Abstract
Background
In the United Kingdom, patients with locally advanced rectal cancer routinely receive neoadjuvant chemoradiotherapy. However, the effects of this on physical fitness are unclear. This pilot study is aimed to investigate the effect of neoadjuvant chemoradiotherapy on objectively measured in vivo muscle mitochondrial function and whole-body physical fitness.
Methods
We prospectively studied 12 patients with rectal cancer who completed standardized neoadjuvant chemoradiotherapy, recruited from a large tertiary cancer centre, between October 2012 and July 2013. All patients underwent a cardiopulmonary exercise test and a phosphorus magnetic resonance spectroscopy quadriceps muscle exercise-recovery study before and after neoadjuvant chemoradiotherapy. Data were analysed and reported blind to patient identity and clinical course. Primary variables of interest were the two physical fitness measures; oxygen uptake at estimated anaerobic threshold and oxygen uptake at Peak exercise (ml.kg−1.min−1), and the post-exercise phosphocreatine recovery rate constant (min−1), a measure of muscle mitochondrial capacity in vivo.
Results
Median age was 67 years (IQR 64–75). Differences (95%CI) in all three primary variables were significantly negative post-NACRT: Oxygen uptake at estimated anaerobic threshold −2.4 ml.kg−1.min−1 (−3.8, −0.9), p = 0.004; Oxygen uptake at Peak −4.0 ml.kg−1.min−1 (−6.8, −1.1), p = 0.011; and post-exercise phosphocreatine recovery rate constant −0.34 min−1 (−0.51, −0.17), p<0.001.
Conclusion
The significant decrease in both whole-body physical fitness and in vivo muscle mitochondrial function raises the possibility that muscle mitochondrial mechanisms, no doubt multifactorial, may be important in deterioration of physical fitness following neoadjuvant chemoradiotherapy. This may have implications for targeted interventions to improve physical fitness pre-surgery.
Trial Registration
Clinicaltrials.gov registration NCT01859442
Introduction
In the UK colorectal cancer is the third commonest cause of cancer death [1], [2]. In 2013, ∼9000 patients were diagnosed with rectal cancer (35% aged >75 y), of whom ∼4600 underwent major resection with a 90-day elective postoperative mortality of 2.5% [3]. 25% are locally advanced (Tumour, Node, Metastasis (TNM) stage - T3/T4N+) cancers (i.e. resection margin threatened) considered for neoadjuvant chemoradiotherapy (NACRT) to control local disease and to achieve tumour downsizing and negative resection margins [4]–[8]; however, external beam radiation and oral or intravenous fluoropyrimidines causes dose-limiting toxicity, reaching Grade 3–5 in 20% (Common Terminology Criteria for Adverse Events, Version 3.0). It is unknown to what extent NACRT affects physical fitness in this patient cohort.
Poor physical fitness, assessed by cardiopulmonary exercise testing (CPET), is linked to poor postoperative outcomes after major surgery [9]–[15]. CPET provides an integrated quantitative assessment of the cardiorespiratory system at rest and under the stress of maximal exercise, testing the physiological reserve required to withstand the stress of surgery. Subjective assessment tools have been used to predict surgical outcomes, but there is little evidence linking objectively-measured physical fitness and surgical outcome in this group. The UK National Bowel Cancer Audit found the American Society of Anaesthesiologists – Physical Status (ASA-PS) score (a categorical descriptor of fitness for surgery) to be the strongest predictor of death within 30 days of surgery [16]. Only two trials have suggested that rectal cancer patients with a higher subjective performance status (WHO Score >1) have worse post-operative outcome after combined chemotherapy or chemo-radiation and surgery [17], [18]. Studies investigating objective changes in physical fitness in patients receiving neoadjuvant cancer treatments are lacking [19]. We have previously demonstrated a significant reduction in objectively measured physical fitness with neoadjuvant chemotherapy in upper gastrointestinal cancer which was associated with reduced 1 year survival [20] and a similar reduction in fitness with neoadjuvant chemoradiotherapy in rectal cancer which was associated with in-hospital morbidity [21]. Whether and how this impaired physical fitness relates to changes in mitochondrial function is unknown.
Skeletal muscle mitochondrial function can be studied non-invasively in vivo using phosphorus magnetic resonance spectroscopy (31P MRS) [22]; this can usefully be combined with CPET measurements [23], [24] of whole-body fitness, to which muscle mitochondrial function makes a substantial contribution. Good correlations are observed between in vivo and in vitro measures of mitochondrial function in health and chronic conditions (e.g. type 2 diabetes) makes the assessment of mitochondrial function by 31P MRS an attractive and reliable modality, especially for repeated measurements [24]–[26].
The primary aim of this pilot study was to evaluate changes in objectively-measured physical fitness and skeletal muscle mitochondrial function after standardized NACRT, in patients scheduled for rectal cancer surgery. An exploratory aim was to observe changes in physical activity (PA) in the same patient cohort.
Methods
Patients and clinical methods
The protocol for this trial and supporting TREND checklist are available as supporting information; see Checklist S1 and Protocol S1. This nested mechanism pilot study forms part of a larger clinical trial which began in March 2011. Ethics approval for the main trial was given by the North West – Liverpool East Research and Ethics Committee (11/H1002/12) in March 2011, with a subsequent amendment (11/H1002/12c) adding 31P MRS measurements for this nested mechanism pilot sub-study approved in January 2012. The larger trial was registered with clinicaltrials.gov (NCT01325909 – March 2011), and initially this NCT registration was taken to cover all aspects of the larger trial, including the present pilot study. Subsequently the pilot study was registered separately (NCT 01859442 – May 2013), and as a result of this change of approach this specific registration post-dated the recruitment of the first patients reported here, for which studies commenced in October 2012. The authors confirm that all ongoing and related trials for this intervention are registered. Written informed consent was obtained from all patients. All patients were followed up until surgery, with the last patient exiting the study in October 2013.
We recruited consecutive patients between October 2012 and July 2013 who were referred to the Colorectal Multi-Disciplinary Team (MDT), age ≥18 y, with locally advanced resectable rectal cancer (circumferential resection margin threatened), scheduled for standardized NACRT on the basis of Tumour, Node, Metastasis (TNM) classification >T2/N+ with no distant metastasis [27] and WHO Performance Status <2 [28]. Predefined exclusion criteria were: inability to give informed consent, non-resectable disease, standard MR exclusion criteria, inability to exercise studies due to leg dysfunction, and patients who declined surgery or NACRT, or who received non-standard NACRT.
TNM staging involved flexible sigmoidoscopy for histological diagnosis, colonoscopy, chest, abdomen and pelvis computer-aided tomography (CT) and a 1.5T pelvic magnetic resonance imaging (MRI). All patients then underwent standardised NACRT for 5 weeks. Standardized radiotherapy consisted of 45 Gy in 25 fractions on weekdays using a 3-dimensional conformal technique with CT guidance. A boost dose was given (5.4 Gy in 3 fractions) to the primary tumour only. Oral capecitabine (825 mg.m−2) was given twice daily on radiotherapy days. No patients received brachytherapy. The colorectal multidisciplinary team (MDT) was blind to CPET results, which therefore did not influence perioperative management. All patients underwent total mesorectal excision (TME) surgery [29]. A defunctioning stoma was constructed at the discretion of the surgeon. No deviations from the protocol were encountered.
CPET
CPET (Geratherm Respiratory GmbH; Love Medical Ltd, Manchester, United Kingdom) and the estimation of the estimated anaerobic threshold followed a standard protocol described in detail elsewhere [14]. Patient characteristics recorded included age, gender, height, weight, diagnosis, staging, surgical procedure planned, WHO classification, ASA-PS, and diagnoses of diabetes, ischaemic heart disease, cerebrovascular disease, or heart failure. Resting flow-volume loops were used to derive Forced Expiratory Volume over 1 second (FEV1) and Forced Vital Capacity (FVC). Ventilation and gas exchange variables included oxygen uptake (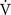 o2), ventilatory equivalents for oxygen and carbon dioxide (
o2), ventilatory equivalents for oxygen and carbon dioxide (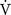 E/
E/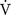 o2;
o2; 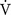 E/
E/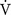 co2) and oxygen pulse (
co2) and oxygen pulse (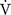 o2/heart rate), all measured both at estimated anaerobic threshold (
o2/heart rate), all measured both at estimated anaerobic threshold ( L) and at peak exercise.
L) and at peak exercise.
NACRT associated toxicity and CPET-related adverse events were discussed at the weekly MDT meeting. Toxicity events were graded on the National Cancer Institute Common Terminology Criteria (version 3.0), and acute radiation-induced skin toxicity using the Radiation Therapy Oncology Group scoring system.
Physical activity
PA was measured during a continuous 72 h period using a biaxial accelerometer (SenseWear armband BodyMedia Inc., Pittsburgh, USA), worn over the right triceps during weekdays at baseline and post-NACRT. Step count while active averaged over the whole 72 h was used as a measure of PA. All patients were instructed not to change their PA, and to continue performing normal activities of daily living throughout the study period.
MRS methods
31P MRS assessments of muscle mitochondrial function in quadriceps were carried out using a Siemens 3T Trio MR scanner (Siemens AG, Erlangen, Germany) using an isometric knee extension exercise protocol. Subjects lay supine (secured with a Velcro strap across the hips) with the right knee flexed over a rigid foam support in a custom-built rig permitting isometric knee extension exercise against a strap across the anterior lower shin/ankle connected to an aluminium bar fitted with a strain gauge. 31P MRS data were acquired from right quadriceps muscle using a dual-tuned 18 cm/15 cm diameter 31P/1H surface coil (RAPID Biomedical, Rimpar, Germany), Velcro-strapped to the anterior thigh (midway between anterior superior iliac spine and patella). After automated set-up and manual shimming using tissue water signal, a 4-scan fully relaxed (TR = 10s) spectrum and a 32-scan partially saturated (TR = 2s) resting spectrum were collected. The exercise protocol consisted of 5 min rest followed by 2 bouts of isometric exercise (paced at 2 s on, 2 s off by an audible cue, exercise force being fed back visually via an LED display) each followed by 7 min recovery, while spectra were collected (TR = 2s) every 8 s. Two exercise intensities were used, corresponding to 70% and 90% of maximal voluntary contraction established in 3 brief trials prior to MRS acquisition (in pilot experiments these intensities gave acceptable PCr depletion with minimal acidification in typical subjects). Block MRS data output files from exercise-recovery acquisitions were converted to text using a specially-written MATLAB routine. All 31P MRS data were processed using the java-based Magnetic Resonance User Interface (jMRUI v.3.0), using the AMARES time-domain fitting algorithm. Data were fitted assuming Lorentzian lineshapes for phosphocreatine (PCr), inorganic phosphate (Pi) and ATP (β-ATP a 1∶2∶1 triplet, α-ATP and γ-ATP both 1∶1 doublets). The chemical shift of Pi relative to PCr was used by standard means to determine intracellular pH. PCr recovery time courses were fitted to a monoexponential function to estimate the recovery rate constant (kPCr min−1): in the absence of appreciable changes in pH, this is an accepted measure of effective muscle mitochondrial function. As kPCr did not differ significantly between the two exercise intensities, and as pH changes were small throughout, values of kPCr are presented as mean of the two intensities [30].
All patients underwent CPET, PA monitoring and 31P MRS 2 weeks before NACRT (baseline) and immediately post-NACRT (post-NACRT) that is within 48 hours of finishing NACRT. CPET data were reported by two experienced assessors blind to patient demographics and data time-points. Any CPET or exercise-related adverse events were discussed at the weekly colorectal MDT meeting. 31P MRS analysis was performed blind to clinical details and CPET data.
Our primary outcome variables include 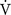 o2 at
o2 at  L,
L, 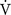 o2 Peak and kPCr, assessed with CPET and 31P MRS; an exploratory outcome variable is the number of steps whilst active, assessed by PA monitoring. A comparison of these variables is made between baseline and post-NACRT.
o2 Peak and kPCr, assessed with CPET and 31P MRS; an exploratory outcome variable is the number of steps whilst active, assessed by PA monitoring. A comparison of these variables is made between baseline and post-NACRT.
Statistical methods
Our aim was to recruit 12 patients to a pilot study who would undergo standardised NACRT, CPET and 31P MRS scans at baseline (pre-NACRT) and immediately post-NACRT. No formal sample size calculation was performed as this study was designed as a nested pilot study: the target number was based on experience and reports of similar clinical 31P MRS studies and pragmatic considerations of recruitment logistics and funding. Descriptive statistics are reported as mean (SD) or median and inter-quartile range (IQR) depending on the distribution and categorical statistics as frequency (percentage). Normality was assessed by the Shapiro-Wilk test. Pre- and post-NACRT data were compared using paired t-tests.
For the primary analysis, baseline and post-NACRT measurements for 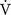 o2 at
o2 at  L,
L, 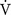 o2 Peak and kPCr were compared using a paired t-test as an intention to treat. Formal comparisons were considered to be statistically significant at p<0.05. Spearman's correlation coefficient (r) was used to describe the strength of association between changes in
o2 Peak and kPCr were compared using a paired t-test as an intention to treat. Formal comparisons were considered to be statistically significant at p<0.05. Spearman's correlation coefficient (r) was used to describe the strength of association between changes in 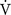 o2 at
o2 at  L and change in haemoglobin. For PA these comparisons were considered as exploratory and tested against the uncorrected 5% significance level; the need to square-root transform PA makes it impossible to recover the differences and confidence intervals on a meaningful scale, so only p-values and predicted means are presented. These analyses were conducted using Stata version 12 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP.)
L and change in haemoglobin. For PA these comparisons were considered as exploratory and tested against the uncorrected 5% significance level; the need to square-root transform PA makes it impossible to recover the differences and confidence intervals on a meaningful scale, so only p-values and predicted means are presented. These analyses were conducted using Stata version 12 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP.)
Results
Patient flow and characteristics
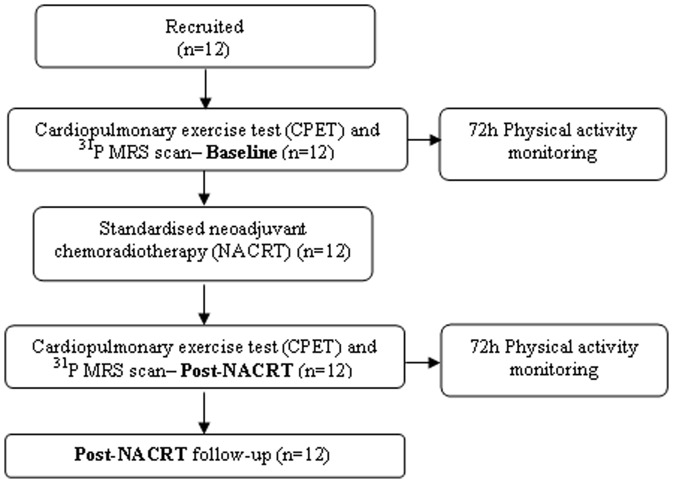
Fifteen patients were eligible for surgery, of which 3 were recruited into a different trial; 12 patients (10 males and 2 females) were recruited and underwent CPET and 31P MRS prior to starting a standardised course of NACRT (Figure 1). All CPETs were carried out 48±5 hours before the 31P MRS scan both at baseline and post-NACRT. All patients underwent CPET and 31P MRS immediately after (post-NACRT) finishing NACRT (at a maximum of 48±5 hours). Table 1 describes baseline patient characteristics and Table 2 describes patient characteristics between baseline and post-NACRT. There were no significant changes in BMI or lung function following NACRT, but there was a small but significant fall in haemoglobin. 83% of diagnosed rectal cancers were T3 with threatened circumferential resection margins.
Figure 1. Showing flow of patients through the study protocol.
Post-NACRT tests carried out within 48±5 hours of finishing NACRT period.
Table 1. Baseline patient characteristics.
| Mean (SD) | |
| Age (years) | 69 (10) |
| Number (%) | |
| Gender Male:Female | 10 (83): 2 (17) |
| Currently smoking | 2 (17) |
| Past medical history | |
| Diabetes | 1 (8) |
| Ischaemic heart disease | 2 (17) |
| Heart failure & cerebrovascular disease | 0 |
| American Society of Anaesthesiologists (ASA) score | |
| 1 | 6 (50) |
| 2 | 6 (50) |
| World Health Organisation performance status | |
| 0 | 9 (75) |
| 1 | 3 (25) |
| Tumour distance from anal verge (cm) | |
| <5.0 cm | 6 (50) |
| 5.1–10.0 cm | 5 (42) |
| >10.1 cm | 1 (8) |
| International Union against Cancer Tumour Node Metastasis (TNM) MRI staging | |
| cT3 | 10 (83) |
| cT4 | 2 (17) |
| cN0 | 3 (33) |
| cN1 | 4 (17) |
| cN2 | 5 (50) |
| cM0 | 12 (100) |
Table 2. Patient characteristics at Baseline and post-NACRT.
| Baseline | Post-NACRT | Mean difference | P | |
| mean (SD) | mean (SD) | (95% CI) | ||
| BMI (kg.m−2) | 26.8 (3.9) | 26.3 (3.2) | −0.5 (−0.5, 1.6) | 0.291 |
| FEV1 (l) | 3.0 (0.7) | 2.9 (0.8) | −0.1 (−0.1, 0.4) | 0.153 |
| FVC (l) | 4.3 (0.8) | 4.2 (0.9) | −0.2 (−0.1, 0.4) | 0.188 |
| FEV1/FVC (%) | 72 (7.3) | 73 (7.3) | 1.2 (−3.1, 0.8) | 0.215 |
| Haemoglobin (g.dl−1) | 13.2 (1.5) | 12.9 (1.5) | −0.4 (0.5, 0.7) | 0.034 |
Chemoradiotherapy and acute toxicity
The mean cumulative dose of capecitabine was 96% (range 84–100%) of the planned treatment dose; 1 patient needed dose reduction. All but 1 patient received at least 45 Gy radiotherapy, and all completed the full 25 fractions. 2 patients (including 1 receiving a diverting stoma because of obstructive symptoms prior to NACRT) experienced grade 3 toxicity, notably diarrhoea and radiation dermatitis, but no grade 4 toxicity was reported.
The effect of NACRT on physical fitness, physical activity and mitochondrial function
Table 3 shows CPET, PA and 31P MRS-derived variables pre- and post-NACRT. No exercise or MRS adverse events were encountered until end of follow-up. Post-NACRT, there were significant decreases in both absolute (ml.min−1) and relative (ml.kg−1.min−1) 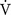 o2, in O2 pulse at
o2, in O2 pulse at  L and at Peak exercise, in baseline heart rate, in kPCr and in the number of steps (although this is strongly influenced by an apparent outlier; difference in the number of steps was not significant after the outlier was removed); Figure 2 shows mean and individual values of kPCr,
L and at Peak exercise, in baseline heart rate, in kPCr and in the number of steps (although this is strongly influenced by an apparent outlier; difference in the number of steps was not significant after the outlier was removed); Figure 2 shows mean and individual values of kPCr, 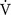 o2 at
o2 at  L and
L and 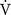 o2 at Peak, while Figure 3 shows the mean number of daily steps.
o2 at Peak, while Figure 3 shows the mean number of daily steps. 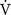 E/
E/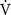 co2 and work rates at
co2 and work rates at  L and Peak exercise did not change. No significant relationship was found between the change in
L and Peak exercise did not change. No significant relationship was found between the change in 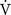 o2 at
o2 at  L and change in haemoglobin (r = 0.27; p = 0.396).
L and change in haemoglobin (r = 0.27; p = 0.396).
Table 3. Patient data at Baseline and post-NACRT.
| Baseline | Post-NACRT | Mean difference | P | |
| mean (SD) | mean (SD) | (95% CI) | ||
| kPCr 1 (min−1) | 1.5 (0.5) | 1.1 (0.3) | −0.4 (−0.5, −0.2) | 0.001 |
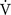 o2 at o2 at  L (ml.kg−1.min−1)
L (ml.kg−1.min−1) |
12.3 (3.4) | 9.9 (2.2) | −2.4 (−3.8, −0.9) | 0.004 |
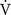 o2 at o2 at  L (L.min−1)
L (L.min−1) |
0.9 (0.3) | 0.7 (0.2) | −0.2 (−0.1, −0.3) | 0.003 |
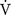 o2 Peak (ml.kg−1.min−1) o2 Peak (ml.kg−1.min−1) |
18.7 (6.1) | 14.7 (4.0) | −4.0 (−6.8, −1.1) | 0.011 |
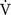 o2 Peak (L.min−1) o2 Peak (L.min−1) |
1.4 (0.5) | 1.1 (0.3) | −0.3 (−0.1, −0.5) | 0.013 |
O2 pulse at  L (ml.beat−1)
L (ml.beat−1) |
8.9 (2.9) | 7.7 (2.2) | −1.2 (−0.2, −2.1) | 0.022 |
| O2 pulse at Peak (ml.beat−1) | 10.5 (3.6) | 8.7 (2.2) | −1.8 (−0.3, −3.3) | 0.024 |
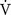 E/
E/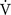 co2 at co2 at  L
L
|
34.5 (6.4) | 34.2 (4.3) | −0.3 (2.5, −3.1) | 0.825 |
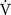 E/
E/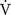 co2 at Peak co2 at Peak |
35.9 (4.6) | 37.1 (4.8) | 0.3 (−3.7, 1.4) | 0.080 |
| Average number of steps | 5352 (3913) | 3725 (2217) | −1627 (−59, 3195) | 0.039 |
Abbreviations: kPCr, post-exercise phosphocreatine recovery rate constant; 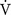 o2 at
o2 at  L, oxygen uptake at estimated anaerobic threshold;
L, oxygen uptake at estimated anaerobic threshold; 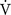 o2 Peak, oxygen uptake at peak exercise; O2 pulse at
o2 Peak, oxygen uptake at peak exercise; O2 pulse at  L, oxygen pulse at estimated anaerobic threshold; O2 pulse at Peak, oxygen pulse at peak exercise;
L, oxygen pulse at estimated anaerobic threshold; O2 pulse at Peak, oxygen pulse at peak exercise; 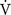 E/
E/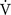 co2 at
co2 at  L, ventilatory equivalents for carbon dioxide at estimated anaerobic threshold;
L, ventilatory equivalents for carbon dioxide at estimated anaerobic threshold; 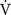 E/
E/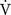 co2 at
co2 at  L, ventilatory equivalents for carbon dioxide at peak exercise; Work rate at
L, ventilatory equivalents for carbon dioxide at peak exercise; Work rate at  L, work rate at estimated anaerobic threshold; Work rate at Peak, work rate at peak exercise.
L, work rate at estimated anaerobic threshold; Work rate at Peak, work rate at peak exercise.
Figure 2.
31P MRS (kPCr) and CPET (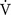 o2 at
o2 at  L and
L and 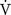 o2 at Peak) data at Baseline (before NACRT)) and at 48±5 hours post-NACRT: lines link data-points (closed circles) for individual patients, and open circles show overall mean±SEM. Mean changes (SEM) between baseline and post-NACRT are for kPCr −0.4(0.1) min−1, p = 0.001; for
o2 at Peak) data at Baseline (before NACRT)) and at 48±5 hours post-NACRT: lines link data-points (closed circles) for individual patients, and open circles show overall mean±SEM. Mean changes (SEM) between baseline and post-NACRT are for kPCr −0.4(0.1) min−1, p = 0.001; for 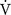 o2 at
o2 at  L −2.4(0.7) ml.kg−1.min−1, p = 0.004; and for
L −2.4(0.7) ml.kg−1.min−1, p = 0.004; and for 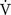 o2 Peak −4.0(1.3) ml.kg−1.min−1, p = 0.011.
o2 Peak −4.0(1.3) ml.kg−1.min−1, p = 0.011.
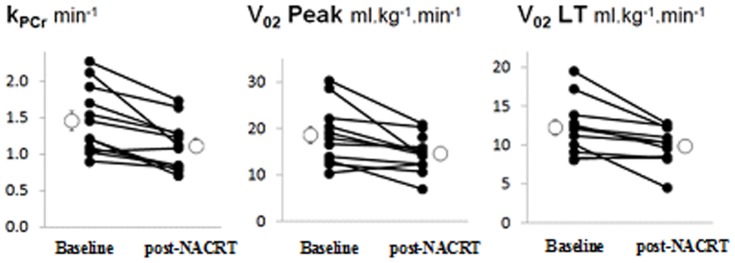
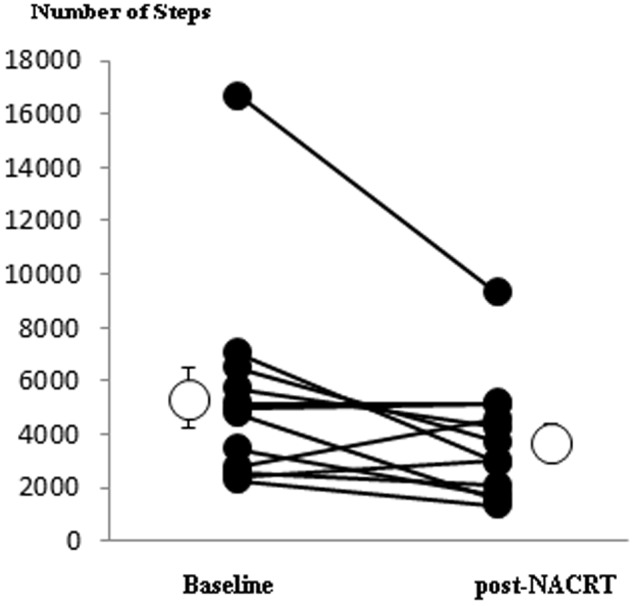
Figure 3. Averaged number of steps at Baseline (before NACRT)) and at 48±5 hours post-NACRT: lines link data-points (closed circles) for individual patients, and open circles show overall mean±SEM. Mean change (SEM) between baseline and post-NACRT is −1627(712) steps, p = 0.039.
Discussion
This is the first study to identify mitochondrial abnormalities accompanying changes in physical fitness following neoadjuvant chemoradiotherapy. The benefits of NACRT for locally advanced rectal cancer are improved local disease control and possibly overall and cancer-specific survival; however its effect on objectively measured physical fitness has not been measured, nor has the mechanism of this been explored in vivo.
This pilot study shows a significant reduction in both whole-body physical fitness measured by CPET ( o2 at
o2 at  L −2.36 ml.kg−1.min−1,
L −2.36 ml.kg−1.min−1,  o2 at Peak −3.95 ml.kg−1.min−1) and in vivo muscle mitochondrial function kPCr (−0.34 min−1) between baseline and post-NACRT. We also found a significant decline in PA with NACRT (−1627 steps). This acute decline in mitochondrial function may account for the rapid loss in fitness and activity over the neoadjuvant treatment period, clinically important in the context of fitness for surgery and perioperative risk however causality cannot be established as controlling for PA in a clinical setting is difficult, and statistical adjustment for PA in a small patient group is not feasible. These findings however illuminate a potential mechanistic link that might be contributing to the changes in objectively measured whole body physical fitness with NACRT, consistent with the results of our earlier pilot study [21].
o2 at Peak −3.95 ml.kg−1.min−1) and in vivo muscle mitochondrial function kPCr (−0.34 min−1) between baseline and post-NACRT. We also found a significant decline in PA with NACRT (−1627 steps). This acute decline in mitochondrial function may account for the rapid loss in fitness and activity over the neoadjuvant treatment period, clinically important in the context of fitness for surgery and perioperative risk however causality cannot be established as controlling for PA in a clinical setting is difficult, and statistical adjustment for PA in a small patient group is not feasible. These findings however illuminate a potential mechanistic link that might be contributing to the changes in objectively measured whole body physical fitness with NACRT, consistent with the results of our earlier pilot study [21].
Cancer-induced cachexia can cause major loss of skeletal muscle, resulting in fatigue and higher mortality [31],[32]. In our cohort cancer progression is not a contributing factor as tumours were downstaged. Furthermore BMI and weight remained stable. The small but statistically significant fall in haemoglobin is unlikely to be functionally relevant, and showed no correlation with the CPET or 31P MRS changes.
Several publications postulate mechanisms by which chemotherapy may contribute to skeletal muscle dysfunction [33]. Oxidative damage [34] resulting from doxorubicin-based chemotherapy in haematological malignancies causes sarcopaenia [35], up-regulation of E3 ubiquitin-ligase/MAFbx [35] and mitochondrial death [36]. Drugs with a quinone moiety can directly interact with oxygen to generate reactive oxygen species (ROS) causing oxidative stress-mediated injury to cardiac muscle, kidneys and brain tissue [37], while other chemotherapeutic agents decrease antioxidant levels [37]; however these chemotherapy regimens were not used in this cohort. At subcellular levels, mitochondria are major targets for chemotherapy-induced oxidative stress [36]. Chemotherapy is known to affect cardiorespiratory (causing exercise intolerance) [38] and microcirculatory function [39], PA [33], but these multifactorial physiological mechanisms remain elusive.
Mitochondrial function measured by 31P MRS is impaired in a variety of chronic diseases, as well as primary mitochondrial disease. In peripheral arterial occlusive disease [30], [40] this mainly reflects impaired O2 delivery to the muscle. In cardiac failure [41] and COPD [42], [43] it is likely to reflect a multifactorial pathology including reduced PA, mitochondrial density [44] and ROS mechanisms. Similar mechanisms might mediate the decline in physical fitness in our cohort. The acute decline in PA seen might also contribute to changes in mitochondrial function and physical fitness. A better understanding and quantification of the potential mechanisms involved if oxidative injury is present in this patient cohort is essential to design intervention strategies that will attenuate the toxicity of chemoradiotherapy agents without compromising their anticancer effects [36]
Our findings have potential clinical implications because reduced physical fitness is associated with increased perioperative morbidity and mortality after major intra-abdominal surgery, especially colorectal surgery [14], [15], [45] Our data provides the first direct evidence that the benefits of NACRT in tumour downsizing may be at least partly offset by increased perioperative risk as a result of reduced physical fitness, related to reduced skeletal muscle mitochondrial function and activity however causality cannot be established. This proposed mechanism merits further investigation, as does the possibility of preventive interventions e.g. by exercise training during the pre-operative period (currently the focus of our published exercise training study [46]). This is an interventional pilot study in the same patient cohort scheduled to undergo standardised NACRT and a 6-week structured, tailored exercise training programme (exercise group n = 22) or a control period (n = 13). Here 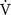 o2 at
o2 at  L significantly reduced between baseline and post-NACRT (−1.9 ml.kg−1.min−1). In the exercise group
L significantly reduced between baseline and post-NACRT (−1.9 ml.kg−1.min−1). In the exercise group 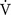 o2 at
o2 at  L significantly improved between post-NACRT and week 6 post-NACRT (+2.1 ml.kg−1.min−1) whereas control group values were unchanged (−0.7 ml.kg−1.min−1). Furthermore a randomised controlled study investigating the potential benefits in physical fitness and quality of life of a 9-week structured responsive endurance training programme following NACRT prior to elective rectal cancer surgery (PB-PG-0711-25093) is currently recruiting.
L significantly improved between post-NACRT and week 6 post-NACRT (+2.1 ml.kg−1.min−1) whereas control group values were unchanged (−0.7 ml.kg−1.min−1). Furthermore a randomised controlled study investigating the potential benefits in physical fitness and quality of life of a 9-week structured responsive endurance training programme following NACRT prior to elective rectal cancer surgery (PB-PG-0711-25093) is currently recruiting.
This study demonstrates an acute decline in objectively-measured physical fitness, activity as well as a decline in mitochondrial function using validated and robust methodology. Particular strengths of our study are the low risk of confounding by indication [47], the blinded physiological evaluations, the standardization of the NACRT and the homogenous cancer cohort. Limitations lie in the observational design, the small sample size of what was designed as a pilot study and that no adjusting for multiple testing was performed.
In conclusion, NACRT before major rectal cancer surgery significantly reduces physical fitness objectively assessed by CPET and muscle mitochondrial function assessed by 31P MRS. The existence of abnormalities at both skeletal-muscle and whole-body level may perhaps suggest a potential mechanistic relationship in NACRT which merits further investigation, which could potentially inform development of tailored interventions in the perioperative period to improve both physical fitness and mitochondrial function in patients with operable rectal cancer.
Supporting Information
TREND statement checklist.
(PDF)
Study protocol for patients consented to this trial.
(DOCX)
Acknowledgments
Aintree University Hospitals Colorectal Multi Disciplinary Team; Institute of Ageing and Chronic Disease, University of Liverpool; University Southampton NHS Foundation Trust - University of Southampton NIHR Respiratory Biomedical Research Unit.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. Data will be made available on Clinical trials.gov NCT01859442 http://clinicaltrials.gov/show/NCT01859442.
Funding Statement
The research was funded in part from the British Oxygen Company Chair of the Royal College of Anaesthetists (MPWG), awarded by the National Institute of Academic Anaesthesia. Some of this work was undertaken at University Southampton NHS Foundation Trust - University of Southampton NIHR Respiratory Biomedical Research Unit which received a portion of funding from the UK Department of Health Research Biomedical Research Units funding scheme. All funding was unrestricted. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Statistical Bulletin Cancer registrations in England (2010), 1–11. Available: http://www.ons.gov.uk/ons/search/index.html?newquery=cancer+registrations. Accessed 03/03/2014
- 2.Statistical Bulletin Cancer Incidence and Mortality in the United Kingdom, 2008-10 (2012), 1–24. Available http://www.ons.gov.uk/ons/search/index.html?newquery=cancer+registrations. Accessed 03/03/2014
- 3.Association of Coloproctology of Great Britain and Ireland. National Bowel Cancer Audit Annual Report (2013) Available: http://www.acpgbi.org.uk/content/uploads/Bowel-Cancer-Audit-2013_INTERACTIVE-PDF_01-07-13.pdf. Accessed 03/03/2014
- 4. Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U, et al. (2004) Operative Versus Nonoperative Treatment for Stage 0 Distal Rectal Cancer Following Chemoradiation Therapy. Ann Surg 240(4):711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chau I, Brown G, Cunningham D, Tait D, Wotherspoon A, et al. (2006) Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J Clin Oncol 24(4):668–674. [DOI] [PubMed] [Google Scholar]
- 6. Mohiuddin M, Winter K, Mitchell E, Hanna N, Yuen A, et al. (2006) Randomized phase II study of neoadjuvant combined-modality chemoradiation for distal rectal cancer: Radiation Therapy Oncology Group Trial 0012. J Clin Oncol 24(4):650–655. [DOI] [PubMed] [Google Scholar]
- 7. Bosset JF, Collette L, Calais G, Mineur L, Maingon P, et al. (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355(11):1114–1123. [DOI] [PubMed] [Google Scholar]
- 8. Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, et al. (2006) Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol 24(28):4620–4625. [DOI] [PubMed] [Google Scholar]
- 9. Lai CW, Minto G, Challand CP, Hosie KB, Sneyd JR, et al. (2013) Patients' inability to perform a preoperative cardiopulmonary exercise test or demonstrate an anaerobic threshold is associated with inferior outcomes after major colorectal surgery. Br J Anaesth 111(4):607–611. [DOI] [PubMed] [Google Scholar]
- 10. Wilson RJT, Davies S, Yates D, Redman J, Stone M (2010) Impaired functional capacity is associated with all-cause mortality after major elective intra-abdominal surgery. Br J Anaesth 105(3):297–303. [DOI] [PubMed] [Google Scholar]
- 11. Snowden CP, Prentis J, Jacques B, Anderson H, Manas D, et al. (2013) Cardiorespiratory fitness predicts mortality and hospital length of stay after major elective surgery in older people. Ann Surg 257(6):999–1004. [DOI] [PubMed] [Google Scholar]
- 12. West M, Jack S, Grocott MPW (2011) Perioperative cardiopulmonary exercise testing in the elderly. Best Pract Res Clin Anaesthesiol 25(3):427–437. [DOI] [PubMed] [Google Scholar]
- 13. Carlisle J, Swart M (2007) Mid-term survival after abdominal aortic aneurysm surgery predicted by cardiopulmonary exercise testing. Br J Surg 94(8):966–969. [DOI] [PubMed] [Google Scholar]
- 14. West MA, Lythgoe D, Barben CP, Noble L, Kemp GJ, et al. (2014) Cardiopulmonary exercise variables are associated with postoperative morbidity after major colonic surgery: a prospective blinded observational study. Br J Anaesth 112(4):665–671. [DOI] [PubMed] [Google Scholar]
- 15. Hennis PJ, Meale PM, Grocott MPW (2011) Cardiopulmonary exercise testing for the evaluation of perioperative risk in non-cardiopulmonary surgery. Postgr Med J 87(1030):550–557. [DOI] [PubMed] [Google Scholar]
- 16. Finan P, Greenaway K (2012) Association of Coloproctology of Great Britain and Ireland. National Bowel Cancer Audit Annual Report 47–59. [Google Scholar]
- 17. Swellengrebel HAM, Marijnen CAM, Verwaal VJ, Vincent A, Heuff G, et al. (2011) Toxicity and complications of preoperative chemoradiotherapy for locally advanced rectal cancer. Br J Surg 98(3):418–26. [DOI] [PubMed] [Google Scholar]
- 18. Marijen C, Kapiteijn E, vab de Velde C, Martijn H (2002) Acute side effects and complications after short-term preoperative radiotherapy combined with total mesorectal excision in primary rectal cancer: Report of a mulitcenter randomized trial. J Clin Oncol 20(3):817–25. [DOI] [PubMed] [Google Scholar]
- 19. Jones LW, Eves ND, Haykowsky M, Joy AA, Douglas PS (2008) Cardiorespiratory exercise testing in clinical oncology research: systematic review and practice recommendations. Lancet Oncol 9(8):757–65. [DOI] [PubMed] [Google Scholar]
- 20. Jack S, West M, Raw D, Marwood S, Ambler G, et al. (2014) The effect of neoadjuvant chemotherapy on physical fitness and survival in patients undergoing oesophagogastric cancer surgery. Eur J Surg Oncol doi:10.1016/j.ejso.2014.03.010 (in press) [DOI] [PubMed] [Google Scholar]
- 21. West M, Loughney L, Barben C, Sripadam R, Kemp G, et al. (2014) The effects of neoadjuvant chemoradiotherapy on physical fitness and morbidity in rectal cancer surgery patients. Eur J Surg Oncol doi:10.1016/j.ejso.2014.03.021 (in press) [DOI] [PubMed] [Google Scholar]
- 22. Kemp GJ, Brindle KM (2012) What do magnetic resonance-based measurements of Pi→ATP flux tell us about skeletal muscle metabolism? Diabetes 61(8):1927–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cannon DT, Howe FA, Whipp BJ, Ward SA, McIntyre DJ, et al. (2013) Muscle metabolism and activation heterogeneity by combined 31P chemical shift and T2 imaging, and pulmonary O2 uptake during incremental knee-extensor exercise. J Appl Physiol 115(6):839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Praet SFE, De Feyter HMM, Jonkers RAM, Nicolay K, van Pul C, et al. (2006) 31P MR spectroscopy and in vitro markers of oxidative capacity in type 2 diabetes patients. Magn Reson Mater Phy 19(6):321–331. [DOI] [PubMed] [Google Scholar]
- 25. Larson-Meyer D, Newcomer B, Hunter G, Joanisse D, Weinsier RL, et al. (2001) Relationship between in vivo and in vitro measurements of skeletal musle oxidative metabolism. Muscle Nerve 24:1665–1676. [DOI] [PubMed] [Google Scholar]
- 26. McCully KK, Turner TN, Langley J, Zhao Q (2009) The reproducibility of measurements of intramuscular magnesium concentrations and muscle oxidative capacity using 31P MRS. Dyn Med 8(5):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sobin LH, Fleming ID (1997) TNM Classification of Malignant Tumors,. Fifth Edition Cancer. 80(9):1803–1804. [DOI] [PubMed] [Google Scholar]
- 28. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, et al. (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5(6):649–655. [PubMed] [Google Scholar]
- 29. MacFarlane JK, Ryall RDH, Heald RJ (1993) Mesorectal excision for rectal cancer. Lancet 20 341(8843):457–60. [DOI] [PubMed] [Google Scholar]
- 30. Kemp GJ, Roberts N, Bimson WE, Bakran A, Harris PL, et al. (2001) Mitochondrial function and oxygen supply in normal and in chronically ischemic muscle: a combined 31P magnetic resonance spectroscopy and near infrared spectroscopy study in vivo. J Vasc Surg 34(6):1103–1110. [DOI] [PubMed] [Google Scholar]
- 31. Tisdale MJ (2001) Cancer anorexia and cachexia. Nutrition 17(5):438–442. [DOI] [PubMed] [Google Scholar]
- 32. Gilliam LAA, St Clair DK, Clair DKS (2011) Chemotherapy-Induced Weakness and Fatigue in Skeletal Muscle: The Role of Oxidative Stress. Antioxid Redox Signal 15(9):2543–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Powers SK, Jackson MJ (2010) Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol Rev 88(4):1243–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tozer RG, Tai P, Falconer W, Ducruet T, Karabadjian A, et al. (2008) Cysteine-rich protein reverses weight loss in lung cancer patients receiving chemotherapy or radiotherapy. Antioxid Redox Signal 10(2):395–402. [DOI] [PubMed] [Google Scholar]
- 35. Yamamoto Y, Hoshino Y, Ito T, Nariai T, Mohri T, et al. (2008) Atrogin-1 ubiquitin ligase is upregulated by doxorubicin via p38-MAP kinase in cardiac myocytes. Cardiovasc Res 79(1):89–96. [DOI] [PubMed] [Google Scholar]
- 36. Chen Y, Jungsuwadee P, Vore M, Butterfield D, St Clair D (2007) Collateral damage in cancer chemotherapy: Oxidative stress in nontargeted tissues. Mol Interv 7:147–156. [DOI] [PubMed] [Google Scholar]
- 37. Ladas EJ, Jacobson JS, Kennedy DD, Teel K, Fleischauer A, et al. (2004) Antioxidants and cancer therapy: a systematic review. J Clin Oncol 22(3):517–528. [DOI] [PubMed] [Google Scholar]
- 38. Jones LW, Eves ND, Haykowsky M, Freedland SJ, Mackey JR (2009) Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol 10(6):598–605. [DOI] [PubMed] [Google Scholar]
- 39. Karvunidis T, Chvojka J, Lysak D, Sykora R, Krouzecky A, et al. (2012) Septic shock and chemotherapy-induced cytopenia: effects on microcirculation. Intensive Care Med 38(8):1336–1344. [DOI] [PubMed] [Google Scholar]
- 40. Pipinos II, Shepard AD, Anagnostopoulos PV, Katsamouris A, Boska MD (2000) Phosphorus 31 nuclear magnetic resonance spectroscopy suggests a mitochondrial defect in claudicating skeletal muscle. J Vasc Surg 31(5):944–952. [DOI] [PubMed] [Google Scholar]
- 41. Massie BM, Conway M, Yonge R, Frostick S, Sleight P, et al. (1987) 31P nuclear magnetic resonance evidence of abnormal skeletal muscle metabolism in patients with congestive heart failure. Am J Cardiol 60(4):309–315. [DOI] [PubMed] [Google Scholar]
- 42. Puente-Maestu L, Lázaro A, Humanes B (2013) Metabolic derangements in COPD muscle dysfunction. J Appl Physiol 114(9):1282–1290. [DOI] [PubMed] [Google Scholar]
- 43. Puente-Maestu L, Tejedor A, Lázaro A, de Miguel J, Alvarez-Sala L, et al. (2012) Site of mitochondrial reactive oxygen species production in skeletal muscle of chronic obstructive pulmonary disease and its relationship with exercise oxidative stress. Am J Respir Cell Mol Biol 47(3):358–362. [DOI] [PubMed] [Google Scholar]
- 44. Gosker HR, Hesselink MKC, Duimel H, Ward KA, Schols MWJ (2007) Reduced mitochondrial density in the vastus lateralis muscle of patients with COPD. Eur Respir J 30(1):73–79. [DOI] [PubMed] [Google Scholar]
- 45. West MA, Parry M, Lythgoe D, Barben C, Kemp G, et al. (2014) Cardiopulmonary exercise variables are associated with postoperative morbidity after rectal cancer surgery. Br J Surg 101(9):1166–72. [DOI] [PubMed] [Google Scholar]
- 46. West M, Loughney L, Lythgoe D, Barben C, Sripadam R, et al. (2014) The effect of prehabilitation on objectively measured physical fitness following neoadjuvant treatment in preoperative rectal cancer patients – a blinded interventional pilot study. Br J Anaesth. in press. [DOI] [PubMed] [Google Scholar]
- 47. Grocott MPW, Pearse RM (2010) Prognostic studies of perioperative risk: robust methodology is needed. Br J Anaesth 105(3):243–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TREND statement checklist.
(PDF)
Study protocol for patients consented to this trial.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. Data will be made available on Clinical trials.gov NCT01859442 http://clinicaltrials.gov/show/NCT01859442.