Abstract
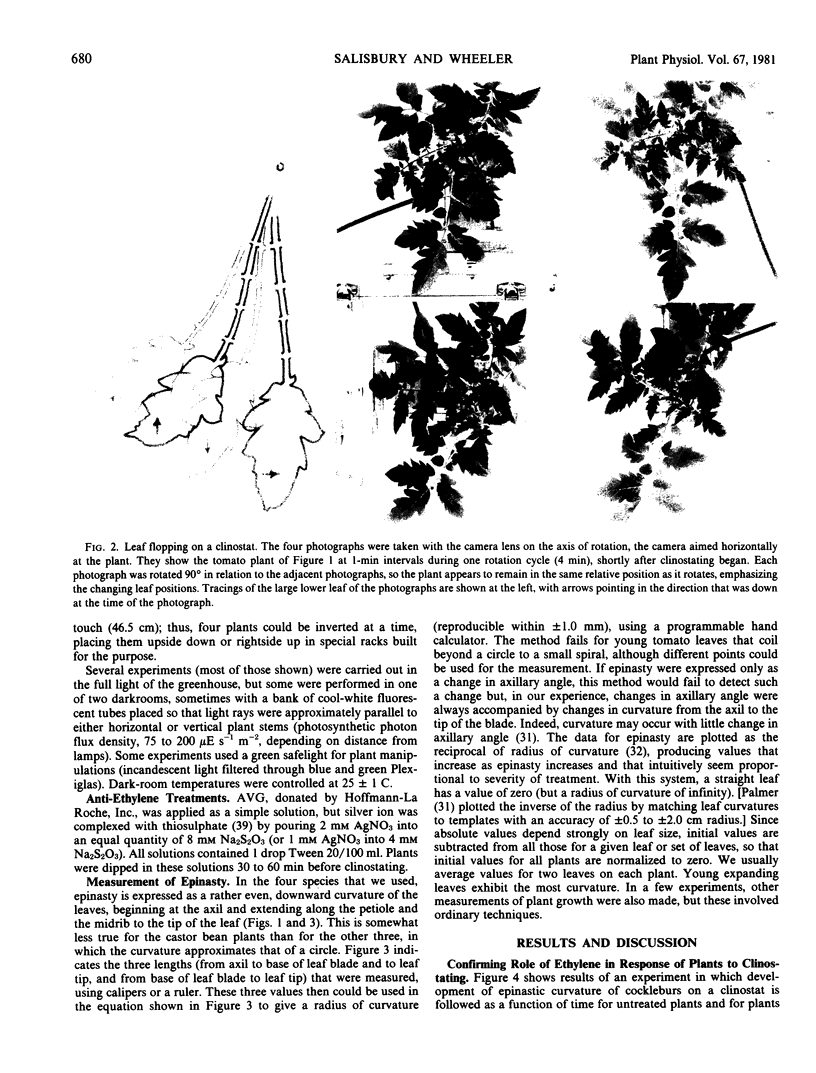
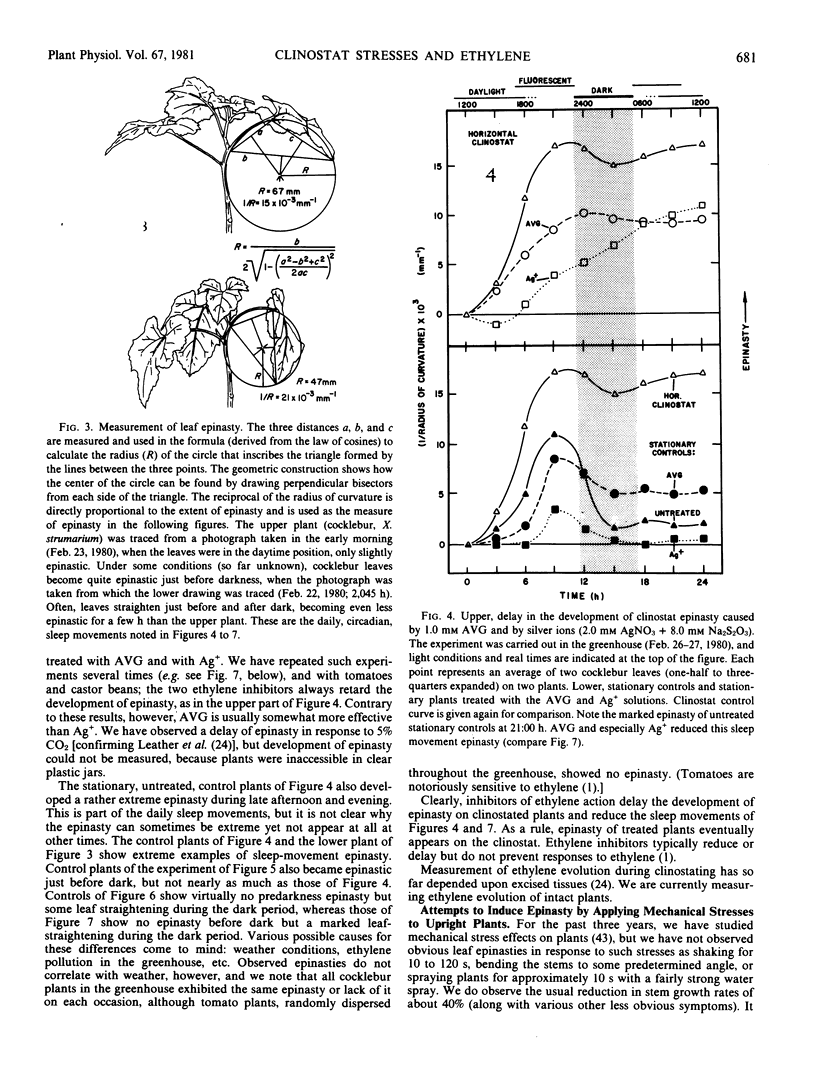
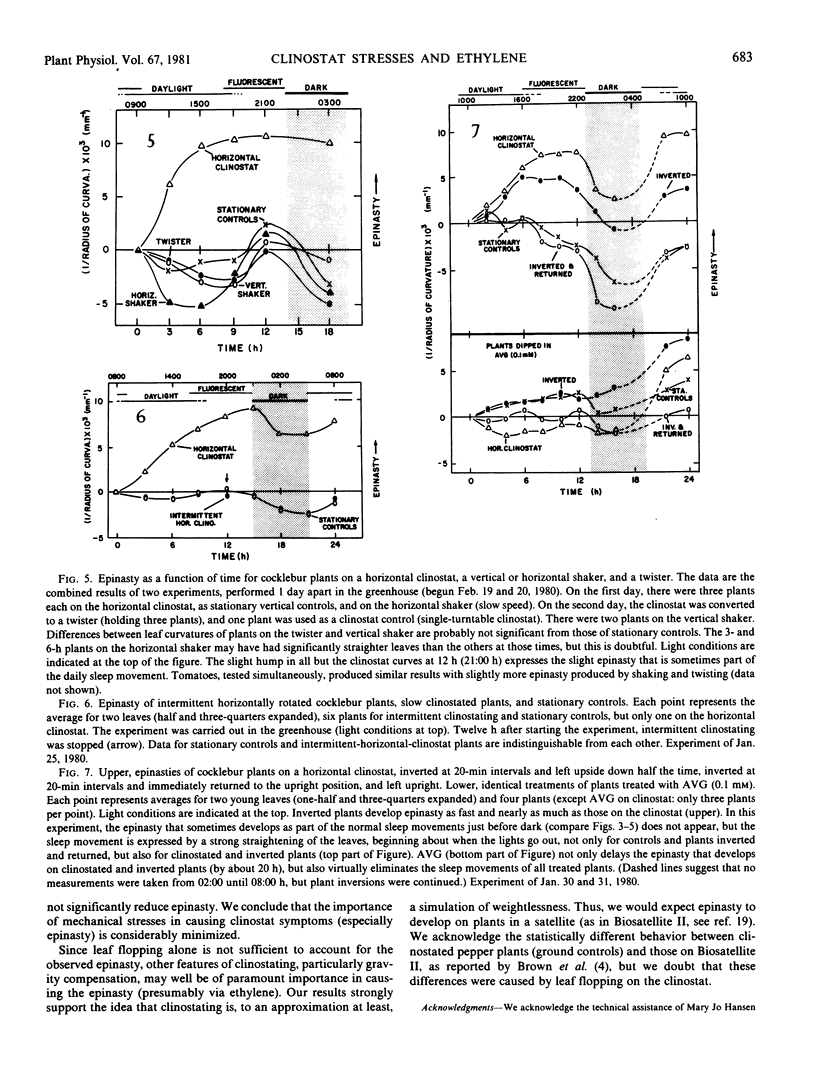
The severe epinasty and other symptoms developed by clinostated leafy plants could be responses to gravity compensation and/or the mechanical stresses of leaf flopping. Epinasty in cocklebur (Xanthium strumarium L.), tomato (Lycopersicon esculentum Mill.), and castor bean (Ricinus communis L.) is delayed by inhibitors of ethylene synthesis and action (aminoethoxyvinylglycine and Ag+), confirming the role of ethylene in clinostat epinasty. To test the possibility that clinostat mechanical stresses (leaf flopping) cause ethylene production and, thus, epinasty, vertical plants were stressed with constant, gentle, horizontal, or vertical shaking or with a quick, back-and-forth rotation (twisting). Clinostat leaf flopping was closely approximated but with a minimum of gravity compensation, by turning plants so their stems were horizontal, rotating them quickly about the stem axis, and then returning them to the vertical, repeating the treatment every four minutes (clinostat rotation time). None of these mechanical stresses produced significant epinasties, but vigorous hand-shaking (120 seconds per day) generated minor epinasties, as did Ag+ applied daily (concentrations high enough to cause leaf browning). Plants gently inverted every 20 minutes developed epinasty at about the same rate and to about the same extent as clinostated plants, but plants inverted every 20 minutes and immediately returned to the upright position did not become epinastic. It is concluded that clinostat epinasty is probably caused by disturbances in the gravity perception mechanism, rather than by leaf flopping.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer E. M. Effect of silver ion, carbon dioxide, and oxygen on ethylene action and metabolism. Plant Physiol. 1979 Jan;63(1):169–173. doi: 10.1104/pp.63.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. H., Dahl A. O., Chapman D. K. Limitation on the use of the horizontal clinostat as a gravity compensator. Plant Physiol. 1976 Aug;58(2):127–130. doi: 10.1104/pp.58.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. H., Dahl A. O., Chapman D. K. Morphology of Arabidopsis Grown under Chronic Centrifugation and on the Clinostat. Plant Physiol. 1976 Mar;57(3):358–364. doi: 10.1104/pp.57.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedolph R. R., Naqvi S. M., Gordon S. A. Effect of Gravity Compensation on the Geotropic Sensitivity of Avena Seedlings. Plant Physiol. 1965 Sep;40(5):961–965. doi: 10.1104/pp.40.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedolph R. R., Naqvi S. M., Gordon S. A. Role of Indole-3-acetic Acid in Modification of Geotropic Responses in Clinostat Rotated Avena Seedlings. Plant Physiol. 1966 May;41(5):897–902. doi: 10.1104/pp.41.5.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedolph R. R., Oemick D. A., Wilson B. R., Smith G. R. Causal basis of gravity stimulus nullification by clinostat rotation. Plant Physiol. 1967 Oct;42(10):1373–1383. doi: 10.1104/pp.42.10.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshizaki T. Influence of gravitational forces on plants. Environ Biol Med. 1973;2(2):47–79. [PubMed] [Google Scholar]
- Leather G. R., Forrence L. E. Increased Ethylene Production during Clinostat Experiments May Cause Leaf Epinasty. Plant Physiol. 1972 Feb;49(2):183–186. doi: 10.1104/pp.49.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon C. J. Choice of rotation rate for the horizontal clinostat. Plant Physiol. 1970 Sep;46(3):355–358. doi: 10.1104/pp.46.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon C. J. Lateral transport of auxin mediated by gravity in the absence of special georeceptor tissue. Plant Physiol. 1971 Nov;48(5):642–644. doi: 10.1104/pp.48.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe F. C. LIMITATIONS OF THE KLINOSTAT AS AN INSTRUMENT FOR SCIENTIFIC RESEARCH. Science. 1904 Sep 16;20(507):376–379. doi: 10.1126/science.20.507.376-b. [DOI] [PubMed] [Google Scholar]
- Shen-Miller J., Gordon S. A. Gravitational compensation and the phototropic response of oat coleoptiles. Plant Physiol. 1967 Mar;42(3):352–360. doi: 10.1104/pp.42.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver I. L. The dynamics of a discrete geotropic sensor subject to rotation-induced gravity compensation. J Theor Biol. 1976 Sep 21;61(2):353–362. doi: 10.1016/0022-5193(76)90023-0. [DOI] [PubMed] [Google Scholar]
- Sobick V., Sievers A. Responses of roots to simulated weightlessness on the fast-rotating clinostat. Life Sci Space Res. 1979;17:285–290. doi: 10.1016/b978-0-08-023416-8.50042-8. [DOI] [PubMed] [Google Scholar]
- Tibbitts T. W., Hertzberg W. M. Growth and epinasty of marigold plants maintained from emergence on horizontal clinostats. Plant Physiol. 1978 Feb;61(2):199–203. doi: 10.1104/pp.61.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor T. S., Vanderhoef L. N. Mechanical inhibition of hypocotyl elongation induces radial enlargement: implications for cytokinin action. Plant Physiol. 1975 Dec;56(6):845–846. doi: 10.1104/pp.56.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler R. M., Salisbury F. B. Gravitropism in Higher Plant Shoots: I. A ROLE FOR ETHYLENE. Plant Physiol. 1981 Apr;67(4):686–690. doi: 10.1104/pp.67.4.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]





