Abstract
Purpose
Wound care for partial-thickness burns should alleviate pain, decrease hospital length of stay, and be readily applied to a variety of wounds. The effectiveness of Biobrane (UDL Laboratories, Rockford, IL) is compared with that of Beta Glucan Collagen (BGC; Brennan Medical, St. Paul, MN) in a retrospective cohort study.
Methods
A retrospective chart review of all children treated at a tertiary care pediatric hospital between 2003 and 2009 identified patients with partial-thickness burns treated with Biobrane. These patients were compared with historical controls treated with BGC.
Results
A total of 235 children between the ages of 4 weeks and 18 years with an average of 6.0% body surface area partial-thickness burns were treated with Biobrane. In a multivariate statistical analysis, patients treated with Biobrane healed significantly faster than those treated with BGC (Biobrane vs BGC: median, 9 vs 13 days; P = .019; hazard ratio, 1.68). In addition, patients who required inpatient treatment trended toward having shorter length of hospital stay in the Biobrane group (2.6 vs 4.1 days, P = .079).
Conclusion
Partial-thickness burn care consists of early debridement and application of a burn wound dressing. Biobrane dressings result in faster healing compared with BGC and may decrease hospital length of stay for patients requiring inpatient admission.
Keywords: Pediatric burns, Partial-thickness, Burn treatment, Burn dressings, Biobrane
Burn injuries in children account for more than 50,000 hospitalizations per year and are the most common cause of death in the home [1]. Scald burns are the leading mechanism of burn injury in children younger than 5 years, with flame burns being more common in older children [2]. Partial-thickness burns are exceedingly common in children and present a challenge in treatment. Partial-thickness burns are characterized as superficial or deep and typically present with pain, fluid-filled blisters, and redness. Unlike full-thickness burns, effective wound care in the treatment of partial-thickness burns is crucial to good wound-healing outcome. The management of these burns relies on preserving the unburned dermal and epidermal appendages in the wound bed, promoting reepithelialization. The optimal partial-thickness burn wound dressing provides protection from bacterial contamination, decreased heat and water loss from the wound, decreased pain as sensory nerve terminals are covered, elimination of daily dressings changes, and ease of instructing patient/family on home [3,4]. In addition, increasing pressure to be cost-effective in the use of inpatient services makes effective outpatient treatment of partial-thickness burns attractive [5].
The main principles of treatment in partial-thickness burns are early debridement of nonviable tissue and subsequent application of an appropriate dressing. Biobrane (UDL Laboratories, Rockford, IL), a biosynthetic material that promotes reepithelialization, was chosen as our burn dressing of choice in these patients because of its versatility, ease of use, and minimization of frequent dressing changes [6,7]. This report describes our experience with the use of Biobrane for primary coverage of partialthickness burns. Using patients treated in a similar fashion with Beta Glucan Collagen Matrix (BGC; Brennan Medical, St. Paul, MN) from a previous study as historical controls, we compared outcome measures with the current Biobrane-treated patients.
1. Methods
A retrospective chart review was performed on 818 consecutive pediatric burn patients seen at a tertiary care pediatric hospital between January 2003 and May 2009. All new burn patients are entered into a prospective pediatric burn registry maintained by a pediatric burn nurse specialist. Permission to retrospectively review the electronic medical record was obtained from the institutional review board of the Medical University of South Carolina (HR 19222). Patient data, including age, sex, extent of burn injury, inpatient or outpatient treatment, length of hospital stay, wound care regimen, number of outpatient visits, and days to heal, were collected from the electronic medical records of inpatient and outpatient encounters. Days to heal was defined as the number of days between the burn injury and a physical examination by a clinician documenting complete epithelialization of the wound not requiring further wound care. Wounds were assessed at 24 to 48 hours to determine extent of Biobrane adherence, then at 5 to 7 days, and again at 9 to 12 days.
Burn patients typically presented to the emergency department and were initially assessed by house staff or pediatric burn nurse specialists. Candidates for placement of Biobrane wound dressings were those patients younger than 18 years who were suspected to have fresh (generally <24 hours from time of injury), clean, partial-thickness burn injury not involving the face, ears, or genitalia/perineum. These were the patients enrolled in the study. Biobrane was not a treatment option for those patients with obvious full-thickness burns or for those injuries caused by chemicals or electricity because there is a high degree of suspicion of full-thickness injury with these mechanisms. Those patients who presented with another form of treatment already initiated were not automatically excluded from consideration for Biobrane use unless the wound showed development of eschar.
After cleansing and debridement of the burn wound, Biobrane was applied directly to the wound, including an extension of the Biobrane beyond the wound margin to assure complete coverage. Biobrane was secured to the area with tape strips, followed by fluff gauze dressings and gauze wrap to wick drainage away from the wound. This entire dressing was then held firmly in place with an elastic bandage, typically Coban dressing (3M, St. Paul, MN). The dressings were left in place for 24 to 48 hours, at which time the outer wrap and gauze dressings were removed. A complete assessment of the patient and wound was performed, noting adherence of the Biobrane, type and amount of drainage, odor, redness, presence of fever, and other indicators of wound infection. Because this is a pediatric population, in most cases, a light gauze dressing and elastic bandage was replaced to protect the area from mechanical disturbance. At this time, both the parents and the patient, if age appropriate, were instructed on the goals of managing the burn wound with Biobrane. Verbal and written instructions on the care of the dressing were provided.
Approximately 7 to 10 days after Biobrane application, the dry, well-adhered Biobrane was coated with petroleum jelly or antibiotic ointment to begin softening the dressing before removal from the healed wound. Complete removal of the Biobrane dressing with evidence of reepithelialization generally occurred approximately 10 days after application, at which time care of the wound transitioned to moisturizing lotion and sun protection.
The Biobrane cohort identified in this study was compared with historic control patients with partial thickness burns who were treated at our institution between 1997 and 1999 with BGC [3]. Patient demographics and outcome variables were measured and reported in the same manner in both the BGC and Biobrane cohorts, and BGC inclusion criteria were the same as previously mentioned for the Biobrane group (younger than 18 years with a clean, fresh, nonelectrical, nonchemical partial-thickness burn not involving the face, ears, or genitalia/perineum). Wilcoxon signed rank test and Cox proportional hazards model using SAS v9.1 (SAS Institute, Cary, NC) were used to compare the groups.
2. Results
Of the 818 charts reviewed during the study period, 291 (35.6%) patients who had superficial or deep partial-thickness burns treated with Biobrane dressing were identified for this study. The remaining patients were not candidates for Biobrane wound care for numerous reasons, including having burns not anatomically amenable to Biobrane application, having full-thickness burns, being late referrals to our center, or for other reasons (see Table 1).
Table 1.
Criteria for Biobrane exclusion
| Criteria | Number (n = 527) |
% |
|---|---|---|
| Did not see patient for >2 d at burn | 116 | 22 |
| Anatomical: face/ear/foot/fingers/perineum | 82 | 15.6 |
| Third-degree burn | 67 | 12.7 |
| <1% burn | 59 | 11.2 |
| Silvadene already initiated | 46 | 8.7 |
| Not a burn wound | 42 | 8.0 |
| Blisters intact | 41 | 7.8 |
| First-degree burn | 17 | 3.2 |
| Breakdown (scar) | 16 | 3.0 |
| Unknown | 15 | 2.8 |
| Healed burn | 6 | 1.1 |
| BGC used | 5 | 0.95 |
| Scattered burn | 5 | 0.95 |
| Ingestion | 4 | 0.80 |
| Chemical burn | 4 | 0.80 |
| Inhalation only | 1 | 0.20 |
| Electrical burn | 1 | 0.20 |
Table 2 summarizes the data collected for this study. Of the 291 partial-thickness burn patients treated with Biobrane, 235 had complete data and were included in this analysis. The Biobrane-treated patients identified in this current study were compared with historical controls of pediatric patients with partial-thickness burns treated with BGC. There were no differences in patient's ages (P = .89). Percent body surface area burned differed significantly between groups (P = .0008), so a Cox proportional hazards model was used for covariate analysis of outcome variables. Biobrane-based wound dressing demonstrated significantly fewer days to heal (P = .019). A Cox proportional hazards test was performed and demonstrated a hazard ratio of 1.68 (95% confidence interval, 1.09–2.60) (see Fig. 1). The median hospital length of stay (1 day) and number of outpatient visits (3 visits) were the same in both groups. In the Biobrane cohort, 46% of patients were treated on an outpatient basis only, compared with 23% in the BGC cohort; however, this difference did not reach statistical significance (P = .18).
Table 2.
Summary of data
| Biobrane | BGC | P | |
|---|---|---|---|
| n | 235 | 43 | |
| Age (y), mean ± SD (range) | 5.8 ± 5.2 (4 wk to 18 y) | 5.5 ± 4.7 (6 wk to 16 y) | .89a |
| %BSA, mean ± SD (range) | 6.0 ± 5.3 (0.5–30) | 9.3 ± 8.0 (1–35) | .0008a |
| LOS (d); mean, median ± SD (range) | 2.6, 1 ± 6.1 (0–41) | 4.1, 1 ± 6.4 (1–24) | .079b |
| OP visits; mean, median ± SD (range) | 3.9, 3 ± 2.4 (1–22) | 3.8, 3 ± 2.1 (1–11) | .65a |
| Days to heal, median ± SD (range) | 9 ± 5.0 (1–41) | 13 ± 8.2 (5–43) | .019b |
| Outpatient only, n (%) | 109 (46%) | 10 (23%) | .18b |
LOS indicates length of inpatient stay; OP, outpatient visits; BSA, body surface area.
Wilcoxon signed rank test.
Cox proportional hazards model.
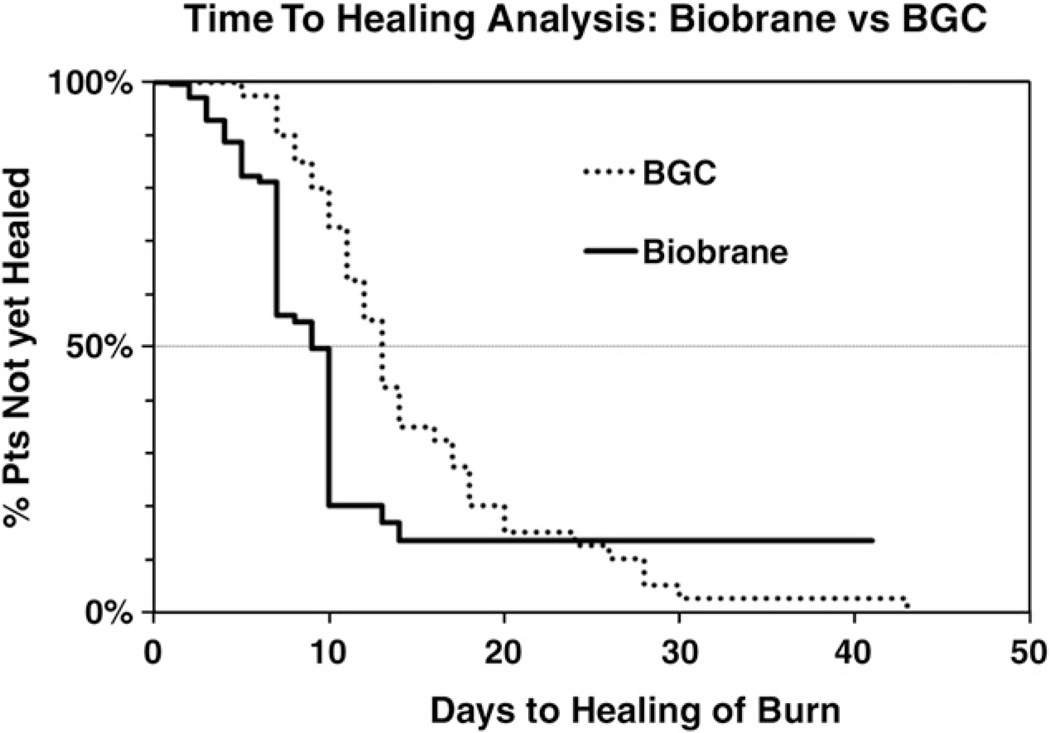
Fig. 1.
Time-to-healing analysis of Biobrane vs BGC in pediatric partial-thickness burns. Using the Cox proportional hazards model, the days to complete healing of the burn is demonstrated for Biobrane (solid line) vs BGC (dotted line). The hazard ratio associated with healing time comparing Biobrane vs BGC was 1.68, meaning that a patient receiving the Biobrane treatment who has not yet healed by a certain day has 1.68 times the chance of being healed by the next day compared with someone in the BGC group (hazard ratio, 1.68*; 95% confidence interval, 1.09–2.60). *Cox proportional hazards test.
3. Discussion
Several treatment options exist for the treatment of partial-thickness wounds. Thorough cleansing, wound debridement, and application of antimicrobial agents such as mafenide acetate (Sulfamylon; UDL Laboratories), 1% silver sulfadiazine (Silvadene; Monarch Pharmaceuticals, Bristol, TN), or bacitracin were the standard of care until synthetic skin substitutes and modern topical agents were developed [8]. Contemporary dressings, including Biobrane, BGC, Mepilex Ag (Molnlycke, Goteborg, Sweden), and Aquacel Ag and Duoderm (both from ConvaTec, Skillman, NJ), among others, have been shown to decrease hospital stay, nursing care times, and pain medications [3,4,9,10]. Biobrane was the first of these synthetic skin substitutes used to promote epithelialization on partial-thickness burns and donor harvest sites [11]. Beta Glucan Collagen Matrix combines β-glucan with collagen in a mesh-reinforced wound dressing. β-Glucan, a complex carbohydrate derived from oats, is known to stimulate macrophages, contributing to wound healing [12]. Duoderm is an occlusive hydrocolloid dressing that offers barrier protection and has been shown to be effective in treating partial-thickness burns with a reduced cost compared with Biobrane [13], although Duoderm is not transparent. Aquacel Ag is a newer, transparent dressing for partial-thickness burns that has been found to be associated with decreased cost and hospital length of inpatient stay compared with Silvadene [10,14]. These advanced dressings augment the traditional principles of controlling the growth of microorganisms while restoring skin architecture and improving the physiology of healing [15].
Biobrane is a biocomposite material made from nylon mesh covered by porcine type 1 collagen that facilitates ingrowth of fibrin. Blood and serum clot in the mesh, promoting adhesion to the wound and a matrix for tissue ingrowth [16]. Biobrane has several advantages over other synthetic skin substitutes and topical dressings in the treatment of partial-thickness burns. First, the application of the dressing after cleansing and debridement is uncomplicated and can be easily taught to resident physicians, nurses, and other medical providers. Biobrane has excellent elasticity and is readily applied to burned skin involving joints. In contrast, BGC must be applied to a flat surface with minimal movement. Moreover, unlike other available advanced burn dressings, such as Mepilex Ag and Duoderm, Biobrane is transparent, aiding in early detection of infection and ease of burn wound assessment without removal of the dressing. Biobrane also offers improvement in ease of dressing changes, as opposed to Silvadene, which is usually removed daily and reapplied. Rare adverse reactions to Biobrane, including contact dermatitis and punctate scarring, have been recorded as case reports in the literature [17]. These characteristics made Biobrane the most suitable choice for use by our pediatric burn program in treating children with partial-thickness burn.
Biobrane has been shown to decrease pain and hospitalization time without additional cost compared with Silvadene in treatment of pediatric burns [2,9,18]. In our series, Biobrane decreased the number of days to heal and shortened the length of stay for inpatient treatment. In addition, Biobrane provided effective primary wound care treatment in more than 90% of the patients treated in the study period. A total of 26 patients (9%) with partial-thickness burns treated with Biobrane went on to require skin grafting in our series. These burns were typically larger contact burns, including several grease burns to the hands. Six (2%) of all Biobrane-treated patients had nonadherence of the dressing at first dressing change, leading to eventual need for skin grafting.
One of the goals of this retrospective study was to compare our recent experience using Biobrane in the treatment of pediatric partial-thickness burns to a similar group of children treated with BGC. Although the study is limited by the use of a historical control group, Biobrane is associated with a higher percentage of outpatient-only cases. Biobrane is suitable for outpatient treatment because of the ease of instructing parents and caregivers in dressing management. Caregivers are instructed to remove the outer gauze and elastic bandage every 24 to 48 hours and assess the Biobrane for adherence and signs of infection and redress. In addition, the increased proportion of outpatient-only treatments may be attributed to both the overall comfort level of our nursing and resident staff in dealing with Biobrane dressings, an important factor in a successful burn program. As opposed to BGC, which is opaque, clinical assessment of the wound for infection and nonadherence is straightforward, and parents can be instructed to monitor for these issues.
Biobrane is a versatile, effective dressing for partial-thickness burns from infancy to adulthood. In our pediatric surgery practice, Biobrane has been easy to apply, practical for outpatient wound care, and is well suited to infrequent dressing changes, minimizing the child's pain and discomfort. Our data suggest that, in comparison with BGC, a Biobrane-based wound care regimen leads to more rapid healing and may lead to shorter inpatient stays. As the financial burden of medical care steadily increases, decreasing inpatient and outpatient costs associated with pediatric partial-thickness burns is highly desirable. As newer burn wound products, such as Aquacel Ag (Aubrey Inc, Carlsnad, CA), Mepilex Ag, and AWBAT, are introduced to the market, carefully controlled studies are needed to assess outcomes and prove superiority to older products with established safety and efficacy profiles. This study provides a clinical benchmark to which other therapies should be compared.
References
- 1.Brigham PA, McLoughlin E. Burn incidence and medical care use in the United States: estimates, trends, and data sources. J Burn Care Rehabil. 1996;17:95–107. doi: 10.1097/00004630-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Bishop JF. Pediatric considerations in the use of Biobrane in burn wound management. J Burn Care Rehabil. 1995;16:311–314. doi: 10.1097/00004630-199505000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Delatte SJ, Evans J, Hebra AV, et al. Effectiveness of Beta-glucan collagen for treatment of partial-thickness burns in children. J Pediatr Surg. 2001;36:113–118. doi: 10.1053/jpsu.2001.20024. [DOI] [PubMed] [Google Scholar]
- 4.Honari S. Topical therapies and antimicrobials in the management of burn wounds. Crit Care Nurs Clin North Am. 2004;16:1–11. doi: 10.1016/j.ccell.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Lukish JR, Eichelberger MR, Newman KD, et al. The use of a bioactive skin substitute decreases length of stay for pediatric burn patients. J Pediatr Surg. 2001;36:1118–1121. doi: 10.1053/jpsu.2001.25678. [DOI] [PubMed] [Google Scholar]
- 6.Greenwood JE, Clausen J, Kavanagh S. Experience with Biobrane: uses and caveats for success. ePlasty. 2009;9:e25. [PMC free article] [PubMed] [Google Scholar]
- 7.Barret JP, Dziewulski P, Ramzy PI, et al. Biobrane versus 1% silver sulfadiazine in second-degree pediatric burns. Plast Reconstr Surg. 2000;105:62–65. doi: 10.1097/00006534-200001000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Demling RH, LaLonde C. Burn trauma. New York (N.Y.): Thieme Medical Publishers Inc; 1989. [Google Scholar]
- 9.Lal S, Barrow RE, Wolf SE. Biobrane improves wound healing in burned children without increased risk in infection. Shock. 1999;14:314–319. doi: 10.1097/00024382-200014030-00013. [DOI] [PubMed] [Google Scholar]
- 10.Paddock HN, Fabia R, Giles S, et al. A silver impregnated antimicrobial dressing reduces hospital length of stay for pediatric patients with burns. J Burn Care Res. 2007;28:409–411. doi: 10.1097/BCR.0B013E318053D2B9. [DOI] [PubMed] [Google Scholar]
- 11.Smith DJ. Use of Biobrane in wound management. J Burn Care Rehabil. 1995;16:317–320. doi: 10.1097/00004630-199505000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Estrada A, Yun CH, Van Kessel A, et al. Immunomodulatory activities of oat Beta-glucan in vitro and in vivo. Microbiol Immunol. 1997;41:991–998. doi: 10.1111/j.1348-0421.1997.tb01959.x. [DOI] [PubMed] [Google Scholar]
- 13.Cassidy C, St Peter SD, Lacey S, et al. Biobrane versus Duoderm for the treatment of intermediate thickness burns in children: a prospective, randomized trial. Burns. 2005;31:890–893. doi: 10.1016/j.burns.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 14.Paddock HN, Fabia R, Giles S, et al. A silver-impregnated antimicrobial dressing reduces hospital costs for pediatric burn patients. J Pediatr Surg. 2007;42:211–213. doi: 10.1016/j.jpedsurg.2006.09.053. [DOI] [PubMed] [Google Scholar]
- 15.Boyce ST. Design principles for composition and performance of cultured skin substitutes. Burns. 2001;27:523–533. doi: 10.1016/s0305-4179(01)00019-5. [DOI] [PubMed] [Google Scholar]
- 16.Klein RL, Rothmann BF, Marshall R. Biobrane: a useful adjunct in the therapy of outpatient burn. Burns. 1984;19:846–847. doi: 10.1016/s0022-3468(84)80382-6. [DOI] [PubMed] [Google Scholar]
- 17.Whitaker IS, Worthington S, Jivan S, et al. The use of Biobrane by burn units in the United Kingdom: a national study. Burns. 2007;33:1015–1020. doi: 10.1016/j.burns.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Lang EM, Eiberg CA, Brandis M, et al. Biobrane in the treatment of burn and scald injuries in children. Ann Plast Surg. 2005;55:485–489. doi: 10.1097/01.sap.0000182652.88669.a6. [DOI] [PubMed] [Google Scholar]