Abstract
Objectives:
To evaluate the safety and effectiveness of once-daily gastroretentive gabapentin (G-GR) for the treatment of postherpetic neuralgia in real-world clinical practice.
Materials and Methods:
Patients aged 18 years and above were divided into 2 cohorts: patients aged 70 years and below and patients above 70 years. All patients were titrated to 1800 mg G-GR/d over 2 weeks and maintained at that dosage for 6 weeks, for 8 weeks total treatment. To reflect clinical practice, exclusion criteria were limited to those in the product label. Efficacy was assessed using a visual analog scale (VAS) and the Brief Pain Inventory. Patient/Clinician Global Impression of Change scales were completed at week 8. Adverse events (AEs) were assessed.
Results:
The efficacy population included 190 patients (110, 70 y and below; 80, above 70 y). The mean percent change in VAS score at week 8 from baseline was −21.3%/−20.4% (70 y and below/above 70 y). The proportion of patients with a ≥30% reduction in VAS score from baseline was 51.8%/55.0% (70 y and below/above 70 y) and was 42.7%/37.5% for a ≥50% reduction. Brief Pain Inventory scores were all significantly reduced by week 8. On the Patient Global Impression of Change instrument, more patients aged 70 years and below reported feeling “much” or “very much” improved from baseline (59.0% vs. 40.3%). G-GR was generally well tolerated. Thirty-seven (18.8%) patients experienced AEs that led to discontinuation. No patients died and 5 (2.5%) patients experienced serious AEs. The most common G-GR-related AEs (70 y and below/above 70 y) were dizziness (11.7%/16.3%) and somnolence (3.6%/8.1%).
Discussion:
In real-world clinical practice, G-GR seems to be an effective, well-tolerated treatment option for patients with postherpetic neuralgia, regardless of age.
Key Words: postherpetic neuralgia, gabapentin, gastroretentive, elderly
Postherpetic neuralgia (PHN) is defined as the persistence of pain for 3 or more months after the resolution of acute herpes zoster.1–3 Gabapentin has been shown to effectively reduce pain resulting from PHN and is considered a first-line treatment.4 However, potential benefits from treatment with gabapentin may be limited by its intrinsic properties, including a short half-life of 5 to 7 hours5 and its uptake by a saturable uptake transporter,6,7 which together require immediate-release gabapentin to be taken at least 3 times per day.
Application of a novel gastroretentive technology to gabapentin to improve its pharmacokinetics led to a formulation that can be administered once daily.8 The efficacy and safety of gastroretentive gabapentin (G-GR) was established in 2 double-blind, placebo-controlled, multicenter phase 3 clinical studies comparing G-GR 1800 mg with placebo.9,10 Integrated analyses of these studies demonstrated that compared with placebo, G-GR significantly reduced the average pain score as early as day 2, that pain scores continued to decrease over time, and that pain scores remained significantly lower for G-GR than for placebo through the end of the treatment (week 10).11 Compared with placebo, more patients treated with G-GR also reported feeling “much” or “very much” improved on the Patient Global Impression of Change (PGIC) and Clinical Global Impression of Change (CGIC) instruments. The most common adverse events (AEs) observed for G-GR-treated patients were dizziness (10.9%), somnolence (4.5%), and headache (4.2%).11,12 These rates of dizziness and somnolence were lower than what was observed in similar clinical studies with immediate-release gabapentin (dizziness, 28.0%; somnolence, 21.4%).13 In the G-GR clinical studies, 6.9% of patients in the placebo arm and 9.7% of patients in the G-GR arm discontinued study treatment due to AEs, most commonly because of dizziness.14
Clinical trials do not always accurately reflect the efficacy and safety of a drug in real-world clinical practice. This phase 4 open-label study was conducted to assess the safety and effectiveness of once-daily G-GR in a relatively unselected group of patients with PHN in real-world use.
MATERIALS AND METHODS
Study Design
Patients from 37 investigational sites in the United States participated in this open-label, single-arm, clinical trial, which was conducted from September 2011 to March 2012. Enrolled patients were divided into 2 cohorts—patients aged 70 years and below and patients above 70 years. The study period included a 2-week titration to 1800 mg G-GR once daily per the approved prescribing information, followed by a 6-week dosage maintenance period, for a total treatment period of 8 weeks. At the end of the study, or early termination, patients underwent a 1-week dose tapering. The study protocol was approved by the appropriate institutional review boards/ethics committees for each center and was conducted in accordance with International Conference on Harmonization Good Clinical Practice guidelines. Written informed consent was obtained from each patient before enrollment.
Patient Selection and Methods
To best reflect the real-world population of patients with PHN, patients were relatively unselected. Adult males or females aged 18 years and above with active PHN were eligible to enroll in this study, regardless of their baseline pain scores. Pregnant women or nursing mothers, patients with hypersensitivity to gabapentin, and patients who had an estimated creatinine clearance of <30 mL/min (as calculated by the Cockroft-Gault Method), or were on hemodialysis were excluded. There were no restrictions on the use of prior medications (including gabapentin and pregabalin). Use of concomitant neuropathic pain medication was permitted and documented through completion of the last study visit (week 8 for patients who continued G-GR therapy or week 9 for patients who underwent dose tapering).
There were 3 office visits during the study: (1) at baseline/dosing, (2) at the end of week 2, and (3) at the end of week 8 (or early termination). There was a follow-up phone call at the end of G-GR dosage tapering. At each office visit, patients completed (1) a visual analog scale (VAS) to estimate their pain intensity during the previous 24 hours and (2) the Brief Pain Inventory (BPI). The PGIC and the investigator-rated CGIC were completed at week 8 (or early termination) visit. A physical examination was performed at the baseline visit. Vital signs and weight were recorded at the baseline and week 8 (or early termination) visits. AEs and the use of concomitant neuropathic pain medications were assessed at each study visit and at the posttapering phone call. The duration of PHN was defined as the number of months between the PHN start date and the date of informed consent.
Treatments
During the 2-week titration period, dosages were increased to 1800 mg/d using the approved dosing schedule (day 1, 300 mg; day 2, 600 mg; days 3 to 6, 900 mg; days 7 to 10, 1200 mg; days 11 to 14, 1500 mg, day 15, 1800 mg). Doses were taken with the evening meal. No dosing adjustments were permitted. Patients continued on a stable dosage of 1800 mg/d for an additional 6 weeks, followed by a 1-week dose taper.
Endpoints
The primary efficacy endpoint was the change in VAS score from baseline to the end of the study (week 8 or early termination) for the efficacy population. Secondary efficacy variables included percent change in the VAS score baseline to week 2 and week 8 (or early termination), the proportion of patients with ≥30% reduction from baseline in VAS score at week 2 and week 8, the proportion of responders with ≥50% reduction from baseline in VAS score at week 2 and week 8, the proportion of responders with ≥20 mm reduction from baseline in VAS score at week 2 and week 8, the mean change from baseline to week 8 (or early termination) in BPI interference scores, and the proportion of patients who were “much” or “very much” improved on the PGIC and CGIC instruments at week 8 (or early termination).
Statistical Methods
The efficacy population included all patients who received ≥1 dose of study drug, completed the VAS at baseline, and completed ≥1 postbaseline VAS. The null hypothesis that the change in VAS scores from baseline to week 8 or early termination equaled 0 was tested at 2-tailed α=0.05 by using a paired t test. Descriptive statistics and Clopper-Pearson exact 95% confidence intervals were calculated for the mean change from baseline. Last observation carried forward (LOCF) methodology was used to impute missing data. For BPI, the average of the 7 interference scores (general activity, mood, walking ability, normal work, relationship, sleep, and enjoyment of life) was calculated, and if any of the interference scores were missing, was used to impute the missing score(s) before computing the average. Data for patients with BPI interference scores of 0 at baseline were not included in the change calculations. If an observation from a scheduled visit was missing, the value for that visit was set to missing. In the case of multiple observations at a specific visit, the observation closest to the target visit was used in the analyses. If observations had the same distance (before and after) from the target visit, data from the later observation were used. If repeated measurements were taken on the same day, the last measurement was used. All endpoint analyses were performed for the subgroups of patients aged 70 years and below and those above 70 years.
The safety population included all patients who received ≥1 dose of study drug. AEs were reported at the week 2 and week 8 clinic visits and during the posttapering phone visit. The incidence of AEs was summarized by type, severity, and relation to study drug. Safety variables included comparison of the incidence and severity of AEs reported during treatment with G-GR, the use of concomitant neuropathic pain medications, and the number of patients who prematurely discontinued G-GR treatment. All AEs were linked to system organ class (SOC) and preferred term (PT) using Medical Dictionary for Regulatory Activities (MedDRA), version 14.0. Patients were counted under multiple SOCs and PTs, but for each SOC and PT, a patient was counted once. If a patient had the same AE on multiple occasions, the highest severity or relationship recorded for the event was presented. If the severity of an AE was missing in the database, it was programmed to be severe. If the relationship was missing, the AE was considered as definitely related.
RESULTS
A total of 201 patients enrolled in the study. The efficacy population included 190 patients and the safety population included 197 patients (Table 1). One hundred ninety-seven (98.0%) patients were dosed: 38 (18.9%) patients discontinued before the end of the 2-week titration period and 139 (69.2%) patients completed 8 weeks of treatment. The most common reasons for study discontinuation included AEs (18.4%) and withdrawal of consent (7.5%) (Table 1). A total of 35 (17.8%) patients had one or more protocol deviations. The most common deviations were being out of the study-visit window (9.6%), dosing errors (3.0%), and dosing noncompliance (3.0%).
TABLE 1.
Patient Disposition

The mean age (SD) of patients in the efficacy population was 67.4 (12.8) years (range, 18 to 92). The majority of patients were white (84.3%) and 92.4% had a baseline VAS score of >20 mm (Table 2). A total of 84 (42.6%) patients were taking a concomitant neuropathic pain medication at baseline, primarily opioids (28.9%) and anticonvulsants (13.2%).The majority (96.8%) of patients did not change concomitant pain medications during the course of the study. Four (2.1%) patients who were taking no medication at baseline added medication for ≥7 days postbaseline, and 2 (1.1%) patients who were taking a neuropathic pain medication at baseline stopped taking one for ≥7 days postbaseline. Adherence to the treatment regimen was high—89.8% of patients completed the 2-week titration period without missing a dose and 67.2% of patients missed no doses throughout the study.
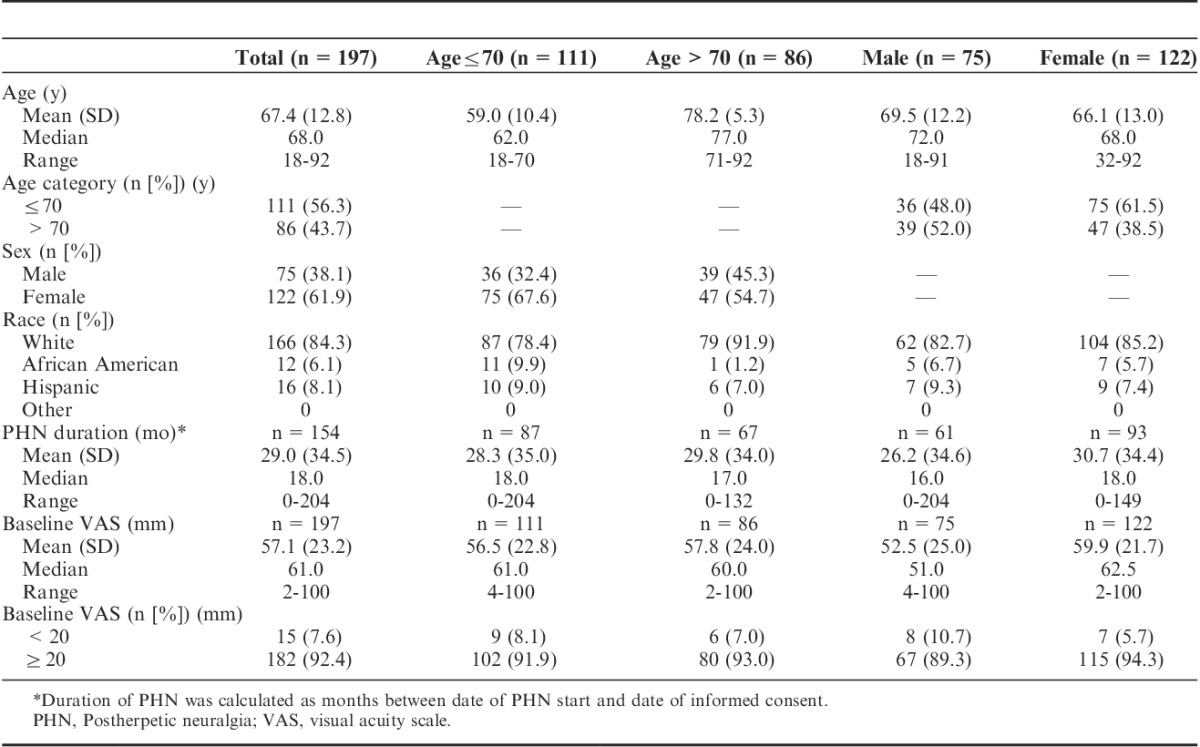
TABLE 2.
Demographics and Baseline Disease Characteristics (Safety Population, n=197)
Efficacy
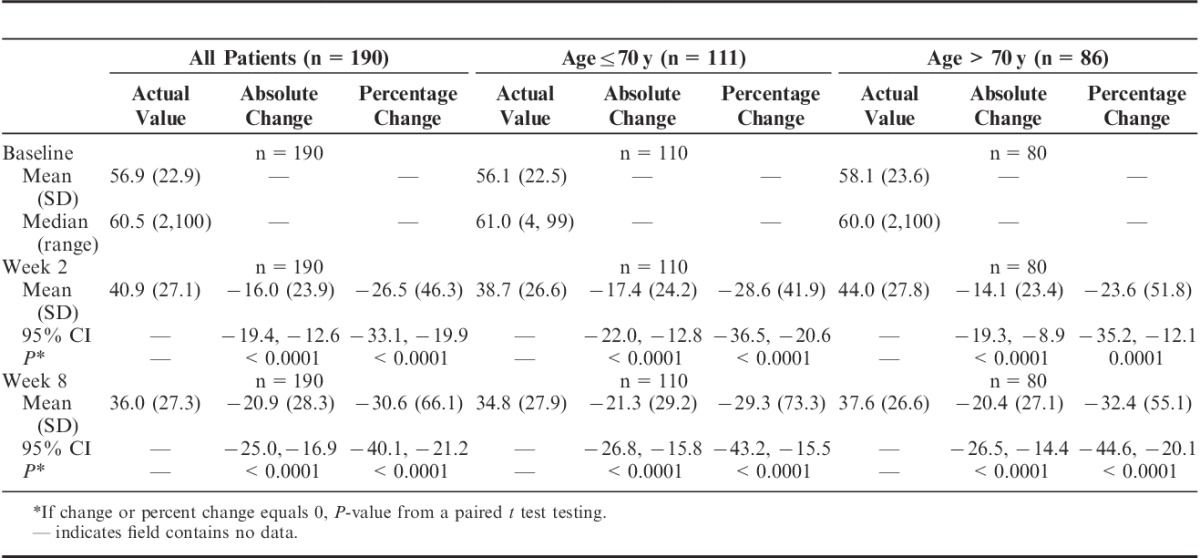
For the primary efficacy endpoint of change in VAS score, the mean change from baseline to week 2 in VAS score was 16.0 mm with a mean percent change from baseline of 26.5%; both were statistically significant (P<0.0001; Table 3). The mean change in VAS score from baseline to week 8 of 20.9 mm with a mean percent change of 30.6% were also both statistically significant (P<0.0001). Mean percent changes in VAS score at weeks 2 and 8 were similar for patients regardless of age (70 y and below vs. above 70 y): week 2: 28.6% versus 23.6%; week 8: 29.3% versus 32.4% (Table 3).
TABLE 3.
Visual Analog Scale, Change From Baseline (Efficacy Population, n=190)
Nearly half (45.8%) of all the patients had a ≥30% reduction in VAS score from baseline to week 2, and 53.2% had ≥30% reduction in VAS score at week 8 (Fig. 1). The proportion of patients who had a ≥50% reduction in VAS score from baseline was 33.2% at week 2 and 40.5% at week 8, and 37.4% of patients had a reduction in VAS score from baseline of ≥20 mm at week 2, which increased to 51.1% by week 8. Analysis of VAS responders at week 8 by age revealed very similar changes in VAS scores from baseline for the 2 age subgroups (70 y and below vs. above 70 y): 51.8% versus 55.0% had a ≥30% reduction; 42.7% versus 37.5% had a ≥50% reduction; and 47.3% versus 56.3% had a reduction of ≥20 mm (Fig. 1).
FIGURE 1.
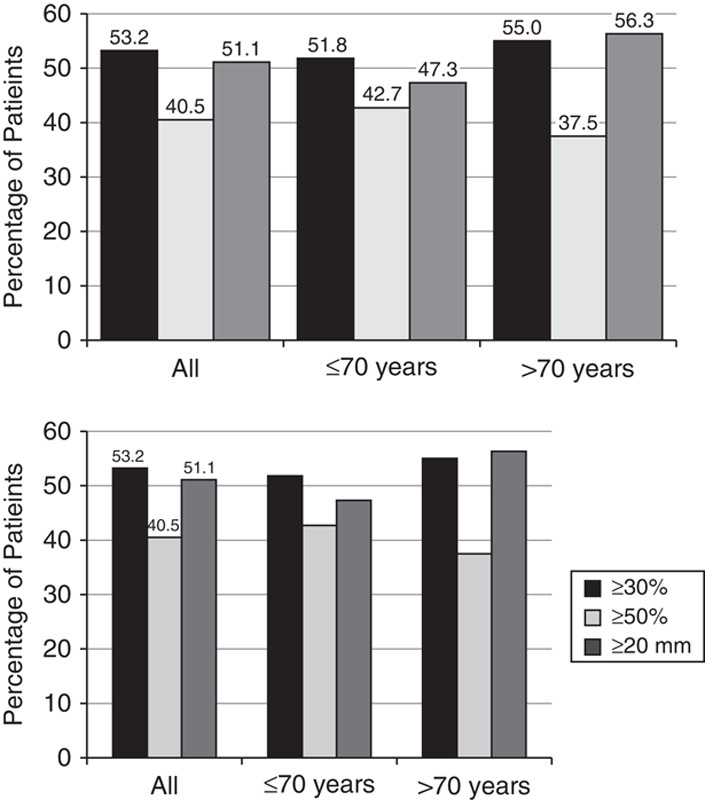
Percentage of patients with a ≥30%, ≥50%, or ≥20 mm reduction in the Visual Analog Scale (VAS) from baseline to Week 8, LOCF. Patients were groups as All, ≤70 years, and >70 years.
The secondary efficacy analyses included the BPI, which measures changes in a patient’s perceived pain (worst pain, least pain, and average pain) and the degree to which pain interferes with their life and activities. The 7 types of interference evaluated by the BPI include general activity, mood, walking ability, normal work, relationship, sleep, and enjoyment of life. In addition, an average of the 7 interference scores was calculated. Patients reported reductions in interference scores at week 2, which continued to decrease until the end of the study (week 8). At week 8, the mean change from baseline in the worst (−2.0), least (−1.0), and average (−1.5) pain scores in the last 24 hours were statistically significant at P<0.0001 (Fig. 2A). Mean changes in the worst, least, and average pain were similar between age subgroups. Decreases in all 7 types of pain interference were also statistically significant at P≤0.0001. However, compared with patients in the above 70-year subgroup, patients in the 70-year and below subgroup reported slightly greater reductions in the individual BPI interference scores (Fig. 2B). The use of concomitant pain medications did not significantly affect pain scores as assessed by the VAS or the BPI, nor were there any differences in the individual BPI interference scores.
FIGURE 2.
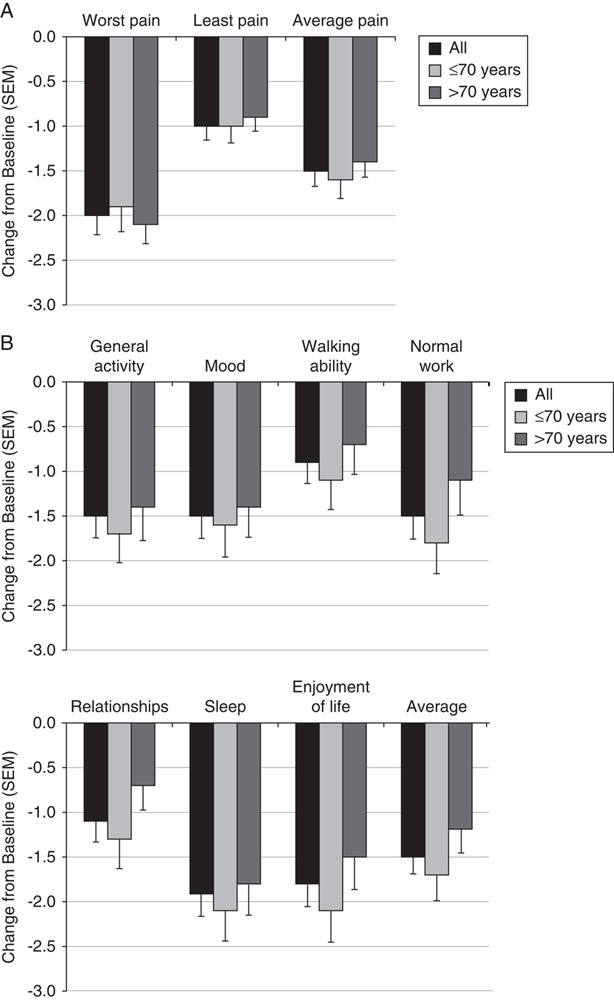
Mean change from baseline to week 8 in pain severity and pain interference for the Brief Pain Index. Patients were classified as all, 70 years and below, and above 70 years. A, Changes in patients’ perceived worst, least, and average pain. B, Changes in 7 types and the average of interference scores.
According to the PGIC instrument, the proportion of all patients who reported feeling “much” or “very much” improved relative to baseline at week 8 was 51.1% (Fig. 3). The CGIC instrument reported a similar proportion of improved patients (53.3%). A higher proportion of younger patients reported feeling “much” or “very much” improved on both instruments compared with patients in the older subgroup (70 y and below vs. above 70 y): PGIC: 59.0% versus 40.3%; CGIC: 61.9% versus 41.6% (Fig. 3). PGIC and CGIC scores did not seem to be affected by the use of concomitant pain medications.
FIGURE 3.
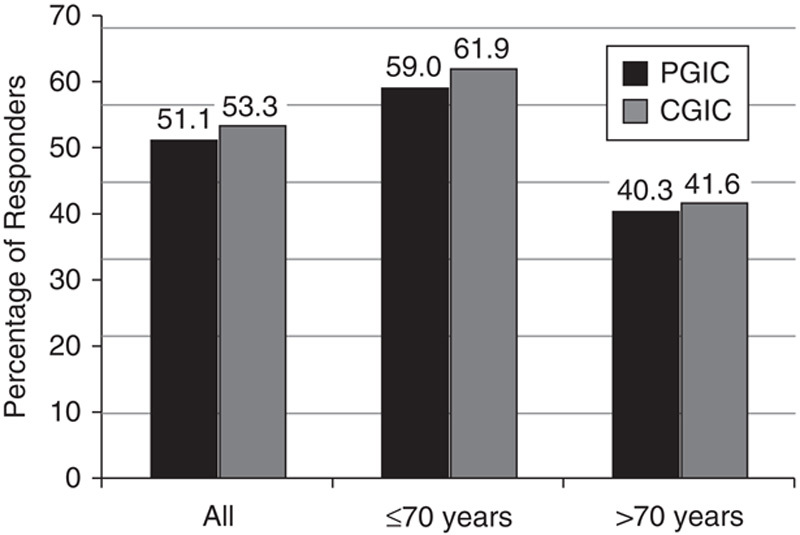
Responders according to the Patient Global Impression of Change (PGIC) and Clinical Global Impression of Change (CGIC). Percentage of patients in the all, 70 years and below, and above 70 years subgroups who reported feeling “much” or “very much” improved compared with their symptoms at baseline.
Safety Results
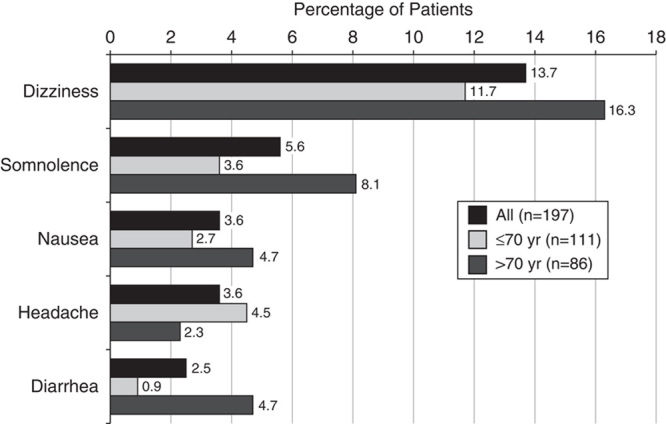
A total of 100 (50.8%) patients in the study experienced an AE and 32% of patients experienced at least 1 G-GR-related AE (Table 4). The G-GR-related AEs experienced by ≥3% of patients were nervous system and gastrointestinal disorders. For the total population, these included dizziness (13.7%), somnolence (5.6%), nausea (3.6%), headache (3.6%), and diarrhea (2.6%) (Fig. 4). The prevalence of these AEs decreased rapidly, and by the end of the titration period had reached sustained levels under 2% (Fig. 5).
TABLE 4.
Summary of Adverse Events (Safety Population, n=197)
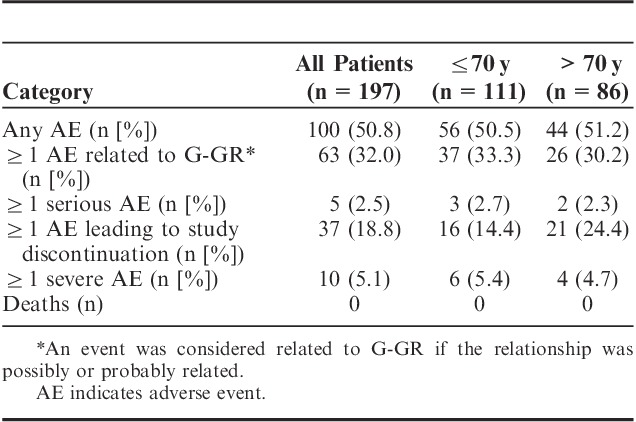
FIGURE 4.
Adverse events occurring in ≥3% of patients. Patients were categorized as all, 70 years and below, and above 70 years groups.
FIGURE 5.
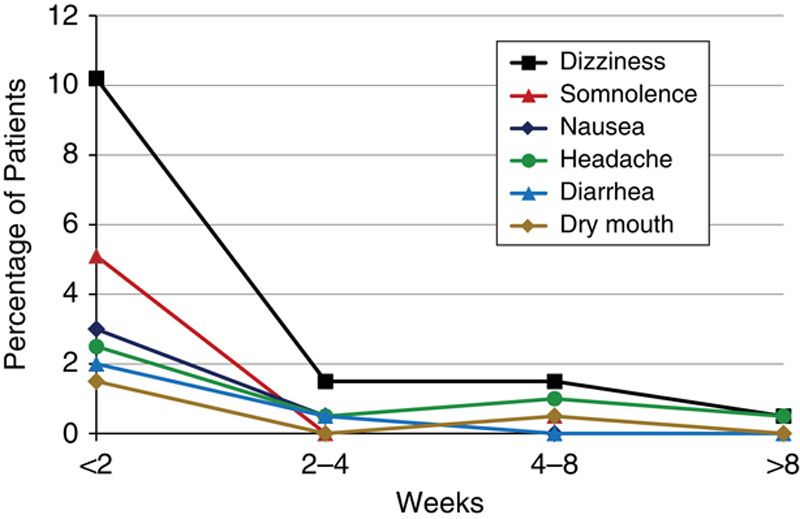
Prevalence of adverse events (AEs) over time. AEs were recorded at the week 2 and week 8 study visits, and during the posttapering phone visit by the week during which they occurred. The titration period comprised the first 2 weeks of the study.
A total of 37 (18.8%) patients experienced AEs that led to study discontinuation (Table 4), most frequently dizziness (6.1%) and somnolence (4.1%). No patient died during the study and 5 (2.5%) patients experienced serious AEs (SAEs). The 5 SAEs, each experienced by 1 patient, included coronary artery disease, duodenal ulcer, gout, pneumonia, hematuria, and confusional state. The patients with the SAEs of moderate pneumonia, severe hematuria, severe confusional state discontinued the study. Only 1 SAE (confusional state) was judged by the investigator to be treatment related. No severe AE was reported by more than 1 patient in the study with the exception of dizziness, which occurred in 3 (1.5%) patients. There were no clinically relevant trends in the change from baseline for the vital sign measurements.
The percentage of patients experiencing ≥1 AE was nearly identical for younger and older patients—50.5% for patients 70 years and below and 51.2% for patients above 70 years (Table 4). The incidence of most AEs occurring in ≥3% of patients was higher in older patients (above 70 vs. 70 y and below)—dizziness (16.3% vs. 11.7%), somnolence (8.1% vs. 3.6%), nausea (4.7% vs. 2.7%), and diarrhea (4.7% vs. 0.9%; Fig. 4). Only headache was experienced more frequently by younger patients (2.7% vs. 0%). More patients above 70 years discontinued from the study due to AEs compared with patients 70 years and below (24.4% vs. 14.4%; Table 4). AE rates were only slightly higher in patients using concomitant therapies compared with those taking none (54.8% vs. 47.8%), even when the concomitant medications included opioids (opioids, 56.1% vs. nonopioids, 51.9%).
DISCUSSION
In this open-label study designed to approximate real-world clinical treatment of patients with PHN, G-GR provided a statistically significant reduction in pain by all measures. As measured by the change from baseline in the VAS score, the reductions in pain were statistically significant at week 2 and decreased further by week 8. The proportion of patients who had a ≥30% or ≥50% reductions in the VAS score also increased from week 2 to week 8, and the reductions were similar in magnitude to those observed at week 10 in the phase 3 clinical trials.11 Although the ability of G-GR to reduce pain was similar for patients in the 2 age subgroups (above 70 vs. 70 y and below), considerably fewer older patients reported feeling “much” or “very much” improved on the PGIC (40% vs. 59%). Also, as assessed by the BPI interference scores, pain appeared to interfere more with the lives of the older patients. These observations are consistent with the results of studies of pain in the elderly, who compared with younger patients, tend to be less tolerant of pain and may be less responsive to treatment.15
To mimic the real-world clinical management of PHN, a condition for which polypharmacy is common, this study permitted the usage of concomitant prescription and/or over-the-counter neuropathic pain medications. Nearly half of the patient population (42.6%) was taking a concomitant pain medication at baseline, with opioids (28.9%) and anticonvulsants (13.2%) used most frequently. However, as measured by the VAS, BPI, CGIC, and PGIC instruments reductions in pain or the effect of pain on quality of life, were similar regardless of whether patients used any concomitant medication, suggesting that G-GR alone provided sufficient pain relief. This conclusion is supported by the observation that only 2.1% of patients added any pain medication during the study.
The overall percent of patients experiencing any AE was very similar between this study and the previous phase 3 studies (51% vs. 54%), as were the profiles of the most common AEs, dizziness and somnolence.11,12 In this study, dizziness was reported for 13.7% of patients compared with 10.9% in the 2 phase 3 studies. Somnolence was reported for 5.6% compared with 4.5% in the phase 3 studies.11,12 These AE rates were considerably lower than those reported with immediate-release gabapentin, where dizziness was reported in 24% to 33% of patients and somnolence in 17% to 27%.2,3 This is especially notable because unlike previous studies of gabapentin in PHN,2,3,9,10,16 patients with known intolerance to gabapentin or pregabalin were not excluded. Moreover, AEs occurred primarily during the titration period and subsided rapidly thereafter to sustained low levels.
Neither patient age nor use of concomitant medication appeared to significantly affect G-GR tolerability. The overall percentage of patients 70 years and below and above 70 years experiencing an AE in the current study (51% for each group) was similar to the rates observed in the phase 3 studies (58% for patients below 65 y and 52% for patients 65 y and above). Although, older patients (above 70 y) in this study experienced slightly more dizziness, somnolence, nausea, and diarrhea, and were more likely to discontinue because of AEs than in the phase 3 studies,12 this was likely due to differences in the study design. In its quest to best approximate real-world usage, this study permitted entry of patients previously unable to tolerate gabapentin or pregabalin and the use of concomitant medications was permitted. Both of these factors could affect tolerability in older, potentially frailer patients. The slight difference in the boundaries of the age subgroups between the current study and that used in the phase 3 studies (70 vs. 65 y) could also affect the comparison as patients in the above 70-year subgroup were older on an average and therefore potentially more susceptible to certain AEs. However, the use of concomitant medication did not seem to affect AE rates, even when those medications included opioids. Furthermore, although nearly half of the patients used concomitant medication(s) throughout the study, and higher numbers of doses per day has been linked to lower adherence to treatment regimens,17 the adherence in this study remained high, suggesting that once-daily dosing may facilitate treatment adherence.
Sleep disturbance, a common problem among patients with PHN,18,19 can complicate disease management. Two primary factors seem to contribute to PHN-associated sleep disturbance. First, the intensity of pain associated with PHN tends to be lowest in the morning, increasing throughout the day, and most severe at night.20 Second, patients who get less sleep or whose sleep quality is poor tend to be less tolerant of pain during the day.21,22 On the basis of the results from the BPI interference subscale sleep question), patients taking G-GR experienced a 37.5% reduction in sleep interference. This positive effect on sleep may result from a dosing schedule that yields peak steady-state gabapentin levels for approximately 6 to 12 hours after dosing,23,24 a time when most patients are in bed for the night. Improved sleep, and perhaps an increased ability to tolerate pain,22 may contribute in part to the increased proportion of patients who reported feeling “much” or “very much” improved on the PGIC after taking G-GR compared with placebo. However, at the end of study, the improvement in sleep over baseline was more pronounced in the subgroup of older patients—55.3% of patients in the above 70-year subgroup experienced decreased sleep interference compared with 26.7% of patients in the 70-year and below subgroup (P<0.0001). In contrast, the greatest improvement as measured by the PGIC was in the younger patients—59.0% reported feeling “much” or “very much” improved compared with 40.3% of patients above 70 years. This disparity suggests that for younger patients who are generally move active, improved sleep is less important to their overall sense of improvement, or alternatively, that younger patients require a greater reduction in sleep interference to enhance their overall sense of improvement.
Although the lack of a control arm in this study might be considered a weakness, this phase 4 study was specifically designed to mimic real-world, clinical practice and enrolled all patients with a diagnosis of PHN. There were no restrictions on the use of concomitant medication or exclusion of patients based on poor previous experience (efficacy or tolerability) with gabapentin or pregabalin and thus no enrichment for patients able to tolerate G-GR or more likely to respond to G-GR treatment. Taken together, these factors should rather be considered strengths of the study.
In summary, although there were some differences in treatment response by age, patients in both age subgroups responded significantly to G-GR treatment, and both efficacy and tolerability were very similar to that observed in the phase 3 studies. Interestingly, older patients reported a greater improvement in sleep, whereas more younger patients reported feeling “much” or “very much” improved. Taken together, it seems that in a real-world clinical setting, once-daily G-GR reduced pain intensity, improved patients’ quality of life, improved sleep, and was well tolerated. In the future, we will report on an analysis of real-world G-GR use from a large health care database.
ACKNOWLEDGMENTS
The authors thank the site investigators (see Appendix 1) for their participation in this study.
APPENDIX 1
List of Study Investigators
Perminder Bhatia (Fresno, CA)
Edward Carlos (Mobile, AL)
Christopher Case (Jefferson City, MO)
Greg Cooper (Toms River, NJ)
Barry J. Cutler (Sunrise, FL)
Michael DeSantis (Hickory, NC)
Vinayak Dongre (Naperville, IL)
Matthew Doust (Phoenix, AZ)
Michael Drass (Altoona, PA)
Edwin Dunteman (St. Louis, MO)
Mandeep Garewal (Ormond Beach, FL)
Jerome Goldstein (San Francisco, CA)
Andrew Gorman (Sun City, AZ)
Arnold Greenberg (San Francisco, CA)
Robert Griffin (Wyomissing, PA)
Jason Haffizulla (Lauderdale Lakes, FL)
Wayne Harper (Raleigh, NC)
Michael Harris (Orem, UT)
Kendrick Henderson (Cordova, TN)
John Hudson (Austin, TX)
David Kaner (Bedford TX)
Herbert G. Markley (Worcester, MA)
Frank Martinez (Corpus Christi, TX)
Laszlo J. Mate (North Palm Beach, FL)
Peter McAllister (Fairfield, CT)
Ida Minevich (Brighton, MA)
Bruce Nicholson (Allentown, PA)
Aris Nikas (San Antonio, TX)
Patrick Peters (San Antonio, TX
Mahendra Sanapati (Evansville, IN )
Sanjiv Sharma (Toms River, NJ
Joseph Stewart (Tuscaloosa, AL)
Marvin Tark (Marietta, GA)
Edward Tavel (Charleston, SC)
Michael M. Tuchman (Palm Beach Gardens, FL)
Jeanette Wendt (Tucson, AZ)
John Wilson (Naples, FL)
Footnotes
Supported by the Depomed Inc., Newark, CA.
M.S. is an employee and a shareholder of Depomed Inc. The remaining authors declare no conflict of interest.
REFERENCES
- 1.Dworkin RH, Portenoy RK. Pain and its persistence in herpes zoster. Pain. 1996;67:241–251. [DOI] [PubMed] [Google Scholar]
- 2.Rice AS, Maton S. Postherpetic Neuralgia Study Group. Gabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled study. Pain. 2001;94:215–224. [DOI] [PubMed] [Google Scholar]
- 3.Rowbotham M, Harden N, Stacey B, et al. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280:1837–1842. [DOI] [PubMed] [Google Scholar]
- 4.Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85:S3–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goa KL, Sorkin EM. Gabapentin. A review of its pharmacological properties and clinical potential in epilepsy. Drugs. 1993;46:409–427. [DOI] [PubMed] [Google Scholar]
- 6.Bockbrader HN. Clinical pharmacokinetics of gabapentin. Drugs Today. 1995;318:613–619. [Google Scholar]
- 7.Stewart BH, Kugler AR, Thompson PR, et al. A saturable transport mechanism in the intestinal absorption of gabapentin is the underlying cause of the lack of proportionality between increasing dose and drug levels in plasma. Pharm Res. 1993;10:276–281. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Cowles VE, Hou E. Pharmacokinetics of gabapentin in a novel gastric-retentive extended-release formulation: comparison with an immediate-release formulation and effect of dose escalation and food. J Clin Pharmacol. 2011;51:346–358. [DOI] [PubMed] [Google Scholar]
- 9.Sang CN, Sathyanarayana R, Sweeney M. Investigators D-S. Gastroretentive gabapentin (G-GR) formulation reduces intensity of pain associated with postherpetic neuralgia (PHN). Clin J Pain. 2012;29:281–288. [DOI] [PubMed] [Google Scholar]
- 10.Wallace MS, Irving G, Cowles VE. Gabapentin extended-release tablets for the treatment of patients with postherpetic neuralgia: a randomized, double-blind, placebo-controlled, multicentre study. Clin Drug Investig. 2010;30:765–776. [DOI] [PubMed] [Google Scholar]
- 11.Rauck RL, Irving GA, Wallace MS, et al. Once-daily gastroretentive gabapentin for postherpetic neuralgia: integrated efficacy, time to onset of pain relief and safety analyses of data from two phase 3 multicenter, randomized, double-blind, placebo-controlled studies. J Pain Symptom Manage. 2013;46:219–228. [DOI] [PubMed] [Google Scholar]
- 12.Irving GA, Sweeney M. Tolerability and safety of gastroretentive once-daily gabapentin tablets for the treatment of postherpetic neuralgia. J Pain Res. 2012;5:203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfizer Inc. Neurontin® (gabapentin) capules, Neurontin® (gabapentin) tablets, Neurontin® (gabapentin) oral solution. US prescribing information. Available at: http://labeling.pfizer.com/ShowLabeling.aspx?id=630. Accessed July 18, 2012.
- 14.Depomed Inc. GRALISE (gabapentin) tablets. US prescribing information. Available at: http://www.gralise.com/lib/PDFS/GRALISE_PI.pdf. Accessed July 2013.
- 15.McCleane G. Pharmacological pain management in the elderly patient. Clin Interv Aging. 2007;2:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irving G, Jensen M, Cramer M, et al. Efficacy and tolerability of gastric-retentive gabapentin for the treatment of postherpetic neuralgia: results of a double-blind, randomized, placebo-controlled clinical trial. Clin J Pain. 2009;25:185–192. [DOI] [PubMed] [Google Scholar]
- 17.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310. [DOI] [PubMed] [Google Scholar]
- 18.van Seventer R, Feister HA, Young JP, Jr, et al. Efficacy and tolerability of twice-daily pregabalin for treating pain and related sleep interference in postherpetic neuralgia: a 13-week, randomized trial. Curr Med Res Opin. 2006;22:375–384. [DOI] [PubMed] [Google Scholar]
- 19.Sabatowski R, Galvez R, Cherry DA, et al. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trial. Pain. 2004;109:26–35. [DOI] [PubMed] [Google Scholar]
- 20.Odrcich M, Bailey JM, Cahill CM, et al. Chronobiological characteristics of painful diabetic neuropathy and postherpetic neuralgia: diurnal pain variation and effects of analgesic therapy. Pain. 2006;120:207–212. [DOI] [PubMed] [Google Scholar]
- 21.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–369. [DOI] [PubMed] [Google Scholar]
- 22.Edwards RR, Almeida DM, Klick B, et al. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137:202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordi T, Hou E, Kasichayanula S, et al. Pharmacokinetics of gabapentin after a single day and at steady state following the administration of gastric-retentive, extended-release, and immediate-release tablets: a randomized, open-label, multiple-dose, three-way crossover, exploratory study in healthy subjects. Clin Ther. 2008;30:909–916. [DOI] [PubMed] [Google Scholar]
- 24.Argoff CE, Chen C, Cowles VE. Clinical development of a once daily gastroretentive formulation of gabapentin for treatment of postherpetic neuralgia: an overview. Expert Opin Drug Deliv. 2012;9:1147–1160. [DOI] [PubMed] [Google Scholar]


