Abstract
The transcriptional regulator HAP4, induced by respiratory substrates, is involved in the balance between fermentation and respiration in S. cerevisiae. We identified putative orthologues of the Hap4 protein in all ascomycetes, based only on a conserved sixteen amino acid-long motif. In addition to this motif, some of these proteins contain a DNA-binding motif of the bZIP type, while being nonetheless globally highly divergent. The genome of the yeast Hansenula polymorpha contains two HAP4-like genes encoding the protein HpHap4-A which, like ScHap4, is devoid of a bZIP motif, and HpHap4-B which contains it. This species has been chosen for a detailed examination of their respective properties. Based mostly on global gene expression studies performed in the S. cerevisiae HAP4 disruption mutant (ScΔhap4), we show here that HpHap4-A is functionally equivalent to ScHap4, whereas HpHap4-B is not. Moreover HpHAP4-B is able to complement the H2O2 hypersensitivity of the ScYap1 deletant, YAP1 being, in S. cerevisiae, the main regulator of oxidative stress. Finally, a transcriptomic analysis performed in the ScΔyap1 strain overexpressing HpHAP4-B shows that HpHap4-B acts both on oxidative stress response and carbohydrate metabolism in a manner different from both ScYap1 and ScHap4. Deletion of these two genes in their natural host, H. polymorpha, confirms that HpHAP4-A participates in the control of the fermentation/respiration balance, while HpHAP4-B is involved in oxidative stress since its deletion leads to hypersensitivity to H2O2. These data, placed in an evolutionary context, raise new questions concerning the evolution of the HAP4 transcriptional regulation function and suggest that Yap1 and Hap4 have diverged from a unique regulatory protein in the fungal ancestor.
Introduction
Evolution of transcription factors and their regulatory networks are particularly interesting to study in the Hemiascomycetes. The phylogenetic distances between species within the phylum are equivalent to the evolution of chordates, and the number of available complete genome sequences and large-scale gene expression data sets is the largest among eukaryotes, and increasing. Moreover this phylum includes S. cerevisiae, arguably the most studied eukaryotic organism ([1], [2]). The ecology of yeast species is diverse and these organisms have developed various strategies to compete for nutrient sources and to adapt to stress conditions. While S. cerevisiae is a predominantly fermentative species, most yeasts do not possess such a strong fermentative capacity and are respiratory-fermentative, being able to both respire and ferment in different proportions. Respiratory metabolism is also strongly linked to iron metabolism and redox state maintenance.
The expression of S. cerevisiae genes is finely regulated by the hierarchy of about 200 interplaying and subordinated transcriptional regulators. Global regulators modulate gene expression profiles according to environmental requirements acting directly on promoters of regulated genes, as well as by communication via other transactivators.
One of these key global regulators in S. cerevisiae is Hap4, the transcriptional activator moiety of the CCAAT-binding HAP complex [3] controlling the fermentation/respiration switch [4] [5] [6].
HAP2, HAP3 and HAP5 (encoding the core DNA-binding elements of the complex), are highly conserved in all eukaryotes ([7], reviewed in [8] and [9]), but this is not the case for HAP4, the activator component of the HAP complex which was originally identified only in S. cerevisiae and later in Kluyveromyces lactis. These two proteins share a 16 aminoacid-long N-terminal motif [10]. Based on this motif, called here (N-Hap4), we have found putative orthologues in other ascomycetes [11] and discerned two different subclasses of Hap4 proteins. One subclass (identified mostly among species distant from S. cerevisiae) contains an additional DNA-binding motif which is the basic region (BR) of the bZIP motif (basic leucine zipper; Prosite PS50217) [12]. BZIP motifs are shared by a family of transcriptional regulators whose archetype is S. cerevisiae Yap1, a schematic representation of which is provided Fig 1. Factors which have this BR domain also have a cysteine rich domain (CRD), present in Yap1.
Figure 1. A schematic representation of the Hap4 and Yap1 proteins.
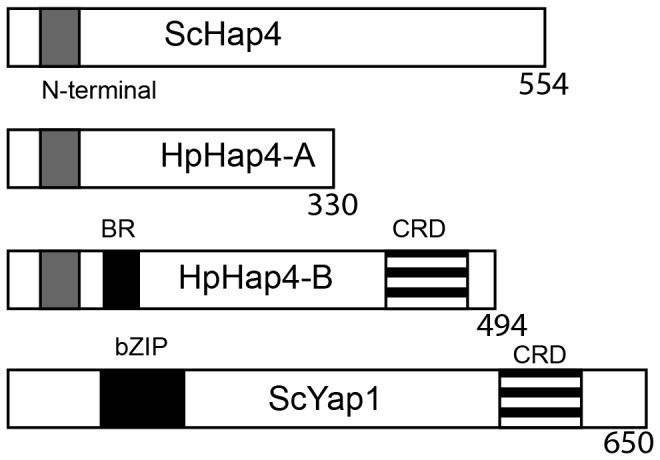
The Saccharomyces cerevisiae Hap4 and Yap1 proteins are schematically represented relative to the two Hansenula polymorpha HpHap4-A and HpHap4-B ones. The main motifs are indicated in grey (N-terminal Hap4), black (bZIP of BR motif; the BR motif is the DNA binding part of the bZIP motif) and striped (CRD or cysteine rich domain).
This prompted us to undertake a detailed study of these two Hap4 types (with and without the bZIP motif) in the yeast Hansenula polymorpha (Taxonomy ID: 870730). Phylogenetically, this is the species closest to S. cerevisiae which contains both Hap4 types. The yeast Hansenula polymorpha is well studied. It possesses unique physiological characteristics (thermo-tolerance, ability to utilise various carbon sources including methanol [13]), and is the model organism to study peroxisome functions [14]. It also presents interesting biotechnological properties for the production of heterologous proteins [15] and the degradation of lignocellulose [16]. Its position in a simplified phylogenetic tree is shown in Fig 2.
Figure 2. A simple phylogenetic tree of the hemiascomycete yeasts.
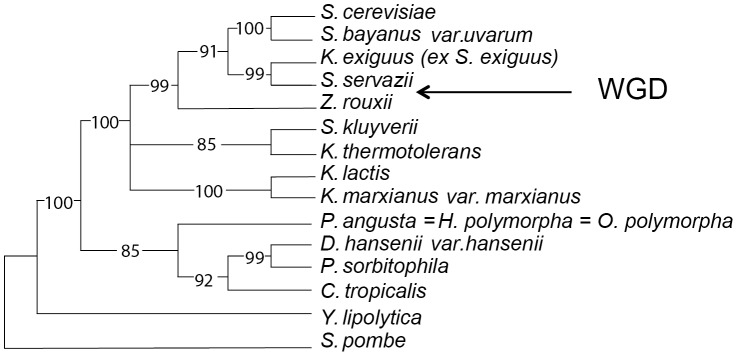
Only a limited number of yeast species are presented which span the complete clade of Hemiascomycete yeasts from S. cerevisiae to Y. lipolytica, S. pombe being the outgroup. H. polymorpha is placed on the tree at the same position as P. angusta which is the anamorphic species. The position of the « Whole Genome Duplication » (WGD) is indicated. The tree is taken from [48].
We previously demonstrated that H. polymorpha HpHap4-A, containing only the N-Hap4 motif, is functionally similar to the S. cerevisiae protein (ScHap4). It restores the ScΔhap4 mutant growth on respiratory substrates, is able to interact with the HAP complex and induces the expression of the Hap4 archetype target CYC1 [17]. We also identified a transactivation domain, different from what was known in S. cerevisiae [11]. Later, we showed that the second Hap4-like protein, HpHap4-B, containing the additional bZIP motif, could act in the same way but to a lesser extent, and that the bZIP motif did not contribute to this ability [12].
A range of Hap4-like proteins were recently reported to participate as repressors in iron homeostasis networks. In Schizosaccharomyces pombe, Php4 (PHP4 is an orthologue of ScHAP4 and does not contain a bZIP motif) acts in iron deficiency conditions as a repressor of genes encoding iron-containing proteins via interactions with the PHP complex (orthologous to ScHAP complex) [18]. PHP4 itself is tightly regulated at transcriptional [19] and post-translational [20] levels according to iron availability. In Aspergillus nidulans, the Hap4-like protein HapX negatively regulates the components of the iron-dependent pathways under iron starvation via its HAP complex [21].
Candida albicans genome contains four different HAP4-like genes. Hap43, the only bZIP containing Hap4-like protein in C. albicans, acts as a transcriptional repressor during iron starvation [22].
Similarly, in H. polymorpha the expression of HpHAP4-B is also induced in conditions of iron chelation and the disruptant strain is sensitive to iron deficiency [12].
This study aims to distinguish between the two types of Hap4-like proteins. Since the H. polymorpha genome sequence was not publicly available when we started this work, we expressed HpHAP4-A and HpHAP4-B in a S. cerevisiae background. Meanwhile, the genome of another H. polymorpha strain was sequenced and is now in open access (http://genome.jgi-psf.org/Hanpo1/Hanpo1.home.html).
In this heterologous system, we clearly observed differences between the two Hap4-like proteins. Moreover, we found that HpHap4-B was also involved in the oxidative stress response, similarly to Yap1. These findings, and the analysis of the presence/absence of the two different motifs in the HAP4-like family of transcriptional factors related to their place in the fungi phylogenetic tree, lead us to propose an evolutionary scenario which is further discussed.
Materials and Methods
Strains and growth conditions
The yeast strains and plasmids used in this study are listed in Table 1. S. cerevisiae strains were grown as previously described [11]. H. polymorpha strains were grown at 37°C in YPD medium (1% yeast extract, 2% peptone, and 1% glucose) or in minimal medium (0.17% w/v yeast nitrogen base without amino acids (Difco) with 0.5% w/v ammonium sulphate as a nitrogen source). Amino acids were added to a final concentration of 50 µg/ml as required. For solid media, agar was added to 2% (w/v) final concentration. Cultivation of Escherichia coli DH5α and standard recombinant DNA techniques were performed essentially as described [23].
Table 1. Strains and plasmids used in this study.
| Strain or plasmid | Genotype or description | Reference or source |
| S. cerevisiae | ||
| W303-1A | MATa ade 2-1 his3-11 leu2-3, 112 trp1-1 ura3-1 | [51] |
| ScΔhap4 | W303-1A Δhap4::kan | This laboratory |
| ScΔyap1 | MATα ade2-1 his3-11 leu2-3,112 trp1-1 ura 3-1 can-100 yap1::HIS3 | [52] |
| H. polymorpha | ||
| NCYC 495 leu1_1 | leu1_1 | [53] |
| NCYC 495 leu1_1 (pYT1) | leu1-1:HpLEU2 | [12] |
| HpΔhap4-A | hap4-AΔ::ScLEU2 | This study |
| HpΔhap4-B | hap4-BΔ::ZeoR | [12] |
| HpΔhap4-AΔhap4-B | hap4-AΔ::ScLEU2 hap4-BΔ::ZeoR | This study |
| Plasmids | ||
| pBFG1 | 2 µm LEU2 pPGK1::3HA | [54] |
| pBFG1-HpHAP4-A | HpHAP4-A in pBFG1 | [11] |
| pBFG1-HpHAP4-B | HpHAP4-B in pBFG1 | [12] |
| pBFG1-HpHAP4-B-bZip | HpHAP4-B with deleted BR motif in pBFG1 | [12] |
| pBFG1-ScYAP1 | ScYAP1 in pBFG1 | [12] |
| pBFG1-ScHAP4 | ScHAP4 in pBFG1 | [11] |
| pYT1 | Complements H. polymorpha leu1_1 | [55] |
| pHap4-A::ScLEU2 | pYT1 HpHAP4-A::ScLEU2 | This study |
| pPICZ-B | Pichia pastoris expression vector | Invitrogen |
| pHap4-B::ZeoR | pPICZ HpHAP4-B::ZeoR | [12] |
Growth sensitivity tests
Dilutions of overnight YPD cultures were plated on minimal glucose medium (W0) with antimycin A (1 µg/ml) or H2O2 (0.5 mM) or minimal xylose medium with 5 mM salicylhydroxamic acid (SHAM) supplemented with necessary amino acids and grown at 37°C for 2 days.
Gel shift experiment
DNA probes were prepared by PCR amplification and end-labelled with polynucleotide kinase and [γ-32P]-ATP. Oligonucleotides used to generate the ARE sequence are P17 (5′-CGACGGCTGCCATTAGTCAGCATGGCGCGCAC-3′) and P18 (5′-GTGCGCGCCATGCTGACTAATGGCAGCCGTCG-3′). Analysis of DNA-binding complexes was performed as previously described by [24].
Construction of the H. polymorpha Δhap4 deletion mutants
H. polymorpha ΔHphap4-A and double ΔHphap4-A ΔHphap4-B deletion mutants were constructed by the gene replacement method with NCYC495 leu1-1 as the parental strain.
A HpHAP4-A deletion cassette was constructed in two steps. First, the HpHAP4-A upstream-flanking fragment of 1,305 bps ending 3 nucleotides upstream of the ATG start codon was isolated by PCR reaction using primers P9 (5′-TGTGGATCCTTCGAACACAAAGCCTAT-3′) and P10 (5′-GGTTCTAGATCATGGAACCCATTGAAT-3′) and H. polymorpha genomic DNA as a template. PCR products were cloned as BamHI-XbaI fragments into plasmid pYT1. This produced the intermediate plasmid pHAP4-A-5′. As a second step, the HpHAP4-A 3′ region of 1,377 bps starting at nucleotide 379 of HpHAP4-A ORF was isolated by PCR with primers P11 (5′-TAACTgCAggTgTCCgACCTgAAAAAT-3′) and P12 (5′-TGGAAGCTTTGAATCCATCGTATAACG-3′) and cloned as a PstI-HindIII fragment into plasmid pHAP4-A-5′, producing plasmid pHAP4-A::ScLEU2. This latter plasmid harbours a deletion cassette on which the HpHAP4-A region, coding aminoacids 1-126, is replaced with the S. cerevisiae LEU2 gene. This deletion cassette was excised with BamHI and HindIII and transformed in the leu1-1 recipient strain.
A HpHAP4-B deletion cassette was constructed in an analogous manner using the positive selection marker of zeocin resistance [12].
The double knockout strain (HpΔhap4-HpΔhap4-B) was obtained by transformation of HpΔhap4-A with the HpHAP4-B deletion cassette.
H. polymorpha NCYC495 leu1-1 or the derivative prototrophic strain (transformed with pYT1 plasmid) were used as wild-type controls throughout this study as indicated.
Microarrays
RNA extraction was performed as described previously by [25] and purified using the RNeasy Kit (Qiagen). All cultures were performed on minimal medium plus galactose (2%) and harvested at OD600 = 0.8–1. For oxidative stress conditions, 0.5 mM H2O2 were added in the media for 1 hour. These conditions were chosen in order to compare with previous studies of the HAP4 and YAP1 genes of S. cerevisiae [5], [26].
RNAs (four independent preparations each time) were prepared from ten different genetic backgrounds in two conditions, with and without oxidative stress (H2O2): ScΔhap4 and ScΔyap1, each containing the empty plasmid BFG1 as a reference (abbreviated as BFG1/BFG1.H202), BFG1 with the ScHAP4 gene (ScHap4/ScHap4.H202), BFG1 carrying the HpHAP4-A gene (HpHap4-A/HpHap4-A.H202), BFG1 carrying the HpHAP4-B gene (HpHap4-B/HpHap4-B.H2O2). The integrity of the total RNA was determined using an Agilent Technologies 2100 Bioanalyzer and the RNA 6000 Lab-Chip kit. Total RNA concentration was determined using a NanoDrop ND-1000 spectrophotometer [27].
For hybridization, Agilent Yeast oligomicroarrays (v2, cat # G4140B, Agilent Technologies, Palo Alto, CA) were used. Target preparation, hybridization and washing were performed according to the manufacturer's instructions.
The slides were scanned using GenePix 4000B scanner at 100% laser power and the PMT voltage was automatically adjusted. The resulting 16 bit images were analysed using the GenePix Pro 6.0 software. Data were processed using the MAnGO software [28]. The background level was calculated using morphological operators and subtracted. Raw data were normalized using the print-tip Loess method [29]. The array data were submitted to the NCBI Gene Expression Omnibus public depository, entry E-MEXP-3173 for experiments in the Δhap4 background and E-MEXP-3131 for experiments in the Δyap1 background.
Statistical comparisons were performed using multiple testing procedures to evaluate statistical significance for differentially expressed genes. On each slide, the normalized expression log-ratios were averaged on all replicates of each probe. A moderated t-test, e.g. a Student-like test [30], was computed. An error rate (p-value) is associated with each test value. Differentially expressed genes are selected with two criteria: p-value <0.005 and fold-change FC>|1.5|.
To reveal the changes in gene expression in the three different contexts (ScHap4, HpHap4-A, HpHap4-B), the heat map hierarchical clustering method of the heat map function of the R package “stats” was applied to the normalized log2 ratios (intensity of WT versus intensity of Mutant) obtained from the whole microarray experiments in the ScΔhap4 genetic background and on the normalized median signals obtained for the different conditions in the ScΔyap1 background.
Principal Component Analysis (PCA) was used to reveal unknown trends in the data and explore the correlations between mutants [31]. This method was applied to the same data (normalized log2 ratios) than those treated with the heat map clustering method.
Gene Ontology categorization was done with T-profiler tool. This tool uses the t-test to score changes in the average activity of pre-defined groups [32].
Real-time RT-PCR experiments
Total RNA was extracted from H. polymorpha wild-type strain (NCYC495 leu1-1) and HpΔHap4-A after growth on glucose and in 0.5 mM H2O2 for wild-type and HpΔHap4-B in the same conditions as described previously. Three independent biological replicas were used. Reverse transcription experiments were performed with 5 µg of total RNA using reverse PCR primers as gene specific primers and superScriptIII as reverse transcriptase (Invitrogen, Carlsbad, CA). PCR primer pairs are listed in Table 2.
Table 2. List of H. polymorpha genes and corresponding primers used for qRT-PCR.
| Hp gene name* | Oligomer name | Sequence 5'-3' |
| HpFTR1 | FTR1For | TCCGGTCCAGGTACTTATAACATC |
| FTR1 Rev | ATCAAAAGCAAGGTCACAATGACT | |
| HpYAP5 | YAP5For | TGGGAATCCGCAAAAGAGAGAAT |
| YAP5Rev | AGAATTCGGGGATCTGAAAAGCA | |
| HpFRE4 | FRE4For | AGCCGAAGTCGATACTGACA |
| FRE4Rev | TCTTGTACGAGCTGGTCGAT | |
| HpFRE3 | FRE3For | GACTCGGAGGAGCCAATCTG |
| FRE3Rev | CCAAGCAAAGTTTCCCGCAA | |
| HpFRE2 | FRE2For | GAGCTGAAGTGGGTGGC |
| FRE2Rev | GGCACAGGTCTGGCTTC | |
| HpCCC1 | CCC1For | CCTAGCTGCCCGTTCAGAAT |
| CCC1Rev | ATCATCGTCTTGGGGTCAGC | |
| HpGPX1 | GPX1For | AAGGTCGACGTGAATGGTCCTAATG |
| GPX1Rev | GATGTCTTCGGAAATCTTGGACGGA | |
| HpARG5,6 | ARG5-6For | AATTGCATCCGGCTCTACATCG |
| ARG5-6Rev | CTGCTGTGAAGATGTTGTCGGT | |
| HpAAD1 ** | AAD1For | TCTGTAGTGAAAGCTGGGTCG |
| AAD1Rev | CTCGTTGACTGGGAAGTAGCA | |
| HpAAD2 ** | AAD2For | GCTTCTTCGGATTGACCCAG |
| AAD2Rev | AGTTTCAGCTTGATAGCGGC | |
| HpACT1 | ACT1For | CTCTGGTGACGGTGTTACCC |
| ACT1 Rev | TGGTCGAAGTCAAGAGCCAC | |
| HpSDH1 | SDH1For | GGCTTGCCATTGGAGGATCT |
| SDH1Rev | CCATGGTGATGGCTCTCGAA | |
| HpCYC1 | CYC1For | AGGTTCTGCTAAGAAGGGTGC |
| CYC1Rev | CGGACATGGTCTGTTCGTTC | |
| HpMDH1 | MDH1For | CGAGGTGCTCAAGTCCAAGA |
| MDH1Rev | AGAGCGTCGTAGGTCTCCTT | |
| HpCOX5-A | COX5-AFor | AGAGCACTCGTCTTACGGGA |
| COX5-ARev | TTCTCGTCTGGGGTGAGGTA |
* The H. polymorpha gene names are given according to their homologues in S. cerevisiae.
**In the case of the Aryl-alcohol dehydrogenase genes (AAD genes, seven in Sc) we found only two genes in Hp and tested both. Priming with the AAD1 oligomers did not work and the experiment was not carried further.
cDNA templates were diluted to 1/500 for expression measurements. Standard conditions were used with the "Maxima SYBR Green qRT-PCR master mix" kit (Fermentas), in a Light Cycler System (Roche Diagnostics; DNA denaturation at 95°C for 10s, followed by 40 cycles of 60°C for 10s and 72°C for 15s). The specificity of each PCR reaction was checked by measuring fluorescent signals during melting curve analysis. Gene expression was calculated relative to the transcripts levels of the gene HpACT1 which was shown in previous experiments to be constitutively expressed in our conditions. The statistical analysis was performed with the REST software (Qiagen).
Results
1. Heterologous transcriptomic assays revealed that the two HpHap4 proteins behaved differently in the ScΔhap4 genetic background
All previous experiments showed that both H. polymorpha Hap4 proteins can functionally replace the S. cerevisiae Hap4 protein; nevertheless, HpHap4-B was always less efficient than HpHap4-A for all criteria examined [12]. We also showed that HpHAP4-B has a role in iron homeostasis, reminiscent of what is observed for its orthologues in Aspergillus nidulans [21], Candida albicans [22] and Schizosaccharomyces pombe [19]. While both genes activate CYC1 expression, we felt this was not enough to monitor their transactivation capacities. Therefore we compared the effects of overexpressing HpHAP4-A, HpHAP4-B and the native ScHAP4 in S. cerevisiae, in transcriptomic assays. First, we examined genes which were up-regulated by ScHAP4 overexpression (Tables S1, S2 and S3) and compared these data to previously reported assays with ScHAP4 [5], [33]. As expected, most genes coding for the respiratory chain (Table S1) and enzymes of the TCA cycle (Table S2) were found to be under the control of ScHAP4, a result coherent with published data and the known function of this regulator. However, we detected a lesser number of genes encoding the mitochondrial translation apparatus than was previously described (Table S3) The reason for this discrepancy is unclear, but we noticed that if this functional class is represented in each experiment, the specific genes among this class are quite variable. Altogether, the overexpression of ScHAP4 enhances, as expected, the expression of the many genes necessary to ensure the proper function of mitochondrial metabolism.
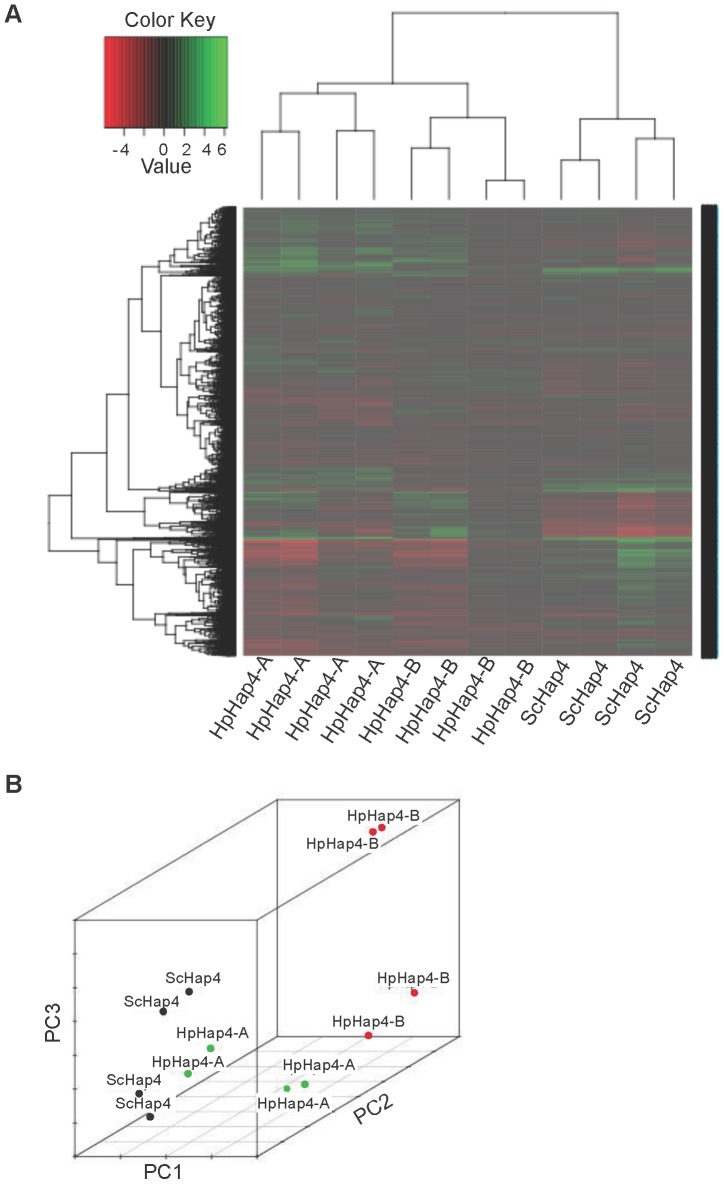
This being established, we examined global gene expression in conditions where either HpHAP4-A or HpHAP4-B was overexpressed (Fig 3A).
Figure 3. Analysis of transcriptomic data of the ScΔhap4 strains.
The analysed data are the normalized log2 ratios (intensity in WT strain versus intensity in the mutant strains) obtained from the microarray experiments (four biological replicates per condition) in the ScΔhap4 genetic background. The WT strain is defined as ScΔhap4 plus the empty plasmid pBFG1 and the three mutant strains as ScΔhap4plus pBFG1 containing the ScHap4, the HpHAP4-A or the HpHAP4-B genes. A: Heat map clustergram. This figure reveals the changes in gene expression between experiments. The experiments and the genes are clustered in a tree from their Pearson correlation coefficient values. Gene expression level is represented on a heat map by colour level (green when overexpressed and red when underexpressed compared to the WT strain). B: Presentation of the experiments in the 3D space of the principal components. The two axes of the figure are the three first principal components determined by Principal Component Analysis (PCA). They explain, respectively, 36%, 27% and 16% of the variance.
Fig 3B is a three dimensional “Principal Component Analysis” (PCA) of the different sets of data which represent in total about 80% of the variability (details for each principal component are given in the legend of the Figure). It clearly separates the HpHAP4-B data from the two groups representing ScHAP4 and HpHAP4-A data. While the latter are not identical, they ought to have some common effects, exemplified when one examines detailed results for genes encoding the respiratory chain components (Table S1) and those for the TCA cycle (Table S2). (i) Most genes from these two lists are indeed regulated by ScHAP4 and HpHAP4-A and one can conclude that in S. cerevisiae, the function of HpHAP4-A partially overlaps that of ScHAP4. (ii) In contrast, HpHAP4-B does not control this same set of genes (none were found significantly regulated by it). The genes differentially regulated by HpHAP4-B are listed Table 3. They are a limited set, involved in cell wall formation, dNTP synthesis or expressed in hypoxia/anaerobiosis. Interestingly, several of them were previously detected in our studies of ScYAP1 [26].
Table 3. Genes up-regulated by HpHAP4-B in the Δhap4 background.
| ARE* | Gene | Presumed function | r-factor** | p-value |
| y | SEO1 | Putative permease; Sulfoxyde Ethionine resistance. | 2.79 | 0.000 |
| y | RPL019B | Ribosomal protein | 2.06 | 0.006 |
| y | AAC3 | ATP/ADP translocase, anaerobically expressed | 2.72 | 0.000 |
| y | COS111 | Detected in mitochondria | 2.07 | 0.004 |
| y | PHO89 | Phosphate metabolism | 2.25 | 0.003 |
| n | BSC1 | Similar to flocculin | 3.23 | 0.000 |
| y | YDL038C | Merged with YDL039C, a pheromone regulated protein | 5.85 | 0.000 |
| y | PRM7 | Pheromone regulated protein; response to drug | 3.46 | 0.000 |
| y | HO | Required for gene conversion of MAT | 3.26 | 0.000 |
| y | HXT15 | Hexose transporter, induced in low level of glucose | 2.06 | 0.000 |
| y | SOR2 | Fructose or mannose metabolism? | 2.01 | 0.000 |
| y | ARO10 | Phenyl pyruvate decarboxylase, first step of the Ehrlich pathway | 4.43 | 0.001 |
| y | YEL057C | Telomere maintenance? | 2.31 | 0.002 |
| y | AGX1 | Alanine:glyoxylate aminotransferase. Glycine synthesis in gly/eth. | 2.01 | 0.000 |
| y | ALG13 | Glycosyltransferase, ER | 2.17 | 0.005 |
| y | FMP48 | Found in mitochondrial proteome | 2.01 | 0.000 |
| y | BIO2 | Biotin synthesis | 2.25 | 0.000 |
| y | PEX18 | Required for peroxisome targeting | 2.15 | 0.002 |
| y | TIR3 | Cell wall mannoprotein/required for anaerobiosis | 2.25 | 0.000 |
| y | RNR2 | dNTP synthesis | 2.05 | 0.002 |
| n | INO1 | Inositol phosphate synthesis | 6.84 | 0.003 |
| y | FAR1 | Cell cycle arrest | 2.31 | 0.000 |
| n | ANB1 | EIF-5A, anaerobiosis gene | 4.67 | 0.000 |
| y | SFC1 | Succinate-fumarate transporter | 2.74 | 0.000 |
| y | HXT16 | Hexose transporter, repressed in high glucose | 2.16 | 0.000 |
| y | PTR2 | Peptide transporter | 4.02 | 0.004 |
| y | RPL38 | Ribosomal protein | 2.07 | 0.002 |
| y | YLR413W | Unknown function | 3.82 | 0.000 |
| y | RPS1A | Ribosomal protein | 2.08 | 0.004 |
| y | HXT2 | Glucose transporter, induced at low levels of glucose | 3.49 | 0.000 |
| n | FET3 | multicopper oxidase; required for high affinity iron uptake | 2.04 | 0.000 |
| y | HAS1 | Helicase, rRNA processing | 2.30 | 0.000 |
| n | RAS1 | Ras protein signal transduction | 2.16 | 0.000 |
| y | YOR121C | Dubious, overlaps YOR120W | 2.09 | 0.003 |
| y | ALD6 | Converts acetaldehyde to acetate. Binds mit OM in oxidative stress | 2.11 | 0.004 |
| y | ODC1 | Mitochondrial transporter, involved in lysine and glutamine biosynthesis. | 2.04 | 0.007 |
| n | GUP2 | Proton symport of glycerol | 2.09 | 0.000 |
| y | NIP7 | Nucleolar protein required for 60S ribosome subunit biosynthesis | 2.14 | 0.000 |
* indicates the presence(y)/absence (n) of a putative Yap1 binding sites in the promoter as obtained from YEASTRACT (http://www.yeastract.com/index.php) database.
** r-factor is the ratio between the normalized value in the strain Δhap4 carrying the empty BFG1 plasmid and the value obtained in the strain Δhap4 carrying BFG1 plus HpHAP4-B.
2. HpHAP4-B is able to replace YAP1 in S. cerevisiae
The additional bZIP-type DNA-binding motif observed in many proteins encoded by putative HAP4 orthologues, especially when the species harbouring them are phylogenetically distant from S. cerevisiae, is a well conserved motif of 25 amino acids which is similar to the DNA-binding motif of the S. cerevisiae YAP family of proteins (Fig 4A). It contains four characteristic amino acid residues, Q234, Q239, A241 and F/Y242 specific of the Yap family [34]. This sequence, called the "Basic Region" (abbreviated here as BR), is necessary for the ScYap1 transactivator to recognize its specific cis-DNA binding sequences (called ARE). The ZIP motif (leucine zipper), which is adjacent to the BR region, was not identified in these HAP4 orthologues; only putative coiled-coiled motifs quite distant to the basic motif could be detected [12].
Figure 4. Functional comparison between ScYAP1 and the HpHAP4-B genes.
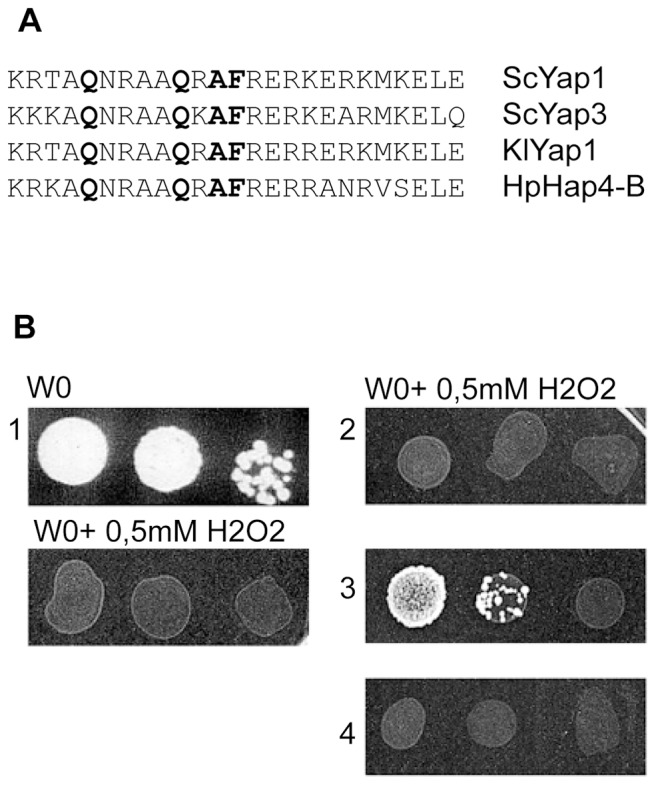
Part A: Comparison of the new bZIP type motif identified in HpHap4-B with the YAP family motif. Q234, Q239, A241 and F/Y242 are four basic regions (DNA-binding sites) characteristic residues of the YAP protein family which are rarely or never observed in other bZIP proteins [34]. Part B: Heterologous complementation of the growth deficiency of S. cerevisiae Δyap1 in the presence of H202 by the HpHAP4-B gene. The gene is expressed on a multicopy plasmid (pBFG1, see Methods ) and the growth monitored on minimal medium (W0) containing 0.5 mM of H202. 1: S. cerevisiae Δyap1 strain (with or without H202). 2:Δyap1 strain with empty plasmid BFG1. 3: Δyap1 with BFG1 plasmid containing HpHap4-B. 4: Δyap1 with BFG1 plasmid containing HpHap4-B with deleted bZIP domain (see Methods). Tests 2, 3 and 4 were performed on medium containing H202. Strains were grown on minimal glucose medium supplemented with the necessary aminoacids at 28°C for 5 days.
This result, as well as the data obtained from transcriptomic studies, prompted us to check if the presence of the BR motif provided the HpHap4-B protein with the capacity to act as ScYap1 does, and to functionally replace it in the S. cerevisiae host. As can be seen in Fig 4B, HpHAP4-B could correct the H2O2 hypersensibility of the ScΔYap1 mutant as observed for the control (ScYAP1). This response is specific for the HpHAP4-B gene; neither HpHAP4-A nor ScHAP4 have this property (data not shown). Note that deletion of the sequence coding for the BR motif in the HpHAP4-B sequence abolished the above-mentioned property (Fig 4B), demonstrating that this capacity is mediated by it.
These data indicate that HpHap4-B is able to activate the expression of (at least some) ScYap1 target genes and to bind to the ARE sequence. To verify this hypothesis, we undertook a gel shift analysis as reported in Fig 5. The HpHap4-B protein slows the migration of the specific, labelled, ARE oligonucleotide on the gel (lane 7) as does ScYap1 (lane 5). This shift was absent in the negative controls (lanes 1–4 and 6), as well as when the BR motif was deleted from the HpHAP4-B sequence (lane 8), which confirms that DNA binding is mediated via the BR sequence.
Figure 5. Gel shift experiment with a synthetic ARE sequence.
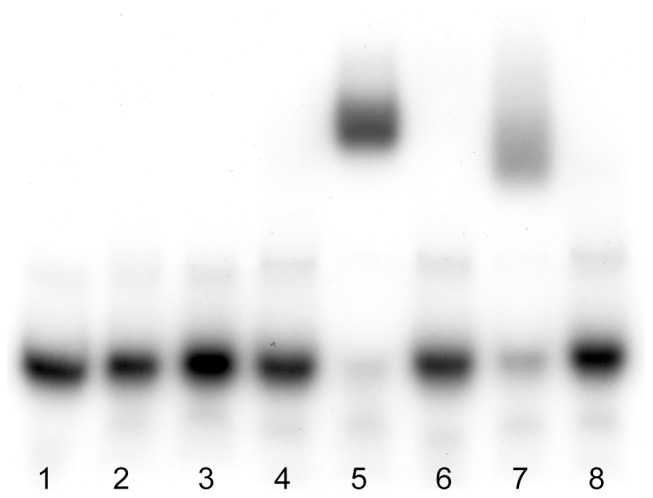
Probe alone (lane 1), S. cerevisiae Δyap1 strain (lane 2), ScΔyap1 with empty plasmid pBFG1 (lane 3), ScΔyap1 with pBFG1 plasmid carrying ScHAP4 (lane 4), pBFG1-ScYAP1(lane 5), pBFG1-HpHAP4-A (lane 6), pBFG1-HpHAP4-B (lane 7) pBFG1-HpHAP4-B-bZIP (devoid of Hphap4-B BR region; lane 8).
3. How does HpHAP4-B correct the defective ScΔyap1 phenotype: global gene expression analysis in the ScΔyap1 context
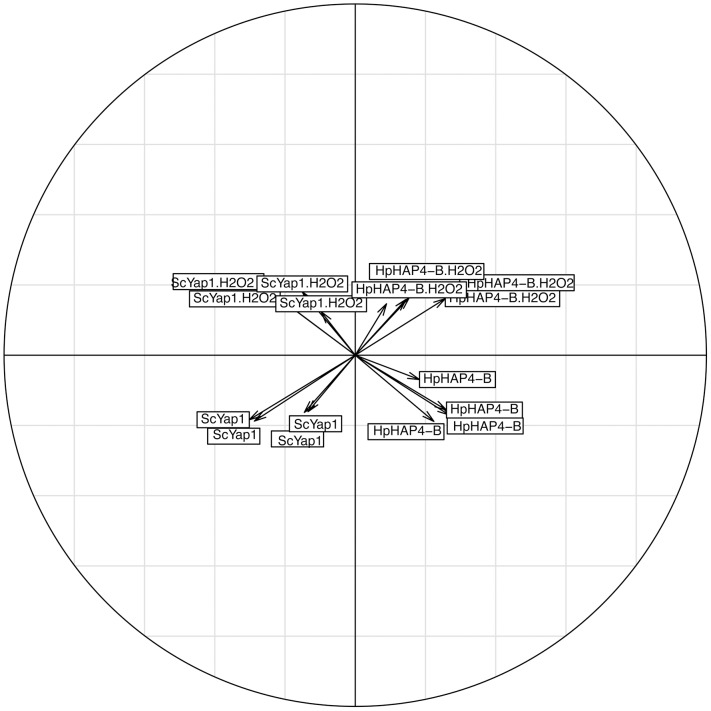
We performed transcriptomic analysis of the effect of HpHAP4-B overexpression in the ScΔyap1 context in the absence and presence of H2O2 and compared the data with the effect of ScYAP1 overexpression in the same conditions. The three first principal components of the PCA analysis performed on the complete set of transcriptomic data explain 94.7% of the variance of the data. The second and the third components explain 7.2% of the variance and separate ScYAP1 action from HpHAP4-B action on one side and the presence and absence of oxidative stress conditions (H2O2) on the other (Fig 6). Since both transactivators restore growth in the presence of H2O2, this strongly suggests that they achieve this endpoint acting upon some common target genes or functions (see below).
Figure 6. Principal Component Analysis of transcriptome data of the ScΔyap1 strains.
The analysed data are the normalized median signals obtained in two mutants and in two different growth conditions (with or without H2O2), compared to the WT by microarray experiments in a ScΔyap1 genetic background. The WT is defined as ScΔyap1 plus the empty plasmid pBFG1, and the mutants as ScΔyap1 plus pBFG1 carrying either the ScYAP1 or the HpHAP4-B gene. For each condition, four independent experiments were performed. The two axes of the figure are the second and the third first principal components and explain 7.2% of the variance.
Target genes regulated by ScYap1 or HpHap4-B were classified according to their gene ontology (GO) functions (Table 4) and one can immediately note striking differences. If ScYap1 is, as expected, mostly geared towards ROS scavengers and chaperones, HpHap4-B is much more biased towards carbon assimilation. Such rapid analysis tells us that while both of them can cope with oxidative stress, their function is clearly different. In addition, a large number of genes can be regulated by both of them (a detailed list is provided in Table S4). For example, if one examines ROS scavenger, the following genes (GSH1, GLR1, GTT2, GTO3 or ECM4, all involved in glutathione metabolism; TRX2, TRR1 or TSA2, involved in thioredoxin metabolism; the superoxide dismutases SOD1 and SOD2 and their chaperone CCS1; CUP1-A and B and CTA1 encoding the catalase A) are up-regulated by ScYAP1 specifically in the presence of H2O2, confirming previous observations [26]. Some of these genes are also regulated by both ScYAP1 and HpHAP4-B (such as GTT2 encoding gluthatione transferase or TSA2 encoding thioredoxin peroxidase) and therefore belong to this common core of targets while others such as CTT1, which encodes the cytoplasmic catalase, or GRX1 (the glutaredoxin which participates to glutathione metabolism) are regulated only by HpHAP4-B. However, the combination of each transactivator target genes is large and varied enough to achieve the response to oxidative stress, even though the sets of genes do not completely overlap.
Table 4. Gene ontology categories of the genes regulated by HpHap4-B and ScYap1.
| HpHAP4-B overexpression in presence of H2O2 | ||||
| Gene ontology category | t-value | E-value | Mean fold change | Number of ORFs |
| Fructose transporter activity | 9,49 | <1.0e–15 | 4.969 | 13 |
| Carbohydrate metabolism | 8,01 | 1.54e–12 | 1.221 | 153 |
| Response to stress | 5,76 | 1.17e–5 | 0.582 | 283 |
| Protein catabolism | 4,45 | 1.19e–2 | 0.722 | 120 |
| Oxidoreductase activity, acting on peroxide as acceptor | 4,39 | 1.56e–2 | 2.572 | 10 |
| ER to Golgi transport | −4.85 | 1.71e–3 | −1.064 | 51 |
| Transporter activity | −4.91 | 1.26e–3 | −0.409 | 330 |
| Biosynthesis | −5.14 | 3.82e–4 | −0.291 | 676 |
| Nucleotidyltransferase activity | −5.21 | 2.62e–4 | −1.119 | 59 |
| Protein biosynthesis | −11.58 | <1.0e–15 | −0.887 | 366 |
| Ribosome biogenesis and assembly | −12.22 | <1.0e–15 | −1.485 | 185 |
| ScYAP1 overexpression in presence of H2O2 | ||||
| Aldehyde metabolism | 7,44 | 1,47E–11 | 6,93 | 18 |
| Oxidoreductase activity, acting on the CH-OH group of donors, NAD or NADP as acceptor | 5,81 | 9,12E–07 | 3,034 | 54 |
| Oxidoreductase activity, acting on CH-OH group of donors | 5,29 | 1,79E–05 | 2,584 | 62 |
| Oxidoreductase activity | 5,14 | 4,01E–05 | 1,348 | 213 |
| Membrane | −3,61 | 4,37E–02 | −0,378 | 767 |
| Intracellular | −3,69 | 3,22E–02 | −0,047 | 3483 |
| Large ribosomal subunit | −4,02 | 8,46E–03 | −1,337 | 113 |
| Protein metabolism | −4,3 | 2,49E–03 | −0,401 | 936 |
| Biosynthesis | −4,3 | 2,49E–03 | −0,487 | 711 |
| Cytosolic ribosome (sensu Eukarya) | −4,33 | 2,17E–03 | −1,28 | 141 |
| Structural constituent of ribosome | −5,15 | 3,80E–05 | −1,294 | 192 |
| Ribosome | −5,21 | 2,76E–05 | −1,195 | 226 |
| Structural molecule activity | −5,29 | 1,79E–05 | −1,075 | 281 |
| Protein biosynthesis | −5,44 | 7,78E–06 | −0,93 | 379 |
| Macromolecule biosynthesis | −5,67 | 2,08E–06 | −0,821 | 503 |
The same holds true for genes encoding heat shock proteins (MDJ1, HSP26, SSE2, HSP42, HSP78, HSP31, HSP104 and others). Globally, stress-induced genes represent 40 to 50% of genes regulated by YAP1 or YAP1 and HpHAP4-B, but only 12% of those genes were regulated only by HpHAP4-B.
We previously reported [26] that ScYAP1 also controls several genes involved in carbon metabolism, which we hypothesized to be necessary for redox balance (several genes of the pentose phosphate pathway were up-regulated in these conditions). When YAP1 is overexpressed, only some of these genes are identified (such as GND2 which catalyses a NADPH regenerating reaction in the pentose phosphate pathway) but we found other genes that should ensure a correct redox balance (several dehydrogenases in particular). Surprisingly, we found many genes involved in carbon metabolism among the HpHAP4-B specifically up-regulated genes (glycolytic genes such as GPM2, MRK1, TLK2, and several coding for enzymes of the carbohydrate storage pathway such as GLK1, TPS1, NTH1 and TPS2).
Finally, genes involved in protein degradation, inositol metabolism, intracellular trafficking and cell wall were found up-regulated both in conditions of the presence and absence of oxidative stress, a situation previously described [26]. Unexpectedly, we also found that a great fraction of the genes which were regulated in common by both transactivators are involved in translation and the majority of them participate in cytoplasmic ribosome biogenesis (about 90% of the genes belonging to the translation functional categories). This amount was much higher than expected as their normal contribution to the functional categories but might be artefactual, reflecting only the effect of overexpression.
The lists of up-regulated genes, which are under control of either ScYAP1 or HpHAP4-B or both in H2O2 stress condition, are presented in Table S4.
4. The two HpHap4 proteins have different functions in their natural host Hansenula polymorpha
All preceding experiments have been performed in a heterologous system, where the H. polymorpha genes were analysed in S. cerevisiae. They all point to the idea that HpHAP4-A seems to be the functional orthologue of the S. cerevisiae HAP4 gene (it regulates mostly the same targets and fully complements ScHAP4 function). Conversely, HpHAP4-B is much less efficient at replacing ScHAP4, can bind the ARE cis-binding site of the ScYAP1 gene and replace it, even though less efficiently, in vivo. Altogether these data indicates that HpHAP4-B should be able to regulate (at least some) ScYAP1 targets. These apparent functional differences remained to be examined in the natural host H. polymorpha.
The two genes were deleted, individually or in combination (double mutant), in H. polymorpha and the resulting phenotypes were analysed. Fig 7 shows the growth of the different mutant strains in presence of various drugs. Growth of strains carrying the single deletion of HpHAP4-A, or the same deletion associated with the HpHAP4-B deletion, are impaired in the presence of 1 µg/ml of antimycin A. This drug is a well-known inhibitor of electron transfer from quinone to cytochrome b in mitochondria [35]. The controls (wild-type strain with or without the empty plasmid) as well as the single deletant HpΔhap4-B, grow normally (Fig 7A). Moreover, the same mutants were also sensitive to salicylhydroxamic acid (SHAM) during growth on non-fermentable sugar xylose (Fig 7B). SHAM is a potential inhibitor of fungal alternative oxidases [36], the branch of mitochondrial electron transport chains that transfers electrons from the ubiquinol pool directly to molecular oxygen. AOX (alternative oxidases) were reported for many fungi (reviewed in [37]). However, to our knowledge they had not yet been described for H. polymorpha.
Figure 7. Growth of different H. polymorpha strains on media containing either antimycin A, SHAM or hydrogen peroxide.

A: H. polymorpha HpΔhap4-A and double knock-out strains, but not HpΔhap4-B deletion mutant, are sensitive to antimycin A on glucose. B: Growth on xylose plus SHAM or H202. 1: HpNCYC495leu1_1 strain. 2: HpNCYC495leu1_1 with pYT1 (empty plasmid, see Material and Methods). 3: HpΔhap4-A. 4: HpΔhap4-B. 5: HpΔhap4-A HpΔhap4-B (double knock-out strain).
HpΔhap4-B mutant and the double disruption mutant were sensitive to H2O2, while the HpΔhap4-A strain grows normally (Fig 7C). These results clearly indicate different functions for the two HpHAP4 paralogues, one related to carbon and energy metabolism and the other involved in oxidative stress.
In order to go beyond the observed phenotype, we examined by qRT-PCR the expression of some genes that could be the targets of these two transactivators in H. polymorpha. Choosing these genes is not an easy task since even if the transactivator function is conserved, it may not be achieved through exactly the same set of target genes. We based our choice on a few genes regulated in the heterologous experiment (see Table S4 for YAP1 and HpHAP4-B) and searched their orthologues. For HpHAP4-A, we selected a few genes encoding components of the respiratory chain or Krebs cycle (HpSDH1, HpMDH1, HpCYTC1, HpCOX5A) and for HpHAP4-B, we selected the targets based on their function in redox control. We examined the expression of several H. polymorpha genes possibly involved in iron homeostasis (HpFRE2, HpFRE3, HpFRE4, HpFTR1, HpCCC1), one gene encoding an aryl-alcohol dehydrogenase (HpAAD2), the gene encoding glutathion peroxidase (HpGPX1) and another encoding one step of the arginine biosynthesis pathway (HpARG5,6). The orthologue of the YAP5 gene, a transcriptional regulator which regulates vacuolar iron storage in S. cerevisiae has also been included. Other genes (such as heat shock proteins) would have been worthy of testing but they were too many copies of the different genes to choose soundly among them. Results are presented in Table 5.
Table 5. qRT-PCR of HpHAP4-A and HpHAP4-B regulation of gene expression in H. polymorpha.
| Part A: regulation by HpHAP4-A in glucose | ||
| Gene | Expression ratio Δhap4-A/WT | P(H1) |
| ACT1 | 1.000 | - |
| SDH1 | 0.502 | 0.032 |
| CYTC1 | 0.610 | 0.000 |
| MDH1 | 0.596 | 0.017 |
| COX5 | 0.629 | 0.097 |
The P(H1) value indicates the probability that the difference between the sample and control groups is due only by chance and was analysed with the REST software (Qiagen, see Material and Methods for more details). Statistically significant results are shown in bold. The case of AAD2* is not conclusive since the data were obtained in two different experiments (two qRT-PCRs, regulated and reproducible and a third one, performed later, which was not).
We assayed HpHAP4-A in glucose which is a fermentescible substrate. If a regulation does exist, we expect the ratio to be around 2, based on our experience with S. cerevisiae. We used galactose in the latter case because it is a fermentescible substrate with no repression of the respiratory function (as does glucose) and we observed regulatory ratio to be around 2 [5]. In H. polymorpha, there is no glucose repression so that the experiment mimics, as far as carbon source is concerned, galactose in S. cerevisiae. We can therefore satisfactorily conclude that the genes HpSDH1, HpCYTC1and HpMDH1 are regulated by HpHAP4-A. The fourth one (HpCOX5) is statistically at the limit and may well be regulated.
As for HpHAP4-B, we observed in presence of oxidative stress transcriptional control of HpFTR1, HpYAP5, HpFRE4 and HpCCR1. The case of HpAAD2 is unclear (see legend). It is also interesting to note that HpYAP5 and HpFRE4 are under control of HpHAP4-B even without any oxidative stress, but for the latter the regulation is opposite.
Although the number of genes which were analysed is limited, these results are compatible with our hypothesis, all the more since HpHAP4-A is induced by respiratory substrates (data not shown) and HpHAP4-B is regulated by iron [12].
5. Distribution of the two motifs (N-Hap4 and BR) in proteins of fungal species
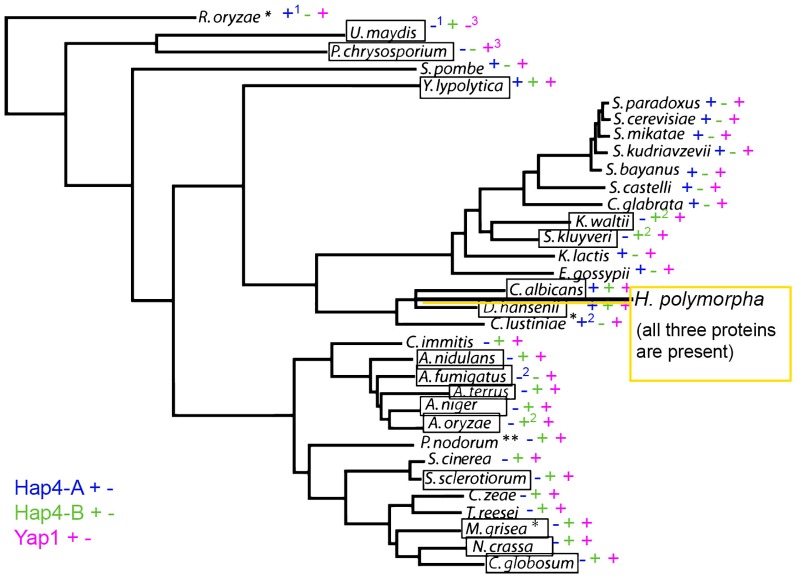
The three types of proteins, Yap1, HpHap4-A and HpHap4-B share specific motifs as indicated in Fig 1: the N-ter motif present in HpHap4-A and HpHAP4-B, the Cysteine Rich domain (or CRD) present in HpHAP4-B and Yap1, and the BR motif present in HpHAP4-B and Yap1. The simultaneous presence of the N-ter and BR motif has already been examined in Ascomycetes, and shown to be associated with Hemiascomytes phylogenetically distant to S. cerevisiae and Euascomycetes for which the genome sequences were available [12]. The N-ter motif of ScHap4 and HpHap4-A is not found in eukaryotes in general, but seems to be fungi-specific. We placed the presence/absence of the Hap4 N-ter and Yap1 BR motifs into the phylogenetic tree and examined the relations between them in fungi for which we disposed of complete genome sequences (Fig 8). When proteins contained both the N-ter and the BR motif, they were similar to Hap4-B and noted as such. The Yap1 type of protein is present in all species. However, the U. maydis Yap1, isolated and characterized as such [38] also has a N-ter motif just upstream the BR motif; this characterizes the protein as belonging to the HpHap4-B rather than to the Yap1 family. The HpHap4 type of protein (containing only the N-ter motif) is present in all hemiascomyceteous yeasts as well as S. pombe but absent in all Pezyzomycotina species.
Figure 8. Phylogenetic tree of fungi in relation with the presence/absence of HpHap4-B-type of proteins.
The phylogenetic tree was adapted from [49]. Species in which proteins such as HpHap4-B (containing both the N- Hap4 and the BR motifs) could be identified are boxed. Aligned with each species, the presence of proteins of the Yap1 type (Yap1 BR motif), HpHap4-B type (BR plus N-ter motifs) and HAP4-A type (N-ter motif) of protein is indicated next to each species. The position of H. polymorpha, which is not on the tree, is indicated, underlining that this yeast belongs to the clade where C. albicans and D. hansenii are localized. * These species were not correctly annotated; for P. nodorum, the BR and N-ter motif are overlapping and degenerate while for U. maydis, the protein annotated as Yap1 turned out to contain also an N-ter motif and belongs therefore to the HpHap4-B family of proteins.
Finally, the HpHap4-B structure containing both the N-ter and the BR motifs is absent in the Saccharomyces group and present in yeasts which are phylogenetically more distant such as H. polymorpha, C. albicans or Y. lipolytica, but surprisingly also in the clade of K. waltii and S. kluyverii. It was also present in all Pezyzomycotina species. Interestingly it was also present in the Basidiomyces U. maydis, a species more basal in the fungal tree than the others.
Discussion
We present here comparative functional studies of the two Hap4-like proteins of the yeast Hansenula polymorpha performed in S. cerevisiae. The H. polymorpha genome sequence has only recently become publicly available. Using S. cerevisiae provided important insights and was probably essential to understand these new features of the Hap4 family of proteins: (i) HpHap4-A and HpHap4-B do not have redundant functions. (ii) HpHap4-A is a functional homologue of ScHap4 since it is essential for respiration and oxidative phosphorylation. The list of target genes, in large part, overlaps the target genes regulated by ScHap4, in particular the genes encoding the components of the respiratory chain. (iii) HpHap4-B bears some relation with ScYap1, which role is to cope with oxidative stress. We deleted the two genes in their natural host, H. polymorpha, and examined the phenotype of the relevant mutants. The results are fully coherent with what was deduced from the heterologous experiment: in the case of HpHAP4-A, presumed to be similar to ScHAP4, the deletion mutant was indeed hypersensitive to antimycin A and SHAM, inhibitors of different components of the respiratory chain. This was not the case for HpHAP4-B which, when deleted, led to a phenotype of hypersensitivity to oxidative stress caused by H2O2, in good agreement with its ability to functionally replace ScYAP1. This hypothesis was reinforced by the qRT-PCR analysis.
What is the mechanism used by HpHAP4-B to regulate gene expression?
Why these differences were observed in S. cerevisiae is surprising. We have previously shown that HpHap4-B can bind the core proteins of the HAP complex, though to a lesser extent than HpHap4-A [12]. Global gene expression studies reveal that its action partially overlaps ScYap1, and all the more so in a Δyap1 context. This indicates that it probably can bind in vivo to the promoter of some genes that are ScYap1 gene targets (via the ARE sequence), extending what we observed in vitro. The mechanism of action of the protein is not yet known. An examination of the cis-binding sites present upstream of the genes regulated by either ScYAP1 only, or HpHAP4-B only or both ScYAP1 and HpHAP4-B, using the YEASTRACT (http://www.yeastract.com/) documented targets, does not show any major differences. Most of the genes have an ARE binding site in their promoter, but conversely, not all genes regulated by YAP1 are regulated by HpHAP4-B. This may reflect that, in S. cerevisiae, some target sites could accept a Yap1 homodimer while some others absolutely require a heterodimer to be made with another Yap protein, an association which HpHap4-B is probably not capable of since it does not contain the "ZIP" part of the bZIP motif [12]; the situation in H. polymorpha may be different and one cannot exclude that promoters of genes regulated by HpHap4-B contain the two motifs (CCAAT and ARE) in close association.
The structure of the protein should also be considered. Conformational changes (the N-Hap4 and BR motifs are next to one another) could lead to the masking of one motif in some conditions. Our results cannot provide definitive answers on the respective role of these two proteins nor on the molecular mechanisms that control them, but may open the way to elaborate plausible hypotheses that should be tested directly in H. polymorpha.
The distribution of the HpHap4-B complex motif suggests a common origin for the Hap4 and Yap1 families of proteins
We have identified the N-Hap4 motif (16 aas), in all ascomycetes and (even though it is less conserved) in some Basidiomycetes. In several species, especially those phylogenetically distant from S. cerevisiae, it is associated with the BR motif of a Yap1p-like bZIP motif. The question of how this new association (N-ter plus BR) is distributed is therefore of interest. From the survey in Fig 8, it is clear that the presence of the HpHap4-B type of protein is scattered among the different clades. For example, outside of the Hemiascomycetes group, it is absent in S. pombe and S. japonicus but present in the Basidyomycetes U. maydis. It is present within one clade containing K. waltii and S. kluyverii and present in all the Pezyzomycotina species we examined, but the motif sequence was found degenerated in P. nodorum. The simplest way to explain these variations is to postulate that the fungal ancestor protein had the three motifs (N-ter, BR and CRD) which have been independently lost in different clades through evolution and speciation; degenerate motifs being witnesses of this evolution. Such a mechanism could have been used for neo- or sub-functionalization of these proteins.
For example, the HpHap4-B type of protein was not detected in S. cerevisiae and related species where the many duplications of the Yap genes family allowed partition of the function. While HpHap4-B is involved in oxidative stress, iron metabolism and storage and carbohydrate control, in S. cerevisiae, Yap1 is more specialized in oxidative stress, Yap5 is an iron responsive transcriptional activator [39] and Hap4 is specialized in the control of carbohydrates.
If this evolutionary scenario is true, one should find, in the different yeast species, proteins with various combinations of the N-Hap4, BR and CRD domains (the cysteine-rich CRD domain is also part of the Yap1 sequence and critical for Yap1-mediated resistance to oxidative stress [40], [41] and can be found in the HpHap4-B type of proteins). It should be also possible to detect “relics” of these specific motifs in some species. It is therefore noteworthy that a close examination of the S. cerevisiae Yap proteins revealed a degenerate N-Hap4-B motif in the Yap7 protein (68% similarity, Fig 9). In addition, the fact that in U. maydis, which is less divergent relative to the putative ancestral fungi than the Hemiascomceteous yeasts, only one protein able to respond to oxidative stress has been found and that it has a Hap4-B type of protein (i.e. containing the three motifs N-ter, BR and CRD) reinforces this scenario [38].
Figure 9. Identification of a “Relic sequence” in S. cerevisiae.
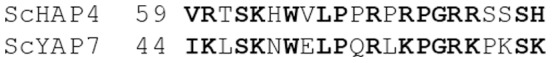
Upper part (Hap4) is the N-ter Hap4 motif with small extensions (the motif itself spans aminoacids 60 to 76). Bottom part (Yap7) is a small sequence in the Yap7 protein which is 68% similar to the upper sequence. Yap7 is a member of the bZIP family of S. cerevisiae transactivators. Its function is not precisely known [50]. Refer to Fig 1 for the various motifs of these proteins.
In mammals, the fact that bZIP proteins can bind CCAAT sequences (cited in [42]) and that the bZIP ancestor is supposed to have functioned as a homodimer [43], [42] fits very well with the presence of a common ancestor for HAP4 and YAP1. It is interesting to note that if we compare the ScHap4 and SpPhp4, proteins which are encoded by the phylogenetically distant yeast species S. cerevisiae and S. pombe, both regulate the mitochondrial components involved in respiration and both do so through the CCAAT-binding sequence. However their action is opposite: ScHap4 activates respiration in response to glucose derepression whereas SpPhp4 represses it in response to iron deficit [19]. This is one of the increasing examples of the complexity of regulatory network evolution where orthologues of target genes and transcriptional regulators are conserved but the regulatory strategy is changed in order to adapt to a different environment (reviewed in [44]).
Relation between carbohydrate metabolism, oxidative status and iron homeostasis
Data obtained in the course of this work, and from the literature, point to an intricate relation between the control of iron homeostasis, oxygen tension and carbon source. Recently, it was demonstrated that the CCAAT-binding factor of Aspergillus nidulans (composed of HapB, HapC and HapE, equivalent to Hap2, Hap3 and Hap5 in S. cerevisiae), senses the redox status of the cell [45], independently of the activator moiety HapX (the equivalent of HpHAP4-B). This relation is not surprising since the presence of excess iron during oxidative stress is deleterious to the cell, allowing the Fenton reaction to take place. Conversely iron is needed for many pathways, among which is the synthesis of the respiratory components. Iron homeostasis control is therefore also linked to the type of carbon sources, respiratory or fermentative, that are available. Carbon source utilization can be different from one species to another and has been studied in detail in the Saccharomyces complex by [46]. These species show a greater variability in terms of the ability to accumulate ethanol in the presence of oxygen and a gradual independence from oxygen in the pre-genome duplication species. In Kluyveromyces lactis, we recently showed that the KlHap1 protein, a transactivator involved in regulation of gene expression in response to heme and oxygen in S. cerevisiae, controls glucose transport [47]. The tight control of these three pathways was probably more primitive in the yeast progenitor but absolutely necessary, and a regulator of the HpHAP4-B type was probably the simplest way to coordinate all these actions. During evolution, more specialized functions appeared, explaining the highly redundant and specialized functions of the many regulators involved in iron control, oxidative stress control and carbon source assimilation in S. cerevisiae. Therefore and according to the environmental niche, proteins of some species lost one or more of the three motifs (N-Hap4, BR and CRD) found in the original protein.
The possibility to test in the future the global regulatory role of the Hap4-B type of proteins in different species will allow us to assess and refine these hypothesis.
Supporting Information
Comparison of the regulatory ratios (WT versus mutant) obtained by overexpression of ScHAP4 or HpHAP4A in ScΔhap4 mutant: genes encoding respiratory chain components.
(DOCX)
Comparison of the regulatory ratios (WT versus mutant) obtained by overexpression of ScHAP4 or HpHAP4A in ScΔhap4 mutant: genes encoding TCA cycle and related pathways enzymes.
(DOCX)
Comparison of the regulatory ratios (WT versus mutant) obtained by overexpression of ScHAP4 or HpHAP4A in ScΔhap4 mutant: genes encoding components of the mitochondrial translation apparatus.
(DOCX)
List of S. cerevisiae genes upregulated in presence of H202 when ScYAP1 or HpHAP4-B were overexpressed in ScΔyap1 mutant.
(DOCX)
Acknowledgments
Access to the HpHAP4-A and HpHAP4-B genes of H. polymorpha was kindly provided by Rhein-Biotech.
Our deep thanks go to Pr. Michael Dubow who proofread the English language and to Pr. F. Confalioneri, Dr. A. Lagorce and M. Dutertre for their help in providing materials and advices for the qRT-PCR experiments.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. Transcriptome data have been deposited at the NCBI Gene Expression Omnibus public depository and are publicly available as E-MEXP-3173 for the Δhap4 experiments and E-MEXP-3131 for the Δyap1 experiments.
Funding Statement
Experiments were funded by recurrent national fundings (CNRS and University) to the IGM (UMR8621). The EC contract GARNISH QLK3-2000-174 to MBF contributed to the start of this work. NP was supported by a French government fellowship for joint PhD, a bilateral CNRS-Ukrainian NAS program and a regional support from Ile de France grant for joint PhD. KS was supported by a FEBS collaborative experimental Scholarship for Central and Eastern Europe and an INTAS fellowship grant for young Scientist (05-109-4177). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Goffeau A (2000) Four years of post-genomic life with 6,000 yeast genes. FEBS Lett 25:37–41. [DOI] [PubMed] [Google Scholar]
- 2. Pena-Castillo L, Hughes TR (2007) Why are there still over 1000 uncharacterized yeast genes? Genetics 176:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Olesen JT, Guarente L (1990) The HAP2 subunit of yeast CCAAT transcriptional activator contains adjacent domains for subunit association and DNA recognition: model for the HAP2/3/4 complex. Genes Dev 4:1714–1729. [DOI] [PubMed] [Google Scholar]
- 4. Blom J, De Mattos MJ, Grivell LA (2000) Redirection of the respiro-fermentative flux distribution in Saccharomyces cerevisiae by overexpression of the transcription factor Hap4p. Appl Environ Microbiol 66:1970–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buschlen S, Amillet JM, Guiard B, Fournier A, Marcireau C (2003) The S. Cerevisiae HAP complex, a key regulator of mitochondrial function, coordinates nuclear and mitochondrial gene expression. Comp Funct Genomics 4:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lascaris R, Bussemaker HJ, Boorsma A, Piper M, van der Spek H, et al. (2003) Hap4p overexpression in glucose-grown Saccharomyces cerevisiae induces cells to enter a novel metabolic state. Genome Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olesen JT, Fikes JD, Guarente L (1991) The Schizosaccharomyces pombe homolog of Saccharomyces cerevisiae HAP2 reveals selective and stringent conservation of the small essential core protein domain. Mol Cell Biol 11:611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maity SN, de Crombrugghe B (1998) Role of the CCAAT-binding protein CBF/NF-Y in transcription. Trends Biochem Sci 23:174–178. [DOI] [PubMed] [Google Scholar]
- 9. Brakhage AA, Andrianopoulos A, Kato M, Steidl S, Davis MA, et al. (1999) HAP-Like CCAAT-binding complexes in filamentous fungi: implications for biotechnology. Fungal Genet Biol 27:243–252. [DOI] [PubMed] [Google Scholar]
- 10. Bourgarel D, Nguyen CC, Bolotin-Fukuhara M (1999) HAP4, the glucose-repressed regulated subunit of the HAP transcriptional complex involved in the fermentation-respiration shift, has a functional homologue in the respiratory yeast Kluyveromyces lactis. Mol Microbiol 31:1205–1215. [DOI] [PubMed] [Google Scholar]
- 11. Sybirna K, Guiard B, Li YF, Bao WG, Bolotin-Fukuhara M, et al. (2005) A new Hansenula polymorpha HAP4 homologue which contains only the N-terminal conserved domain of the protein is fully functional in Saccharomyces cerevisiae. Curr Genet 47:172–181. [DOI] [PubMed] [Google Scholar]
- 12. Sybirna K, Petryk N, Zhou YF, Sibirny A, Bolotin-Fukuhara M (2010) A novel Hansenula polymorpha transcriptional factor HpHAP4-B, able to functionally replace the S. cerevisiae HAP4 gene, contains an additional bZip motif. Yeast 27:941–954. [DOI] [PubMed] [Google Scholar]
- 13. Levine DW, Cooney CL (1973) Isolation and characterization of a thermotolerant methanol-utilizing yeast. Appl Microbiol 26:982–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der Klei IJ, Yurimoto H, Sakai Y, Veenhuis M (2006) The significance of peroxisomes in methanol metabolism in methylotrophic yeast. Biochim Biophys Acta 1763:1453–1462. [DOI] [PubMed] [Google Scholar]
- 15. Gellissen G (2000) Heterologous protein production in methylotrophic yeasts. Appl Microbiol Biotechnol 54:741–750. [DOI] [PubMed] [Google Scholar]
- 16. Voronovsky AY, Ryabova OB, Verba OV, Ishchuk OP, Dmytruk KV, et al. (2005) Expression of xylA genes encoding xylose isomerases from Escherichia coli and Streptomyces coelicolor in the methylotrophic yeast Hansenula polymorpha. FEMS Yeast Res 5:1055–1062. [DOI] [PubMed] [Google Scholar]
- 17. Forsburg SL, Guarente L (1989) Communication between mitochondria and the nucleus in regulation of cytochrome genes in the yeast Saccharomyces cerevisiae. Annu Rev Cell Biol 5:153–180. [DOI] [PubMed] [Google Scholar]
- 18. Mercier A, Pelletier B, Labbe S (2006) A transcription factor cascade involving Fep1 and the CCAAT-binding factor Php4 regulates gene expression in response to iron deficiency in the fission yeast Schizosaccharomyces pombe. Eukaryot Cell 5:1866–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mercier A, Watt S, Bahler J, Labbe S (2008) Key function for the CCAAT-binding factor Php4 to regulate gene expression in response to iron deficiency in fission yeast. Eukaryot Cell 7:493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jbel M, Mercier A, Pelletier B, Beaudoin J, Labbe S (2009) Iron activates in vivo DNA binding of Schizosaccharomyces pombe transcription factor Fep1 through its amino-terminal region. Eukaryot Cell 8:649–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hortschansky P, Eisendle M, Al-Abdallah Q, Schmidt AD, Bergmann S, et al. (2007) Interaction of HapX with the CCAAT-binding complex—a novel mechanism of gene regulation by iron. EMBO J 26:3157–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hsu PC, Yang CY, Lan CY (2011) Candida albicans Hap43 is a repressor induced under low-iron conditions and is essential for iron-responsive transcriptional regulation and virulence. Eukaryot Cell 10:2097–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: a Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 24. Ramil E, Agrimonti C, Shechter E, Gervais M, Guiard B (2000) Regulation of the CYB2 gene expression: transcriptional co-ordination by the Hap1p, Hap2/3/4/5p and Adr1p transcription factors. Mol Microbiol 37:1116–1132. [DOI] [PubMed] [Google Scholar]
- 25. Schmitt ME, Brown TA, Trumpower BL (1990) A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res 18:3091–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dumond H, Danielou N, Pinto M, Bolotin-Fukuhara M (2000) A large-scale study of Yap1p-dependent genes in normal aerobic and H2O2-stress conditions: the role of Yap1p in cell proliferation control in yeast. Mol Microbiol 36:830–845. [DOI] [PubMed] [Google Scholar]
- 27. Imbeaud S, Graudens E, Boulanger V, Barlet X, Zaborski P, et al. (2005) Towards standardization of RNA quality assessment using user-independent classifiers of microcapillary electrophoresis traces. Nucleic Acids Res 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marisa L, Ichante JL, Reymond N, Aggerbeck L, Delacroix H, et al. (2007) MAnGO: an interactive R-based tool for two-colour microarray analysis. Bioinformatics 23:2339–2341. [DOI] [PubMed] [Google Scholar]
- 29. Smyth GK, Speed T (2003) Normalization of cDNA microarray data. Methods 31. [DOI] [PubMed] [Google Scholar]
- 30. Smyth GK (2004) Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3. [DOI] [PubMed] [Google Scholar]
- 31.Mardia KV, Kent JT, Bibby JM (1979) Multivariate analysis. London: Academic press. [Google Scholar]
- 32. Boorsma A, Foat BC, Vis D, Klis F, Bussemaker HJ (2005) T-profiler: scoring the activity of predefined groups of genes using gene expression data. Nucleic Acids Res 33:W592–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lascaris R, Piwowarski J, van der Spek H, Teixeira de Mattos J, Grivell L, et al. (2004) Overexpression of HAP4 in glucose-derepressed yeast cells reveals respiratory control of glucose-regulated genes. Microbiology 150:929–934. [DOI] [PubMed] [Google Scholar]
- 34. Fernandes L, Rodrigues-Pousada C, Struhl K (1997) Yap, a novel family of eight bZIP proteins in Saccharomyces cerevisiae with distinct biological functions. Mol Cell Biol 17:6982–6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaniuga Z, Bryła J, Slater EC (1969) Inhibitors Around the Antimycin-Sensitive Site in the Respiratory Chain. In: Bücher T, Sies H, editors. Inhibitors Tools in Cell Research: Springer Berlin Heidelberg. pp.282–300.
- 36. Hoefnagel MH, Wiskich JT, Madgwick SA, Patterson Z, Oettmeier W, et al. (1995) New inhibitors of the ubiquinol oxidase of higher plant mitochondria. Eur J Biochem 233:531–537. [DOI] [PubMed] [Google Scholar]
- 37. Veiga A, Arrabaca JD, Sansonetty F, Ludovico P, Corte-Real M, et al. (2003) Energy conversion coupled to cyanide-resistant respiration in the yeasts Pichia membranifaciens and Debaryomyces hansenii. FEMS Yeast Res 3:141–148. [DOI] [PubMed] [Google Scholar]
- 38. Molina L, Kahmann R (2007) An Ustilago maydis gene involved in H2O2 detoxification is required for virulence. Plant Cell 19:2293–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li L, Murdock G, Bagley D, Jia X, Ward DM, et al. (2010) Genetic dissection of a mitochondria-vacuole signaling pathway in yeast reveals a link between chronic oxidative stress and vacuolar iron transport. J Biol Chem 285:10232–10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Delaunay A, Isnard AD, Toledano MB (2000) H2O2 sensing through oxidation of the Yap1 transcription factor. EMBO J 19:5157–5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kuge S, Arita M, Murayama A, Maeta K, Izawa S, et al. (2001) Regulation of the yeast Yap1p nuclear export signal is mediated by redox signal-induced reversible disulfide bond formation. Mol Cell Biol 21(18):6139–6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Deppmann CD, Alvania RS, Taparowsky EJ (2006) Cross-species annotation of basic leucine zipper factor interactions: Insight into the evolution of closed interaction networks. Mol Biol Evol 23:1480–1492. [DOI] [PubMed] [Google Scholar]
- 43. Amoutzias GD, Robertson DL, Bornberg-Bauer E (2004) The evolution of protein interaction networks in regulatory proteins. Comp Funct Genomics 5:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lelandais G, Goudot C, Devaux F (2011) The evolution of gene expression regulatory networks in yeasts. C R Biol 334:655–661. [DOI] [PubMed] [Google Scholar]
- 45. Thon M, Al Abdallah Q, Hortschansky P, Scharf DH, Eisendle M, et al. (2010) The CCAAT-binding complex coordinates the oxidative stress response in eukaryotes. Nucleic Acids Res 38:1098–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Merico A, Sulo P, Piskur J, Compagno C (2007) Fermentative lifestyle in yeasts belonging to the Saccharomyces complex. FEBS Journal 274:976–989. [DOI] [PubMed] [Google Scholar]
- 47. Bao WG, Guiard B, Fang ZA, Donnini C, Gervais M, et al. (2008) Oxygen-dependent transcriptional regulator Hap1p limits glucose uptake by repressing the expression of the major glucose transporter gene RAG1 in Kluyveromyces lactis. Eukaryot Cell 7:1895–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Souciet J, Aigle M, Artiguenave F, Blandin G, Bolotin-Fukuhara M, et al. (2000) Genomic exploration of the hemiascomycetous yeasts: 1. A set of yeast species for molecular evolution studies. FEBS Lett 487:3–12. [DOI] [PubMed] [Google Scholar]
- 49. Cornell M, Alam I, Soanes DM, Wong HM, Hedeler C, et al. (2007) Comparative genome analysis across a kingdom of eukaryotic organisms: specialization and diversification in the fungi. Genome Res 1712:1809–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rodrigues-Pousada C, Menezes RA, Pimentel C (2010) The Yap family and its role in stress response. Yeast 27:245–258. [DOI] [PubMed] [Google Scholar]
- 51. Thomas BJ, Rothstein R (1989) Elevated recombination rates in transcriptionally active DNA. Cell 56:619–630. [DOI] [PubMed] [Google Scholar]
- 52. Bossier P, Fernandes L, Rocha D, Rodrigues-Pousada C (1993) Overexpression of YAP2, coding for a new yAP protein, and YAP1 in Saccharomyces cerevisiae alleviates growth inhibition caused by 1,10-phenanthroline. J Biol Chem 268:23640–23645. [PubMed] [Google Scholar]
- 53. Sudbery PE, Gleeson MA, Veale RA, Ledeboer AM, Zoetmulder MC (1988) Hansenula polymorpha as a novel yeast system for the expression of heterologous genes. Biochem Soc Trans 16:1081–1083. [DOI] [PubMed] [Google Scholar]
- 54. Delahodde AF, Pandjaitan R, Corral-Debrinski M, Jacq C (2001) Pse1/Kap121-dependent nuclear localization of the major yeast multidrug resistance (MDR) transcription factor Pdr1. Mol Microbiol 39:304–312. [DOI] [PubMed] [Google Scholar]
- 55. Tan X, Waterham HR, Veenhuis M, Cregg JM (1995) The Hansenula polymorpha PER8 gene encodes a novel peroxisomal integral membrane protein involved in proliferation. J Cell Biol 128:307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of the regulatory ratios (WT versus mutant) obtained by overexpression of ScHAP4 or HpHAP4A in ScΔhap4 mutant: genes encoding respiratory chain components.
(DOCX)
Comparison of the regulatory ratios (WT versus mutant) obtained by overexpression of ScHAP4 or HpHAP4A in ScΔhap4 mutant: genes encoding TCA cycle and related pathways enzymes.
(DOCX)
Comparison of the regulatory ratios (WT versus mutant) obtained by overexpression of ScHAP4 or HpHAP4A in ScΔhap4 mutant: genes encoding components of the mitochondrial translation apparatus.
(DOCX)
List of S. cerevisiae genes upregulated in presence of H202 when ScYAP1 or HpHAP4-B were overexpressed in ScΔyap1 mutant.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. Transcriptome data have been deposited at the NCBI Gene Expression Omnibus public depository and are publicly available as E-MEXP-3173 for the Δhap4 experiments and E-MEXP-3131 for the Δyap1 experiments.