Abstract
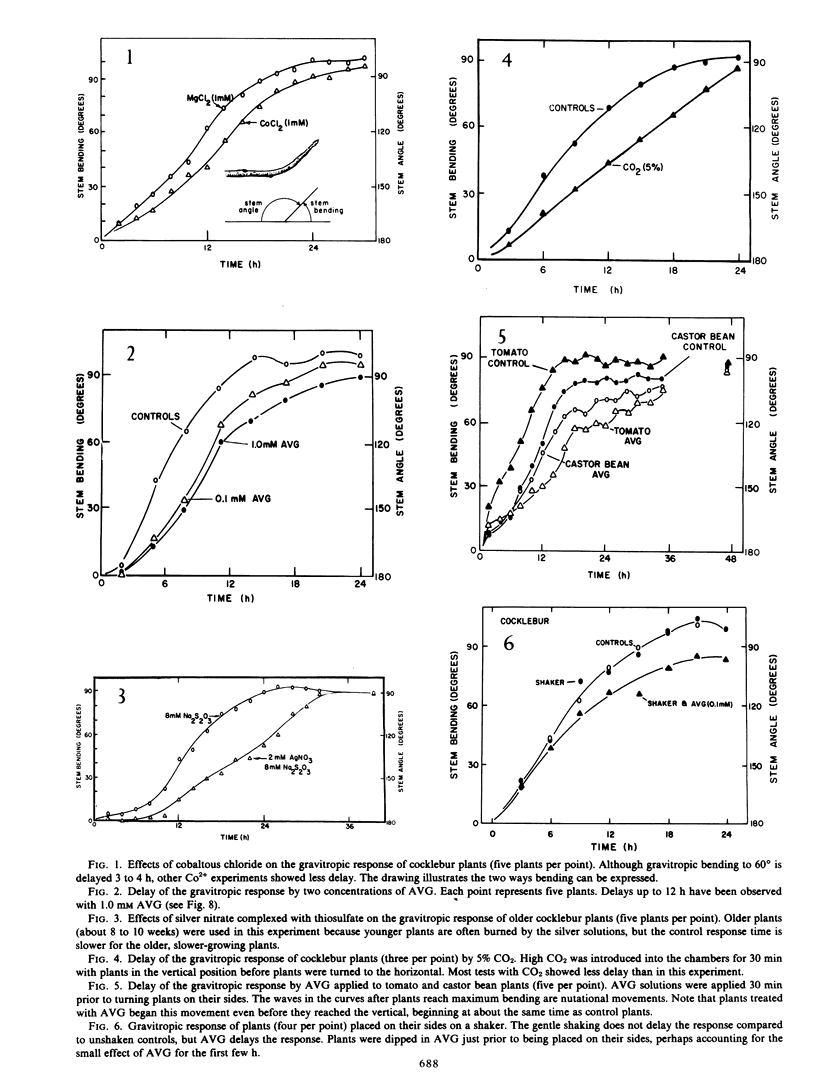
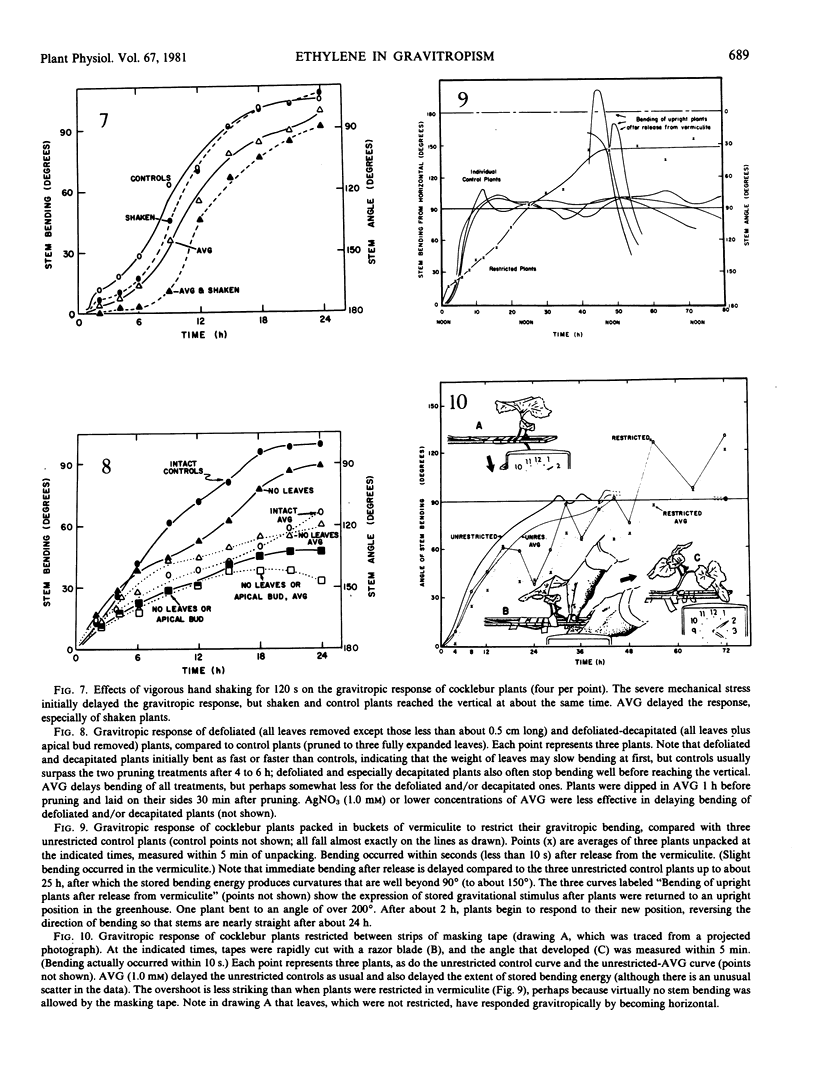
It has long been known that applied ethylene can redirect the gravitropic response, but only occasionally has it been suggested that ethylene normally plays a role in gravitropism. Two inhibitors of ethylene synthesis [Co2+ and aminoethoxyvinylglycine (AVG)] and two inhibitors of ethylene action (Ag+ and CO2) were shown to delay the gravitropic response of cocklebur (Xanthium strumarium L.), tomato (Lycopersicon esculentum Mill.), and castor bean (Ricinus communis L.) stems. Gentle shaking on a mechanical shaker does not inhibit the gravitropic response, but vigorous hand shaking for 120 seconds delays the response somewhat. AVG and Ag+ further delay the response of mechanically stimulated plants. AVG delays the response of defoliated and of decapitated plants. Plants laid on their side and restricted so that they cannot bend upward store both bending energy and gravitropic stimulus; they bend immediately when released from restriction (stored energy) and continue to bend for some hours after (stored stimulus). AVG retards the storage of bending energy but not of stimulus. In gravitropism, graviperception may first stimulate ethylene evolution, which may then influence bending directly, or responses involving ethylene could be more indirect.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B., Rubinstein B. Regulation of Ethylene Evolution and Leaf Abscission by Auxin. Plant Physiol. 1964 Nov;39(6):963–969. doi: 10.1104/pp.39.6.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. E., Lieberman M., Anderson J. D. Inhibition of ethylene production in fruit slices by a rhizobitoxine analog and free radical scavengers. Plant Physiol. 1978 Jun;61(6):886–888. doi: 10.1104/pp.61.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M. Effect of silver ion, carbon dioxide, and oxygen on ethylene action and metabolism. Plant Physiol. 1979 Jan;63(1):169–173. doi: 10.1104/pp.63.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick A. V., Burg S. P. Regulation of root growth by auxin-ethylene interaction. Plant Physiol. 1970 Feb;45(2):192–200. doi: 10.1104/pp.45.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau O. L., Yang S. F. Inhibition of ethylene production by cobaltous ion. Plant Physiol. 1976 Jul;58(1):114–117. doi: 10.1104/pp.58.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leather G. R., Forrence L. E. Increased Ethylene Production during Clinostat Experiments May Cause Leaf Epinasty. Plant Physiol. 1972 Feb;49(2):183–186. doi: 10.1104/pp.49.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon C. J. Auxin control for orientation of pea roots grown on a clinostat or exposed to ethylene. Plant Physiol. 1972 Sep;50(3):417–420. doi: 10.1104/pp.50.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. H. Failure of Ethylene to Change the Distribution of Indoleacetic Acid in the Petiole of Coleus blumei X frederici during Epinasty. Plant Physiol. 1976 Oct;58(4):513–515. doi: 10.1104/pp.58.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury F. B., Wheeler R. M. Interpreting Plant Responses to Clinostating: I. MECHANICAL STRESSES AND ETHYLENE. Plant Physiol. 1981 Apr;67(4):677–685. doi: 10.1104/pp.67.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart E. R., Freebairn H. T. Ethylene, seed germination, and epinasty. Plant Physiol. 1969 Jul;44(7):955–958. doi: 10.1104/pp.44.7.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbitts T. W., Hertzberg W. M. Growth and epinasty of marigold plants maintained from emergence on horizontal clinostats. Plant Physiol. 1978 Feb;61(2):199–203. doi: 10.1104/pp.61.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]



