Abstract
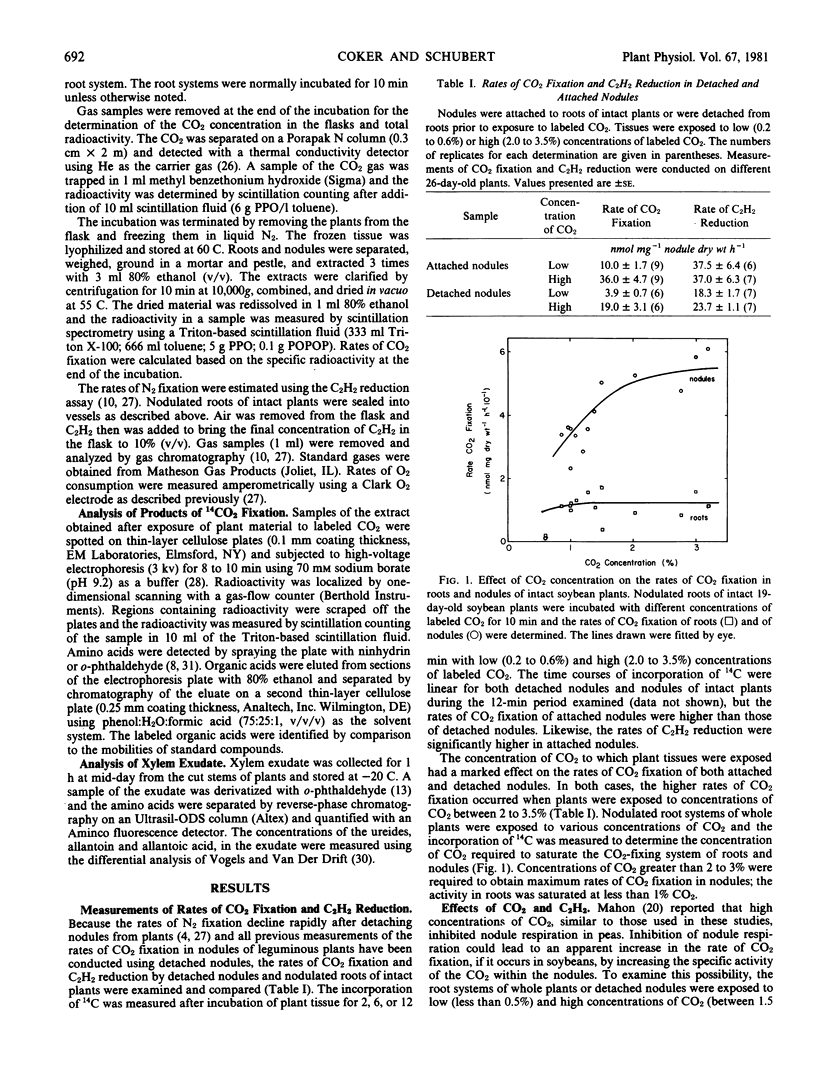
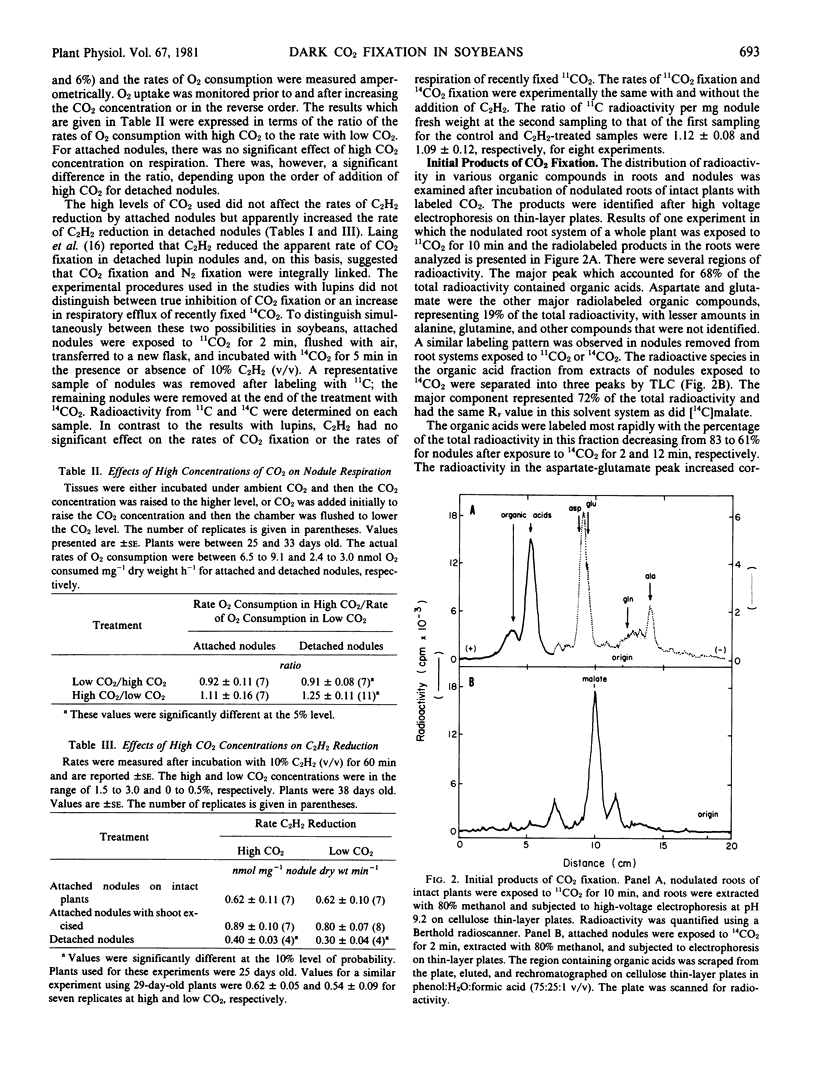
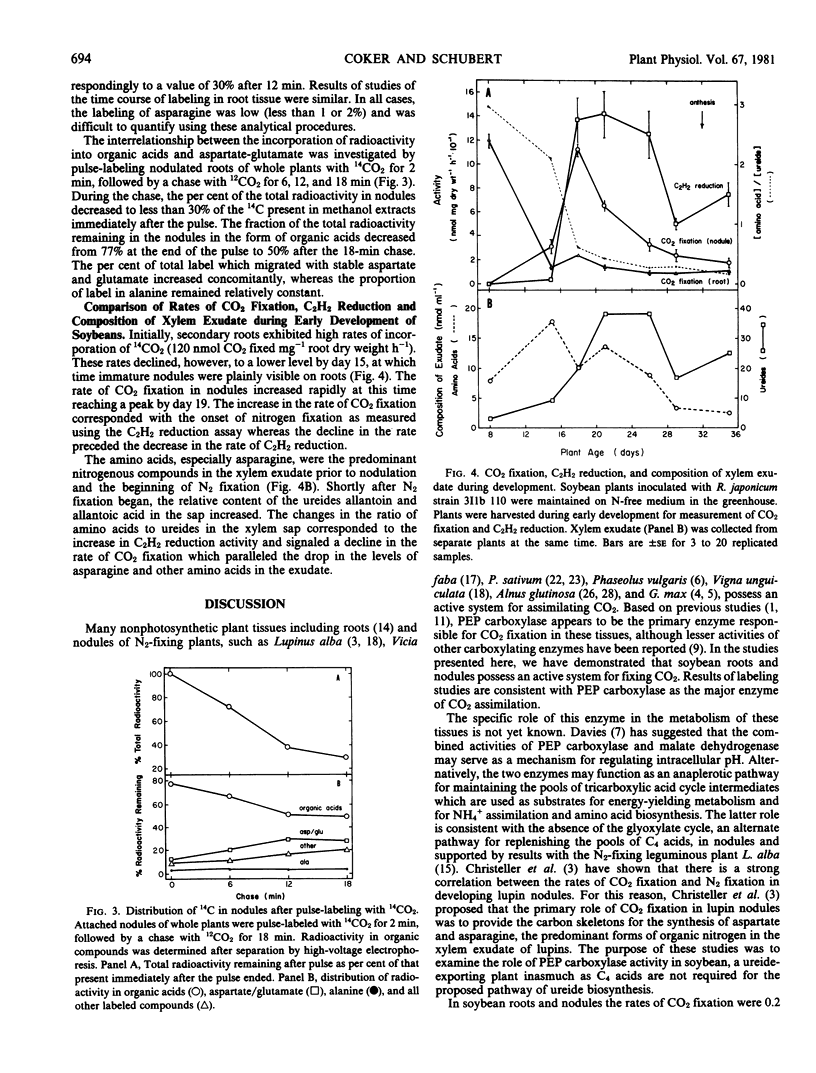
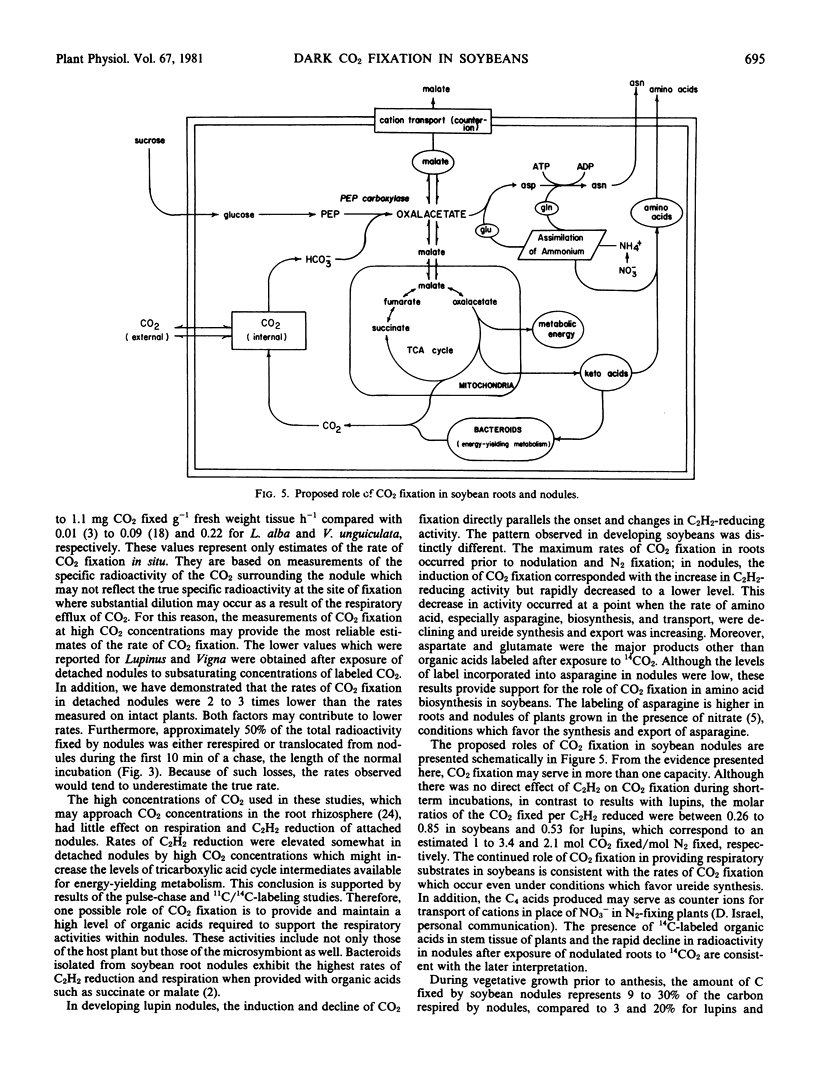
These studies demonstrate that soybean (Merr) roots and nodules possess an active system for fixing CO2. The maximum rates of CO2 fixation observed for roots and nodules of intact plants were 120 and 110 nanomoles CO2 fixed per milligram dry weight per hour, respectively. Results of labeling studies suggest a primary role for phosphoenolpyruvate carboxylase in CO2 assimilation in these tissues. After pulse-labeling with 14CO2 for 2 minutes, 70% of the total radioactivity was lost within 18 minutes via respiration and/or translocation out of nodules. During the vegetative stages of growth of soybeans grown symbiotically, CO2 fixation in nodules increased at the onset of N2 fixation but declined to a lower level prior to the decrease in N2 fixation. This decrease coincided with a decrease in the transport of amino acids, especially asparagine, and an increase in the export of ureides. These findings are consistent with a dual role for CO2 fixation, providing substrates for energy-yielding metabolism and supplying carbon skeletons for NH4+ assimilation and amino acid biosynthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANDURSKI R. S., GREINER C. M. The enzymatic synthesis of oxalacetate from phosphoryl-enolpyruvate and carbon dioxide. J Biol Chem. 1953 Oct;204(2):781–786. [PubMed] [Google Scholar]
- Bergersen J. F., Turner G. L. Nitrogen fixation by the bacteroid fraction of breis of soybean root nodules. Biochim Biophys Acta. 1967 Aug 29;141(3):507–515. doi: 10.1016/0304-4165(67)90179-1. [DOI] [PubMed] [Google Scholar]
- Christeller J. T., Laing W. A., Sutton W. D. Carbon Dioxide Fixation by Lupin Root Nodules: I. Characterization, Association with Phosphoenolpyruvate Carboxylase, and Correlation with Nitrogen Fixation during Nodule Development. Plant Physiol. 1977 Jul;60(1):47–50. doi: 10.1104/pp.60.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEHERTOGH A. A., MAYEUX P. A., EVANS H. J. THE RELATIONSHIP OF COBALT REQUIREMENT TO PROPIONATE METABOLISM IN RHIZOBIUM. J Biol Chem. 1964 Aug;239:2446–2453. [PubMed] [Google Scholar]
- Hardy R. W., Havelka U. D. Nitrogen fixation research: a key to world food? Science. 1975 May 9;188(4188):633–643. doi: 10.1126/science.188.4188.633. [DOI] [PubMed] [Google Scholar]
- Johnson G. V., Evans H. J., Ching T. Enzymes of the glyoxylate cycle in rhizobia and nodules of legumes. Plant Physiol. 1966 Oct;41(8):1330–1336. doi: 10.1104/pp.41.8.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing W. A., Christeller J. T., Sutton W. D. Carbon Dioxide Fixation by Lupin Root Nodules: II. Studies with C-labeled Glucose, the Pathway of Glucose Catabolism, and the Effects of Some Treatments That Inhibit Nitrogen Fixation. Plant Physiol. 1979 Mar;63(3):450–454. doi: 10.1104/pp.63.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell D. B., Rainbird R. M., Atkins C. A., Pate J. S. Economy of Photosynthate Use in Nitrogen-fixing Legume Nodules: Observations on Two Contrasting Symbioses. Plant Physiol. 1979 Nov;64(5):888–891. doi: 10.1104/pp.64.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon J. D. Environmental and genotypic effects on the respiration associated with symbiotic nitrogen fixation in peas. Plant Physiol. 1979 May;63(5):892–897. doi: 10.1104/pp.63.5.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W. Transport of nitrogen in the xylem of soybean plants. Plant Physiol. 1979 Sep;64(3):411–416. doi: 10.1104/pp.64.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Jennings N. T., Evans H. J. Hydrogen Reactions of Nodulated Leguminous Plants: II. Effects on Dry Matter Accumulation and Nitrogen Fixation. Plant Physiol. 1978 Mar;61(3):398–401. doi: 10.1104/pp.61.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels G. D., Van der Drift C. Differential analyses of glyoxylate derivatives. Anal Biochem. 1970 Jan;33(1):143–157. doi: 10.1016/0003-2697(70)90448-3. [DOI] [PubMed] [Google Scholar]
- Wolk C. P., Thomas J., Shaffer P. W., Austin S. M., Galonsky A. Pathway of nitrogen metabolism after fixation of 13N-labeled nitrogen gas by the cyanobacterium, Anabaena cylindrica. J Biol Chem. 1976 Aug 25;251(16):5027–5034. [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]



