Abstract
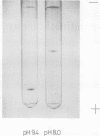
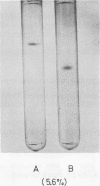
Agmatine iminohydrolase (EC 3.5.3.12) was purified 7,300-fold from extracts of corn shoots by chromatographic separations on diethylaminoethyl-cellulose, Sephadex G-100, and agmatine-affinity column. The enzyme was homogeneous by the criteria of analytical gel electrophoresis. Molecular weight estimated by Bio-Gel P-200 was 85,000, and the enzyme seems to be a dimer with identical subunits (molecular weight, 43,000). The isoelectric point determined by gel electrofocusing was 4.7. The optimal pH and temperature for activity were 6.5 and 60 C, respectively. The activation energy was 10.9 kilocalories per mole. High specificity exists for agmatine, the Km value for agmatine was 1.9 × 10−4 molar, and the enzyme was present in the cytosol. The enzyme was sensitive to Cu2+ and Zn2+ and also was inhibited by p-hydroxymercuribenzoate and arcain.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akamatsu N., Oguchi M., Yajima Y., Ohno M. Formation of N-carbamyl putrescine from citrulline in Escherichia coli. J Bacteriol. 1978 Jan;133(1):409–410. doi: 10.1128/jb.133.1.409-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal. 1970;18:1–53. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Hunninghake D., Grisolia S. A sensitive and convenient micromethod for estimation of urea, citrulline, and carbamyl derivatives. Anal Biochem. 1966 Aug;16(2):200–205. doi: 10.1016/0003-2697(66)90147-3. [DOI] [PubMed] [Google Scholar]
- Kobashi K., Kumaki K., Hase J. I. Effect of acyl residues of hydroxamic acids on urease inhibition. Biochim Biophys Acta. 1971 Feb 10;227(2):429–441. doi: 10.1016/0005-2744(71)90074-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morris D. R., Koffron K. L. Urea production and putrescine biosynthesis by Escherichia coli. J Bacteriol. 1967 Nov;94(5):1516–1519. doi: 10.1128/jb.94.5.1516-1519.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MØLLER V. Simplified tests for some amino acid decarboxylases and for the arginine dihydrolase system. Acta Pathol Microbiol Scand. 1955;36(2):158–172. doi: 10.1111/j.1699-0463.1955.tb04583.x. [DOI] [PubMed] [Google Scholar]
- SZILAGYI I., SZABO I. Microchemical method for the determination of Sakaguchi-positive antibiotics. Nature. 1958 Jan 4;181(4601):52–53. doi: 10.1038/181052a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]