14–26 Mg ha−1) than mixed (10–20 Mg ha−1) or deciduous forests (5–11 Mg ha−1). Here, we are in the range of averaged organic layer C stocks of 22 Mg ha−1 under coniferous species and 13 Mg ha−1 under broad-leaved species in warm temperate moist climates provided by the IPCC guideline (IPCC, 2003).
In our study, the organic layer was identified as a stable C pool. A comparison to most of the existing inventory-based studies showed contradicting results where the organic layer is likely to accumulate C. Generally, soil inventory based studies on organic layer C pool changes are scarce and solely available for North and North-West European Countries. Denmark's soil inventory was assessed by Nielsen et al. (2012) who showed that the organic layer sequestered 0.31 Mg C ha−1 yr−1. The mean sequestration rate in organic layers of podsolic forests soils in Sweden was estimated to be 0.25 Mg ha−1 yr−1 (Berg et al., 2009). Various indirect extrapolation methods for the organic layer of Swedish forest soils estimated sequestration rates ranging from 0.10 to 0.13 Mg ha−1 yr−1 (Liski et al., 2002; Akselsson et al., 2005; Agren et al., 2007). Billett et al. (1990) reported mean accumulation rates of 0.35 Mg C ha−1 yr−1 in organic forest soil horizons from 15 profiles in North-East Scotland, which were sampled in 1949/50 and 1987. Studies with a more local sampling schema also revealed increasing C pools indicated by differing site quality and tree species effects. Most of these studies are spruce dominated. In our study, we clearly detected an increase in the C pool under spruce, whereas deciduous tree species and mixed forests showed decreasing amounts of C (Fig.3). The C sequestration potential under spruce stands was also demonstrated by Prietzel et al. (2006), who showed in a long-term experiment under Scots pine that the organic layer displayed a increase of 0.4 Mg C ha−1 yr−1. Also a model based study of Akselsson et al. (2005) presented sinks for Norway spruce (0.2 Mg C ha−1 yr−1).
Status and trends of the mineral soil carbon pool
Our estimation of the total C pool of the upper 30 cm of the mineral soil is with 55.6 Mg ha−1 (NFSI I) and 61.8 Mg ha−1 (NFSI II) considerably lower than results for temperate forest soils of Belgium of 88 to 96 Mg ha−1 (Lettens et al., 2005; Stevens & van Wesemael, 2008) and France of 70 Mg ha−1 (Martin et al., 2011). A probable overestimation of C stocks was depicted to be partly based on the estimation of soil properties as bulk density and stone content. A recent study from Wiesmeier et al. (2013) conducted in southern Germany, revealed that under comparable environmental conditions the Bavarian forest soils stored 98 Mg C ha−1. This study focused on a depth down to the bedrock or at least to a depth of 1 m, whereas we focused to a depth of only 30 cm. The subsoil contains, however, approx. a third or more of all soil C above the depth of 1 m (Batjes, 1996; Jobbagy & Jackson, 2000; Liski et al., 2002). Despite the limitation to the upper 30 cm, the differentiation between the soil groups was sufficient. The importance of soil specific C stocks has been reported in several studies (Kern, 1994; Morisada et al., 2004; Lettens et al., 2005). Nevertheless, the assignment of mean C stocks to soil groups bear uncertainties due to the varying number of plots per unit as some of the units were represented only by a small number of plots or were completely absent. We could demonstrate that due to the regular sampling grid, the number of plots per soil unit was proportional to the area covered by the soil unit.
We estimated that the mineral soil sequestered 0.41 Mg C ha−1 yr−1. Our results are consistent with findings in a meta-analysis conducted by Luyssaert et al. (2010), suggesting an increase of 0.2 Mg C ha−1 yr−1 in Europe. Lettens et al. (2005) also detected an increase of ca. 0.55–0.73 Mg C ha−1 yr−1 in forest soils of Belgium down to a depth of 30 cm. The Swedish National Forest Soil Inventory (SEPA, 2011) revealed a C increase of 0.17 Mg ha−1 yr−1 in the mineral soil down to the bedrock. Nielsen et al. (2012) identified the forested mineral soils of Denmark as a C pool sequestering 0.08 Mg C ha−1 yr−1. On the other hand, repeated sampling indicated losses of C throughout England and Wales (Bellamy et al., 2005), despite evidence in support of C accumulation. Among other more localized studies, the C sequestration for soil rooting depth was estimated to be between 0.1 Mg ha−1 yr−1 (Nabuurs & Schelhaas, 2002) and 0.9 Mg ha−1 yr−1 (Schulze et al., 2000). Furthermore, a study conducted by Schrumpf et al. (2011) indicated that changes of 0.2–0.3 Mg C ha−1 yr−1 could be a realistic assumption for European forest soils, although local changes may be greater.
Controls of carbon stocks and carbon stock changes
In our study, we clearly showed a site quality effect on the organic layer C pools derived from different parent material (Table2). We agree with results demonstrating that nutrient-rich soils tend to be associated with higher rates of litter decomposition, whereas the accumulation of SOM in less fertile sandy soils occurs due to reduced decomposition (Vesterdal & Raulund-Rasmussen, 1998; Vesterdal, 1999; Ladegaard-Pedersen et al., 2005). Site quality effects on C pool changes were evident due to an increase in the C stocks in dystrophic soils of the lowland, whereas the amount of C stored in the organic layer of high and low base-saturated soils from mountainous sites declined. In a study conducted by Vesterdal (1999) it was demonstrated that the accumulation of C in the litter layer was higher on less fertile sites than on relatively fertile sites. Here, the identified site quality effects refer to the geographical distribution of the substrate groups as dystrophic lowland sites are mainly located in northern Germany, whereas mountainous and hilly sites can be found predominantly in southern Germany. On the other hand, we also detected an increase in organic layer C stocks of dystrophic lowland soils under coniferous forest. These sites are common in northern Germany and usually dominated by pine stands.
Our study revealed a parent material effect on the C pool of mineral soils as calcareous soils stored higher amounts of C than soils derived from noncalcareous material (Fig.4). This was evident for calcareous lowland soils as well as for the Alps, where calcareous substrate is the most abundant and C concentrations are among the highest. Calcareous sites were characterized by low organic layer C stocks, but higher amounts of C in the underlying mineral soil. We suppose that this opposing trend may offset the differences in the organic layer thus showing the highest amounts of C in these soils throughout all depth increments. This was also reported by Vesterdal et al. (2008). Beside the parent material, the soil texture affected the amounts of C as rich-clay soils stored more C compared to sandy soils. Default values according to the IPCC guidelines (IPCC, 2003) for warm temperate moist climates for the uppermost 30 cm of the mineral soil ranged from 34 Mg C ha−1 in sandy soils to 88 Mg C ha−1 in high activity clay soils. Wiesmeier et al. (2013) could not detect any significant differences in C stocks among soils developed from different parent material. In our study, the soil properties are emphasized despite the lower sampling depth, indicating that our approach sufficiently implements the stratification according to soil groups.
We could show that sandy lowland soils sequestered larger amounts of C than clayey-loamy soils of the mountains and hills even though these soils showed low initial C stocks and C concentrations (Table4). These differences in the texture depending C stocks indicate that the extent of decomposition increases from sand to silt to clay-sized complexes (Guggenberger et al., 1995). This can be explained by the positive relation between C and clay content, which may be attributed to the stabilization of SOM by the formation of stable complexes with clay minerals (Torn et al., 1997; Six et al., 2002; Hagedorn et al., 2003). Richter et al. (1999) reported that in a pine forest ecosystem in South Carolina, USA, there was a strong C sink even though the mineral soil accounted for <1% of the C accretion. Despite high inputs of C, they supposed a limited C sequestration by rapid decomposition facilitated by the coarse soil texture and low-activity clay minerals. In our study, the notable increase in the amount of C in sandy soils can be attributed to an increase in C concentrations while simultaneously increasing C/N ratios (Table3). Therefore, we assume a higher C accumulation efficiency of sandy soils compared to other soils due to reduced decomposition. It is thought that the major part of the C pool in the mineral soil consists of the light organic matter fraction which contributes to the labile C pool (Golchin et al., 1994b; Kögel-Knabner et al., 2008). We assume an enhanced contribution of the labile pool to the total C pool in sandy soils compared to clay-rich soils, which is indicated by increasing C concentrations and C/N ratios of sandy soils. A decrease in C concentrations and C/N ratios with the proportion of stabilized SOM has been shown in several studies (Golchin et al., 1994b; Guggenberger et al., 1995; Kögel-Knabner et al., 2008). It should be taken into consideration that this labile pool is prone to disturbance such as climate change, forest fires, and management practices (Jandl et al., 2007; von Lützow & Kögel-Knabner, 2009) and thus crucial for the management of C sequestration (Wiesmeier et al., 2013). The potential of sandy soils to sequester C was highlighted in a study from Stevens & van Wesemael (2008) who showed that soils with high C content tended to lose C, whereas conversely, soils with low C tended to gain C. Schulten & Leinweber (2000) supposed that the importance of the clay fraction can become notably relevant with lower clay content. Therefore, we assume that there is an enrichment of C in fine particle fractions in low-clay sandy lowland soils. Consequently, the relative magnitude of C accumulation is elevated in low clay soils compared to high clay soils.
The bulk density of the uppermost increment ranged from 0.30 to 1.24 g cm−3 (Fig.5). The bulk density increased with increasing depth, which is confirmed in other studies on forest soils (De Vos et al., 2005; Schrumpf et al., 2011). Higher bulk densities usually occur in deeper soils due to the weight of the overburdened soil. We observed values in the 10–30 cm depth increment ranging from 0.57 to 1.48 g cm−3. As confirmed in other studies, the variability of bulk densities increased with depth (Bowman, 1991; Potter et al., 1999). Similar bulk densities for forest soils were reported in previous studies (De Vos et al., 2005; Don et al., 2007; Schrumpf et al., 2011). The observed low bulk density of alpine soils may be attributed to the driving hammer sampling technique. A study from Parfitt et al. (2010) compares the driving hammer method with the carving method. They concluded that the bulk density would be underestimated and the change in soil C due to varying sampling techniques is greater than changes in C over a certain time period.
Similar to other studies, we found a negative relationship of C concentrations with bulk density (Fig.6). This may explain that in most of the soil groups the bulk density decreased, whereas the C concentrations increased (Heuscher et al., 2005; Don et al., 2007; Schrumpf et al., 2011). Decreasing bulk densities between sampling times may result in a SOM gain underestimation due to an increasing thickness of the soil. A comparison of both soil layers may accomplished by a mass weighted calculation, supposed by Ellert et al. (2002). This approach was, however, not applicable as the data were limited to a depth down to 30 cm of the mineral soil. The relative variability of C concentrations was higher compared to the bulk density, which was already demonstrated by other studies (Don et al., 2007; Goidts et al., 2009). Estimates of C stocks are widely used by pedotransfer functions to estimate bulk density from the relation to C concentrations. Here, the correlation between C concentrations and bulk density was not reliable to achieve C stock estimates. In a study from Wiesmeier et al. (2012), the calculation of C stocks resulted in an overestimation of up to 50%. Therefore, the application of modeled parameters is afflicted with high uncertainties and can be deemed problematic due to systematically biased estimations. In contrast to modeled parameters, a considerably low uncertainty was achieved for the inventory data in our study (Fig.7). Consequently, direct measurements by repeated soil inventories are urgently needed to improve modeled estimates.
Due to the availability of relevant soil parameters such as C concentrations and bulk density determined down to a depth of 30 cm, as well as comprehensive field information, a valuable dataset depicting Germany on a national level can be regarded as representative for large-scale studies in Central Europe. This in turn highlights the surveying of pedogenetic properties and forest stand information in large-scale inventories. The bulk density is necessary to calculate C stocks which are based on fine-earth stocks. After comparing the variance of fine-earth stocks with that of the C concentrations, less uncertainty in fine-earth stocks was evident (Fig.8). This contradicts the results of Schrumpf et al. (2011) who demonstrated that on stone rich coniferous forest sites, the fine-earth content had a higher contribution to the variance than that of the C concentrations. It is assumed that the contribution of fine-earth content to the C stock variance increases with the coarse soil fraction. In our study, there are both soils with high and low proportions of coarse soil fractions. Nevertheless, the uncertainty of the measurement and the sample variability were simultaneously considered to improve estimates of C pool changes. This was accomplished for the first time in a national study. However, uncertainties are implied in C stock estimations by small scale spatial heterogeneity as soil properties are correlated over short distances. Schöning et al. (2006) estimated coefficients of variation for the litter layer (38%) and for the mineral soil (30–45%) of a European beech forest. Similar results were obtained by Liski (1995), who demonstrated that the C stocks of soil horizons in a spruce forest were uncorrelated outside a range of 8 m. Uncertainties are also possible due to errors in the measurement of physical and chemical soil parameters. Uncertainties may arise due to changes in laboratory methods between the first and second NFSI as well as from differing analysis methods at various laboratories. Analysis in laboratories were aligned and verified by interlaboratory ring tests. While the ring analyses revealed an approximated 5–20% of uncertainties for organic C concentrations during the NFSI I, the percentage of uncertainty could be lowered to 5% for inorganic C free samples during the NFSI II. The presence of inorganic C increased largely the uncertainty. In our uncertainty budgets we overestimated the uncertainty of C estimation. This is why we applied the uncertainty of inorganic C containing samples to all plots within the major soil groups which contain inorganic C irrespectively of the presence of inorganic in the sampling depth down to a depth of 30 cm.
The depth distribution of volume-based estimates demonstrates that the highest proportion of the C pool increase can be attributed to the upper depth increment (Fig.4). The accumulation of C, especially in the topsoil, may be attributed to the increase in net primary productivity and litter input and/or reduced decomposition rates. In Central Europe, high loadings of N-depositions are thought to be a major disturbance of the C cycle (de Vries et al., 2009; Janssens et al., 2010). Long-term deposition with low to medium intensity leads to an accumulation of C in the soil (de Vries et al., 2009) and to reduced respiration and decomposition rates (Knorr et al., 2005; Janssens et al., 2010). In our study, we found contrasting evidence of elevated N induced C accumulation in the mineral soil and in the organic layers. There were increasing C/N ratios but on average constant C concentrations in the organic layer (Fig.3), meanwhile an increase in both C/N ratios and C concentrations in the topsoil of most groups was also evident (Table3). Increasing C/N ratios indicated by a rise in the C pool in spite of N-deposition is an expected C accumulation response to elevated N (de Vries et al., 2009). On the other hand, long-term experiments revealed that N deposition leads to a decrease in C/N ratios in the organic layer, however, unchanging C/N ratios in the topsoil due to an increase in both C and N concentrations (Pregitzer, 2003). It has been demonstrated that the effect of elevated N on decomposition rates is dependent on litter quality due to increased decomposition rates of high-quality litter and reduced decomposition rates of low quality litter (Knorr et al., 2005; Janssens et al., 2010). Our results are in line with this pattern as under deciduous forests and low C/N ratios C stocks of the organic layer declined; meanwhile they increased or remained unchanged under coniferous forests and high C/N ratios.
In our study, we detected decreasing organic layer C stocks under deciduous trees (Fig.3), whereas the mineral soil C pool generally increased (Fig.4). This clearly shows that apart from N deposition the species composition is another factor affecting C stocks. It was demonstrated by various studies that the incorporated organic layer of broadleaf species in coniferous forests was beneficial for a sustainable C sequestration (Prescott et al., 2004; Jandl et al., 2007; Prietzel & Bachmann, 2012; Wiesmeier et al., 2013). It was assumed when converting monocultures, especially coniferous forests into mixed forests, may alter the plant residues composition. Plant residues are composed of complex mixtures of organic components, mainly polysaccharides and lignin (von Lützow et al., 2006). It has been shown by Kubartova et al. (2009) that decreasing foliar lignin contents may act as key factors responsible for decreased forest floor C/N ratios and C stocks. The gradual incorporation of deciduous tree species into coniferous monocultures produces higher biodegradable plant components than that of homogeneous coniferous forest stands. Accelerated decomposition of easily decomposable plant residues may additionally induce a priming effect, enhancing the microbial degradation of older litter (Fontaine et al., 2003; Prietzel & Bachmann, 2012). The goal of the Federal Government is to create more site-adapted, ecologically orientated forests which will in turn increase the ecological and economic benefits of Germany`s forests. Coniferous forests are being regenerated with broadleaved trees since the first inventory, resulting in multilayer stands. These forest transformations may already be affecting the C pool which could result in a translocation of SOM from the organic layer into the mineral soil. In this study, changes in the C pool under deciduous and mixed forests was not provable due to a change in litter quality as the C/N ratio has increased significantly, indicating a decline in litter quality. Taking site-quality as well as the tree species effects into account, we can conclude that tree species selection combined with a specific parent material can be beneficial in soil C sequestration. Parts of the C accumulation capacity may also result from an uneven age structure caused by post World War II large-scale clear cuts with subsequent replanting (Böttcher et al., 2008) as well as the plantations of the 1970s. Decades later, the soil continuously accumulated C without substantial mechanical soil disturbances. Forests growth in Germany currently supersedes the timber harvest, which may partly explain the C sequestration rate. Results of the German National Forest Inventory revealed a sequestration of tree biomass ranging between 1.2 and 2.5 Mg C ha−1 yr−1 (Dunger et al., 2009). Several authors concluded that the ageing of forests results in increasing C (Jandl et al., 2007; Luyssaert et al., 2008). Nevertheless, it has been shown in previous studies that fluctuations in the C pool of forested soils are neither linear nor consistent. Johnson et al. (2007) detected increasing soil C concentrations from 1972 to 2004, however they found an increase between 1972 and 1982 yet they observed a decline between 1982 and 1993 and another increase in C until 2004. This shows that the C pool affected forest management activities may occur in shorter time periods than the time span necessary to detect changes by resampling (Schrumpf et al., 2011). Therefore, resampling in shorter time intervals is more appropriate in comparison to a single soil inventory repeated after a long time period.
Acknowledgments
We thank the individual Federal states for providing the datasets as well as the Federal Ministry of Food, Agriculture and Consumer Protection (BMELV) for facilitating the data analysis. We acknowledge in particular all site investigators and the persons in charge of the individual Federal research stations for their assistance and support.
References
- Ad-hoc-Arbeitsgruppe Boden. Bodenkundliche Kartieranleitung (KA3) Stuttgart: Schweizerbart; 1982. [Google Scholar]
- Ad-hoc-Arbeitsgruppe Boden. Bodenkundliche Kartieranleitung (KA4) Stuttgart: Schweizerbart; 1994. [Google Scholar]
- Ad-hoc-Arbeitsgruppe Boden. Bodenkundliche Kartieranleitung (KA5) Stuttgart: Schweizerbart; 2005. [Google Scholar]
- Agren GI, Hyvonen R, Nilsson T. Are Swedish forest soils sinks or sources for CO2 - model analyses based on forest inventory data. Biogeochemistry. 2007;82:217–227. [Google Scholar]
- Akselsson C, Berg B, Meentemeyer V, Westling O. Carbon sequestration rates in organic layers of boreal and temperate forest soils - Sweden as a case study. Global Ecology and Biogeography. 2005;14:77–84. [Google Scholar]
- Batjes NH. Total carbon and nitrogen in the soils of the world. European Journal of Soil Science. 1996;47:151–163. [Google Scholar]
- Bellamy PH, Loveland PJ, Bradley RI, Lark RM, Kirk GJD. Carbon losses from all soils across England and Wales 1978-2003. Nature. 2005;437:245–248. doi: 10.1038/nature04038. [DOI] [PubMed] [Google Scholar]
- Berg B, Johansson MB, Nilsson A, Gundersen P, Norell L. Sequestration of carbon in the humus layer of Swedish forests - direct measurements. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestiere. 2009;39:962–975. [Google Scholar]
- BGR. Soil Map of the Federal Republic of Germany 1:1,000,000 (BÜK 1000° N) Hannover: Federal Institute for Geosciences and Natural Resources; 1998. [Google Scholar]
- Billett MF, Fitzpatrick EA, Cresser MS. Changes in the carbon and nitrogen status of forest soil organic horizons between 1949/50 and 1987. Environmental Pollution. 1990;66:67–79. doi: 10.1016/0269-7491(90)90199-m. [DOI] [PubMed] [Google Scholar]
- BMELV. Bundesweite Bodenzustandserhebung im Wald (BZE). Arbeitsanleitung. Bonn: Bundesministerium für Ernährung, Landwirtschaft und Forsten; 1990. [Google Scholar]
- Böttcher H, Kurz WA, Freibauer A. Accounting of forest carbon sinks and sources under a future climate protocol-factoring out past disturbance and management effects on age-class structure. Environmental Science & Policy. 2008;11:669–686. [Google Scholar]
- Bowman RA. Spatial variability of selected carbon, nitrogen, and phosphorus parameters on acid and calcareous rangeland soils. Communications in Soil Science and Plant Analysis. 1991;22:205–212. [Google Scholar]
- De Vos B, Van Meirvenne M, Quataert P, Deckers J, Muys B. Predictive quality of pedotransfer functions for estimating bulk density of forest soils. Soil Science Society of America Journal. 2005;69:500–510. [Google Scholar]
- Dijkstra JPM, Reinds GJ, Kros H, Berg B, de Vries W. Modelling soil carbon sequestration of intensively monitored forest plots in Europe by three different approaches. Forest Ecology and Management. 2009;258:1780–1793. [Google Scholar]
- Don A, Schumacher J, Scherer-Lorenzen M, Scholten T, Schulze ED. Spatial and vertical variation of soil carbon at two grassland sites - Implications for measuring soil carbon stocks. Geoderma. 2007;141:272–282. [Google Scholar]
- Dunger K, Stümer W, Oehmichen K, Riedel T, Bolte A. Der Kohlenstoffspeicher Wald und seine Entwicklung: Ergebnisse einer Kohlenstoffinventur auf Bundeswaldinventur-Basis; die Inventurstudie 2008. Allgemeine Forst Zeitschrift für Waldwirtschaft und Umweltvorsorge. 2009;64:1072–1073. [Google Scholar]
- EEA. Copenhagen: European Environment Agency; 2010a. Raster data on land cover for the CLC 1990 inventory. Available at: http://www.eea.europa.eu/data-and-maps/data/ds_resolveuid/8b12c16a3c93eec2d6944abe42388d90 (accessed 23 September 2013) [Google Scholar]
- EEA. Copenhagen: European Environment Agency; 2010b. Raster data on land cover for the CLC 2006 inventory. Available at: http://www.eea.europa.eu/data-and-maps/data/ds_resolveuid/a645109f7a11d43f5d7e275d81f35c61 (accessed 23 September 2013) [Google Scholar]
- Ellert BH, Janzen HH, Entz T. Assessment of a method to measure temporal change in soil carbon storage. Soil Science Society of America Journal. 2002;66:1687–1695. [Google Scholar]
- Falloon PD, Smith P, Szabo J, Pasztor L. Comparison of approaches for estimating carbon sequestration at the regional scale. Soil Use and Management. 2002;18:164–174. [Google Scholar]
- FAO UNESCO ISRIC. Soil Map of the World. 1990. Revised Legend. Soil Ressources Report 60. Rome.
- FEA. Submission under the United Nations Framework Convention on Climate Change and the Kyoto Protocol. Dessau-Roßlau: Federal Environment Agency; 2012. National Inventory Report for the German Greenhouse Gas Inventory 1990 - 2010. [Google Scholar]
- Fontaine S, Mariotti A, Abbadie L. The priming effect of organic matter: a question of microbial competition? Soil Biology & Biochemistry. 2003;35:837–843. [Google Scholar]
- GAFA. Handbuch Forstliche Analytik. Eine Loseblatt-Sammlung der Analysemethoden im Forstbereich, Gutachterausschuss Forstliche Analytik. Berlin: Bundesministerium für Ernährung und Landwirtschaft; 2006. [Google Scholar]
- Goidts E, van Wesemael B, Crucifix M. Magnitude and sources of uncertainties in soil organic carbon (SOC) stock assessments at various scales. European Journal of Soil Science. 2009;60:723–739. [Google Scholar]
- Golchin A, Oades JM, Skjemstad JO, Clarke P. Soil structure and carbon cycling. Australian Journal of Soil Research. 1994a;32:1043–1068. [Google Scholar]
- Golchin A, Oades JM, Skjemstad JO, Clarke P. Study of free and occluded particulate organic matter in soils by solid state 13C CP/MAS NMR specroscopy and scanning electron microscopy. Australian Journal of Soil Research. 1994b;32:285–309. [Google Scholar]
- Gregorich EG, Janzen HH. Storage of soil carbon in the light fraction and macroorganic matterIn. In: Carter MR, Steward BA, editors. Structure and Organic Matter Storage in Agricultural Soils. Boca Raton: CRC Press; 1996. pp. 167–192. [Google Scholar]
- Guggenberger G, Zech W, Haumaier L, Christensen BT. Land-use effects on the composition of organic matter in particle-size separates of soils: II. CPMAS and solution 13C NMR analysis. European Journal of Soil Science. 1995;46:147–158. [Google Scholar]
- Hagedorn F, Spinnler D, Bundt M, Blaser P, Siegwolf R. The input and fate of new C in two forest soils under elevated CO2. Global Change Biology. 2003;9:862–872. [Google Scholar]
- Heuscher SA, Brandt CC, Jardine PM. Using soil physical and chemical properties to estimate bulk density. Soil Science Society of America Journal. 2005;69:51–56. [Google Scholar]
- IPCC. Good practice guidance for land use, land-use change and forestry. Intergovernmental Panel on Climate Change. 2003. ISBN 4-88788-003-0: 307.
- Jandl R, Lindner M, Vesterdal L, et al. How strongly can forest management influence soil carbon sequestration? Geoderma. 2007;137:253–268. [Google Scholar]
- Janssens IA, Dieleman W, Luyssaert S, et al. Reduction of forest soil respiration in response to nitrogen deposition. Nature Geoscience. 2010;3:315–322. [Google Scholar]
- Jobbagy EG, Jackson RB. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecological Applications. 2000;10:423–436. [Google Scholar]
- Johnson DW, Todd DE, Trettin CF, Sedinger JS. Soil carbon and nitrogen changes in forests of walker branch watershed, 1972 to 2004. Soil Science Society of America Journal. 2007;71:1639–1646. [Google Scholar]
- Kaiser K, Guggenberger G. Mineral surfaces and soil organic matter. European Journal of Soil Science. 2003;54:219–236. [Google Scholar]
- Kern JS. Spatial patterns of soil organic-carbon in the contiguous United-States. Soil Science Society of America Journal. 1994;58:439–455. [Google Scholar]
- Knorr M, Frey SD, Curtis PS. Nitrogen additions and litter decomposition: a meta-analysis. Ecology. 2005;86:3252–3257. [Google Scholar]
- Kögel-Knabner I, Guggenberger G, Kleber M, et al. Organo-mineral associations in temperate soils: integrating biology, mineralogy, and organic matter chemistry. Journal of Plant Nutrition and Soil Science-Zeitschrift Fur Pflanzenernahrung Und Bodenkunde. 2008;171:1436–8730. [Google Scholar]
- König N, Wolff B. Abschlußbericht über die Ergebnisse und Konsequenzen der im Rahmen der bundesweiten Bodenzustandserhebung im Wald (BZE) durchgeführten Ringanalysen. Göttingen: Forschungszentrum Waldökosysteme; 1993. [Google Scholar]
- Kubartova A, Ranger J, Berthelin J, Beguiristain T. Diversity and decomposing ability of saprophytic fungi from temperate forest litter. Microbial Ecology. 2009;58:98–107. doi: 10.1007/s00248-008-9458-8. [DOI] [PubMed] [Google Scholar]
- Ladegaard-Pedersen P, Elberling B, Vesterdal L. Soil carbon stocks, mineralization rates, and CO2 effluxes under 10 tree species on contrasting soil types. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestiere. 2005;35:1277–1284. [Google Scholar]
- Lettens S, van Orshoven J, van Wesemael B, Muys B, Perrin D. Soil organic carbon changes in landscape units of Belgium between 1960 and 2000 with reference to 1990. Global Change Biology. 2005;11:2128–2140. doi: 10.1111/j.1365-2486.2005.001074.x. [DOI] [PubMed] [Google Scholar]
- Liski J. Variation in soil organic carbon and thickness of soil horizons within a boreal forest stand - effect of trees and implications for sampling. Silva Fennica. 1995;29:255–266. [Google Scholar]
- Liski J, Perruchoud D, Karjalainen T. Increasing carbon stocks in the forest soils of western Europe. Forest Ecology and Management. 2002;169:159–175. [Google Scholar]
- von Lützow M, Kögel-Knabner I. Temperature sensitivity of soil organic matter decomposition-what do we know? Biology and Fertility of Soils. 2009;46:1–15. [Google Scholar]
- von Lützow M, Kögel-Knabner I, Ekschmitt K, Matzner E, Guggenberger G, Marschner B, Flessa H. Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions - a review. European Journal of Soil Science. 2006;57:426–445. [Google Scholar]
- Luyssaert S, Schulze ED, Borner A, et al. Old-growth forests as global carbon sinks. Nature. 2008;455:213–215. doi: 10.1038/nature07276. [DOI] [PubMed] [Google Scholar]
- Luyssaert S, Ciais P, Piao SL, et al. The European carbon balance. Part 3: forests. Global Change Biology. 2010;16:1429–1450. [Google Scholar]
- Martin MP, Wattenbach M, Smith P, Meersmans J, Jolivet C, Boulonne L, Arrouays D. Spatial distribution of soil organic carbon stocks in France. Biogeosciences. 2011;8:1053–1065. [Google Scholar]
- Morisada K, Ono K, Kanomata H. Organic carbon stock in forest soils in Japan. Geoderma. 2004;119:21–32. [Google Scholar]
- Mou P, Jones RH, Guo DL, Lister A. Regeneration strategies, disturbance and plant interactions as organizers of vegetation spatial patterns in a pine forest. Landscape Ecology. 2005;20:971–987. [Google Scholar]
- Nabuurs GJ, Schelhaas MJ. Carbon profiles of typical forest types across Europe assessed with CO2FIX. Ecological Indicators. 2002;1:213–223. [Google Scholar]
- Nabuurs GJ, Schelhaas MJ, Mohren GMJ, Field CB. Temporal evolution of the European forest sector carbon sink from 1950 to 1999. Global Change Biology. 2003;9:152–160. [Google Scholar]
- Nielsen O-K, Mikkelsen MH, Hoffmann L, et al. Denmark's National Inventory Report 2012. Emission Inventories 1990-2010 - Submitted under the United Nations Framework Convention on Climate Change 1990-2008. Aarhus: National Environmental Research Institute; 2012. [Google Scholar]
- Oades JM. Retention of organic matter in soils. Biogeochemistry. 1988;5:35–70. [Google Scholar]
- Oostra S, Majdi H, Olsson M. Impact of tree species on soil carbon stocks and soil acidity in southern Sweden. Scandinavian Journal of Forest Research. 2006;21:364–371. [Google Scholar]
- Parfitt RL, Ross C, Schipper LA, Claydon JJ, Baisden WT, Arnold G. Correcting bulk density measurements made with driving hammer equipment. Geoderma. 2010;157:46–50. [Google Scholar]
- Post WM, Kwon KC. Soil carbon sequestration and land-use change: processes and potential. Global Change Biology. 2000;6:317–327. [Google Scholar]
- Potter KN, Torbert HA, Johnson HB, Tischler CR. Carbon storage after long-term grass establishment on degraded soils. Soil Science. 1999;164:718–725. [Google Scholar]
- Prechtel A, von Lutzow M, Schneider BU, Bens O, Bannick CG, Kogel-Knabner I, Huttl RF. Organic carbon in soils of Germany: status quo and the need for new data to evaluate potentials and trends of soil carbon sequestration. Journal of Plant Nutrition and Soil Science. 2009;172:601–614. [Google Scholar]
- Pregitzer KS. Carbon cycling in forest ecosystems with an emphasis on belowground processes. In: Kimble JM, Lal R, Birdsey R, Heath LS, editors. The Potential of U.S. Forest Soils to Sequester Carbon and Mitigate the Greenhouse Effect. Boca Raton: CRC Press; 2003. pp. 93–104. [Google Scholar]
- Prescott CE, Vesterdal L, Simard SW, Preston CM. Influence of initial chemistry on decomposition of foliar litter in contrasting forest types in British Columbia. Canadian Journal of Forest Research. 2004;34:1714–1729. [Google Scholar]
- Prietzel J, Bachmann S. Changes in soil organic C and N stocks after forest transformation from Norway spruce and Scots pine into Douglas fir, Douglas fir/spruce, or European beech stands at different sites in Southern Germany. Forest Ecology and Management. 2012;269:134–148. [Google Scholar]
- Prietzel J, Stetter U, Klemmt HJ, Rehfuess KE. Recent carbon and nitrogen accumulation and acidification in soils of two Scots pine ecosystems in Southern Germany. Plant and Soil. 2006;289:153–170. [Google Scholar]
- Richter DD, Markewitz D, Trumbore SE, Wells CG. Rapid accumulation and turnover of soil carbon in a re-establishing forest. Nature. 1999;400:56–58. [Google Scholar]
- Saby NPA, Bellamy PH, Morvan X, et al. Will European soil-monitoring networks be able to detect changes in topsoil organic carbon content? Global Change Biology. 2008;14:2432–2442. [Google Scholar]
- Schils R, Kuikman P, Liski J, et al. Brussels: European Commission; 2008. Review of existing information on the interrelations between soil and climate change (CLIMSOIL) p. 208. Final report. [Google Scholar]
- Schlichting E, Blume HP. Bodenkundliches Praktikum. Verlag Paul Parey: Hamburg & Berlin; 1966. [Google Scholar]
- Schöning I, Totsche KU, Kögel-Knabner I. Small scale spatial variability of organic carbon stocks in litter and solum of a forested Luvisol. Geoderma. 2006;136:631–642. [Google Scholar]
- Schrumpf M, Schulze ED, Kaiser K, Schumacher J. How accurately can soil organic carbon stocks and stock changes be quantified by soil inventories? Biogeosciences. 2011;8:1193–1212. [Google Scholar]
- Schulp CJE, Nabulars GJ, Verburg PH, de Waal RW. Effect of tree species on carbon stocks in forest floor and mineral soil and implications for soil carbon inventories. Forest Ecology and Management. 2008;256:482–490. [Google Scholar]
- Schulten H-R, Leinweber P. New insights into organic-mineral particles: composition, properties and models of molecular structure. Biology and Fertility of Soils. 2000;30:399–432. [Google Scholar]
- Schulze ED, Hogberg P, Oene Hv. Interactions between the carbon- and nitrogen cycle and the role of biodiversity: a synopsis of a study along a north-south transect through EuropeIn. In: Schulze ED, et al., editors. Carbon and Nitrogen Cycling in European Forest Ecosystems. Heidelberg: Springer Verlag; 2000. pp. 468–492. (Ecological Studies; 142) [Google Scholar]
- Schulze ED, Luyssaert S, Ciais P, et al. Importance of methane and nitrous oxide for Europe's terrestrial greenhouse-gas balance. Nature Geoscience. 2009;2:842–850. [Google Scholar]
- SEPA. Submitted under the United Nations Framework Convention on Climate Change and the Kyoto Protocol. Swedish Environmental Protection Agency; 2011. National Inventory Report 2011 Sweden. National Inventory Report 2011. [Google Scholar]
- Six J, Conant RT, Paul EA, Paustian K. Stabilization mechanisms of soil organic matter: implications for C-saturation of soils. Plant and Soil. 2002;241:155–176. [Google Scholar]
- Stevens A, van Wesemael B. Soil organic carbon dynamics at the regional scale as influenced by land use history: a case study in forest soils from southern Belgium. Soil Use and Management. 2008;24:69–79. [Google Scholar]
- Tan ZX, Lal R, Smeck NE, Calhoun FG, Slater BK, Parkinson B, Gehring RM. Taxonomic and geographic distribution of soil organic carbon pools in Ohio. Soil Science Society of America Journal. 2004;68:1896–1904. [Google Scholar]
- Torn MS, Trumbore SE, Chadwick OA, Vitousek PM, Hendricks DM. Mineral control of soil organic carbon storage and turnover. Nature. 1997;389:170–173. [Google Scholar]
- Trumbore SE, Davidson EA, Decamargo PB, Nepstad DC, Martinelli LA. Belowground cycling of carbon in forests and pastures of Eastern Amazonia. Global Biogeochemical Cycles. 1995;9:515–528. [Google Scholar]
- Vesterdal L. Influence of soil type on mass loss and nutrient release from decomposing foliage litter of beech and Norway spruce. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestiere. 1999;29:95–105. [Google Scholar]
- Vesterdal L, Raulund-Rasmussen K. Forest floor chemistry under seven tree species along a soil fertility gradient. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestiere. 1998;28:1636–1647. [Google Scholar]
- Vesterdal L, Schmidt IK, Callesen I, Nilsson LO, Gundersen P. Carbon and nitrogen in forest floor and mineral soil under six common European tree species. Forest Ecology and Management. 2008;255:35–48. [Google Scholar]
- de Vries W, Posch M. Modelling the impact of nitrogen deposition, climate change and nutrient limitations on tree carbon sequestration in Europe for the period 1900-2050. Environmental Pollution. 2011;159:2289–2299. doi: 10.1016/j.envpol.2010.11.023. [DOI] [PubMed] [Google Scholar]
- de Vries W, Solberg S, Dobbertin M, et al. The impact of nitrogen deposition on carbon sequestration by European forests and heathlands. Forest Ecology and Management. 2009;258:1814–1823. [Google Scholar]
- Wellbrock N, Aydin C-T, Block J, et al. Bodenzustandserhebung im Wald (BZE II) Arbeitsanleitung für die Außenaufnahmen. Berlin: Bundesministerium für Ernährung, Landwirtschaft und Verbraucherschutz; 2006. [Google Scholar]
- Wiesmeier M, Spoerlein P, Geuss U, et al. Soil organic carbon stocks in southeast Germany (Bavaria) as affected by land use, soil type and sampling depth. Global Change Biology. 2012;18:2233–2245. [Google Scholar]
- Wiesmeier M, Prietzel J, Barthold F, et al. Storage and drivers of organic carbon in forest soils of southeast Germany (Bavaria) - Implications for carbon sequestration. Forest Ecology and Management. 2013;295:162–172. [Google Scholar]
- Wirth C, Schulze E-D, Schwalbe G, Tomczyk S, Weber G, Weller E. Dynamik der Kohlenstoffvorräte in den Wäldern Thüringens. Abschlussbericht zur 1. Phase des BMBF-Projektes “Modelluntersuchung zur Umsetzung des Kyoto-Protokolls”. Gotha: Thüringer Landesanstalt für Wald, Jagd und Fischerei; 2004. [Google Scholar]
- Wolff B, Riek W. Deutscher Waldbodenbericht 1996 - Ergebnisse der bundesweiten Bodenzustandserhebung in Wald (BZE) 1987 - 1993. Bonn: Bundesministerium für Ernährung, Landwirtschaft und Forsten; 1996. [Google Scholar]
- Yang YH, Fang JY, Ma WH, Smith P, Mohammat A, Wang SP, Wang W. Soil carbon stock and its changes in northern China's grasslands from 1980s to 2000s. Global Change Biology. 2010;16:3036–3047. [Google Scholar]


