Abstract
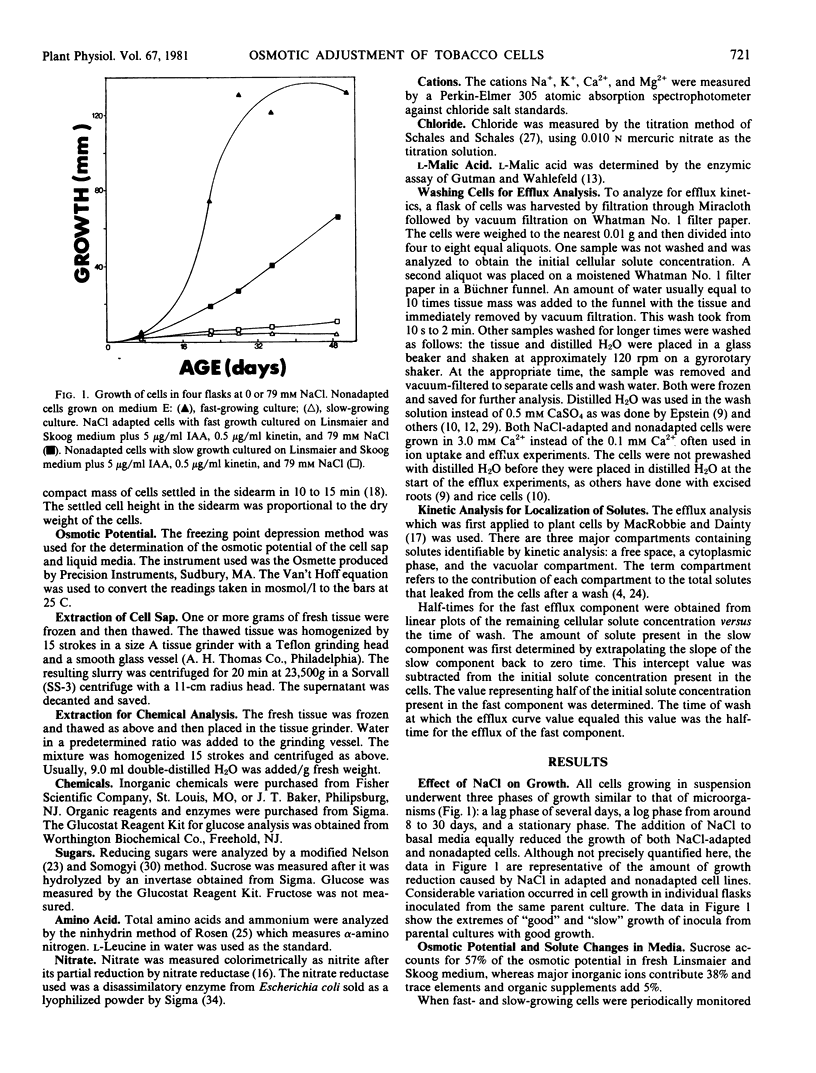
Tobacco cell cultures (var. Samsum) were grown on increasing levels of NaCl to select variants for increased salt tolerance. The osmotic adjustment of NaCl-adapted and nonadapted cell lines was studied. Both cell lines were grown on modified Linsmaier and Skoog medium with or without NaCl. Few differences were found in the response of adapted and nonadapted lines to NaCl.
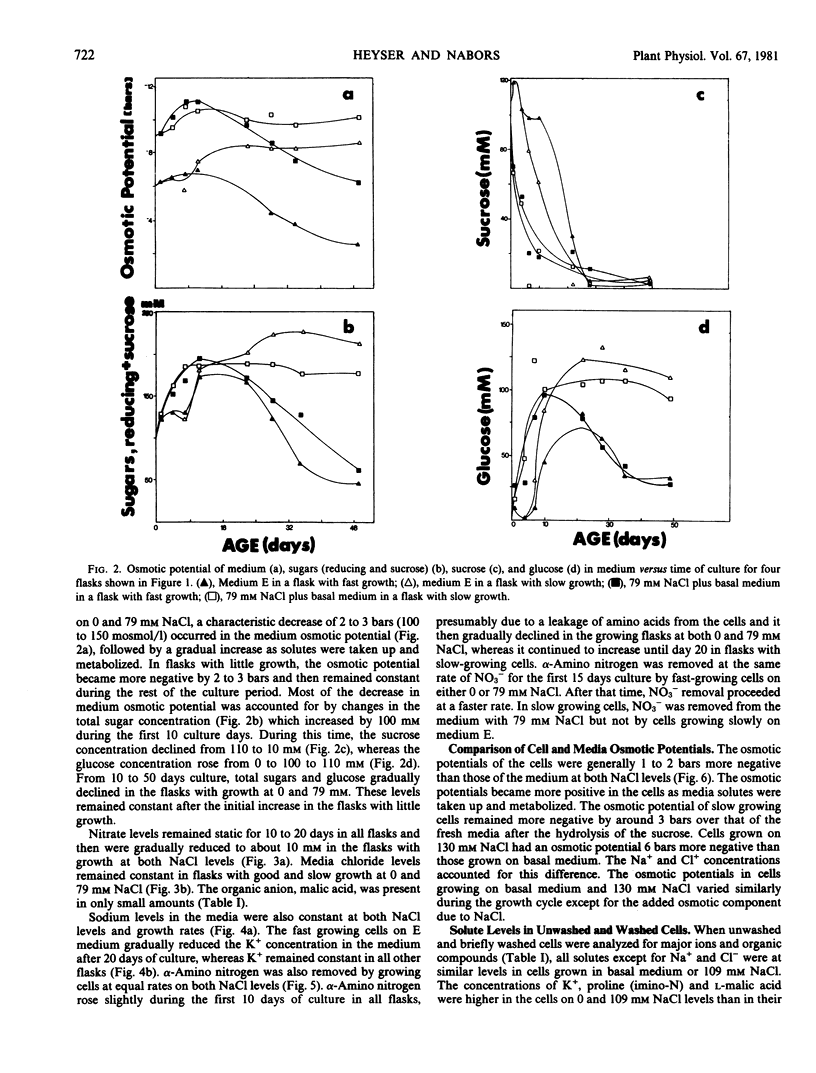
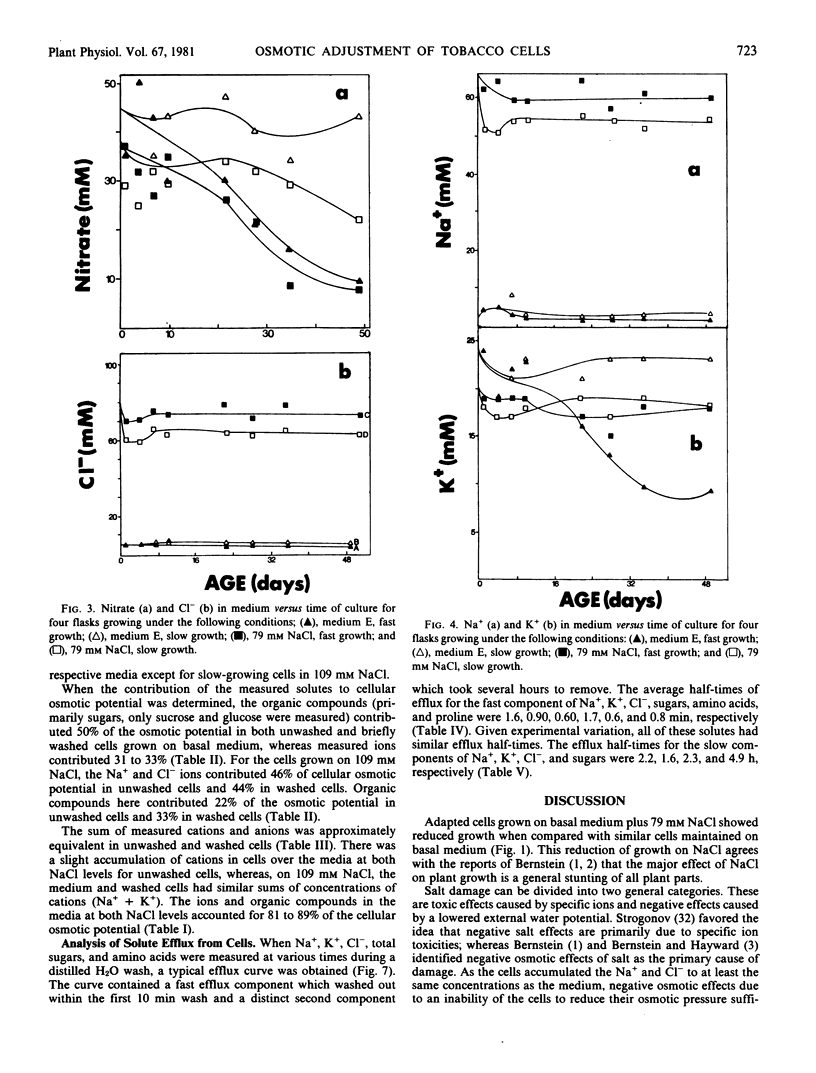
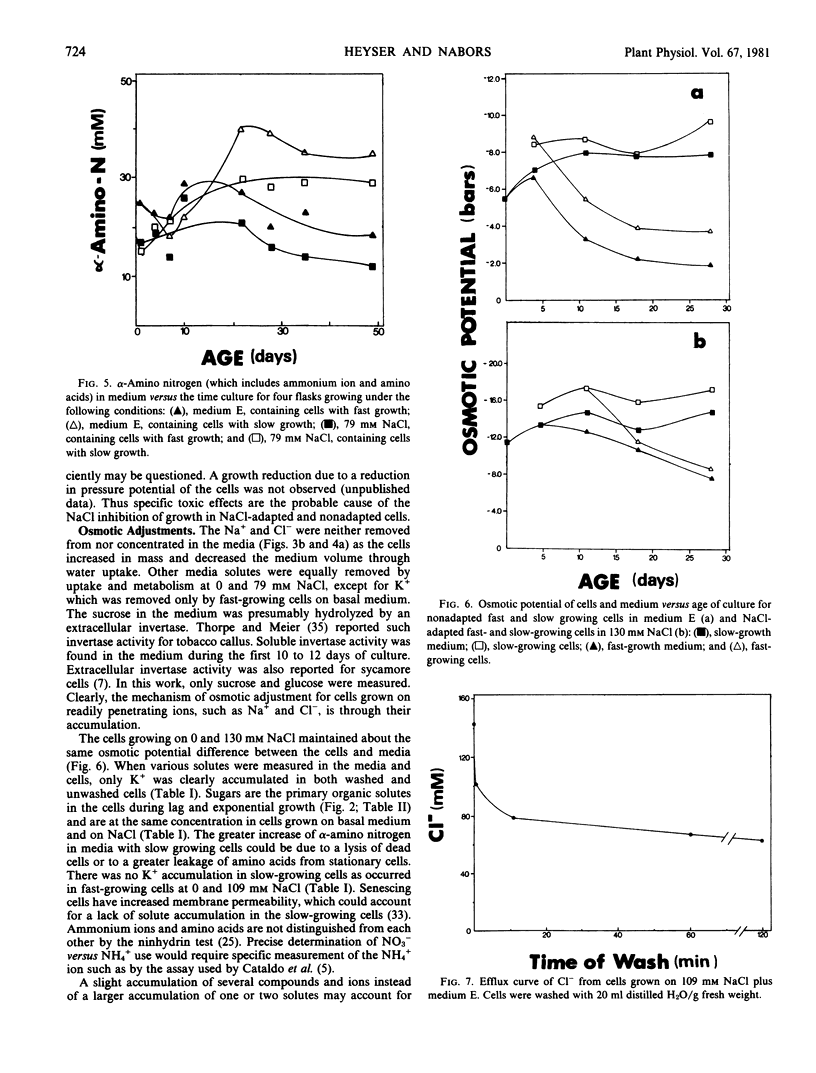
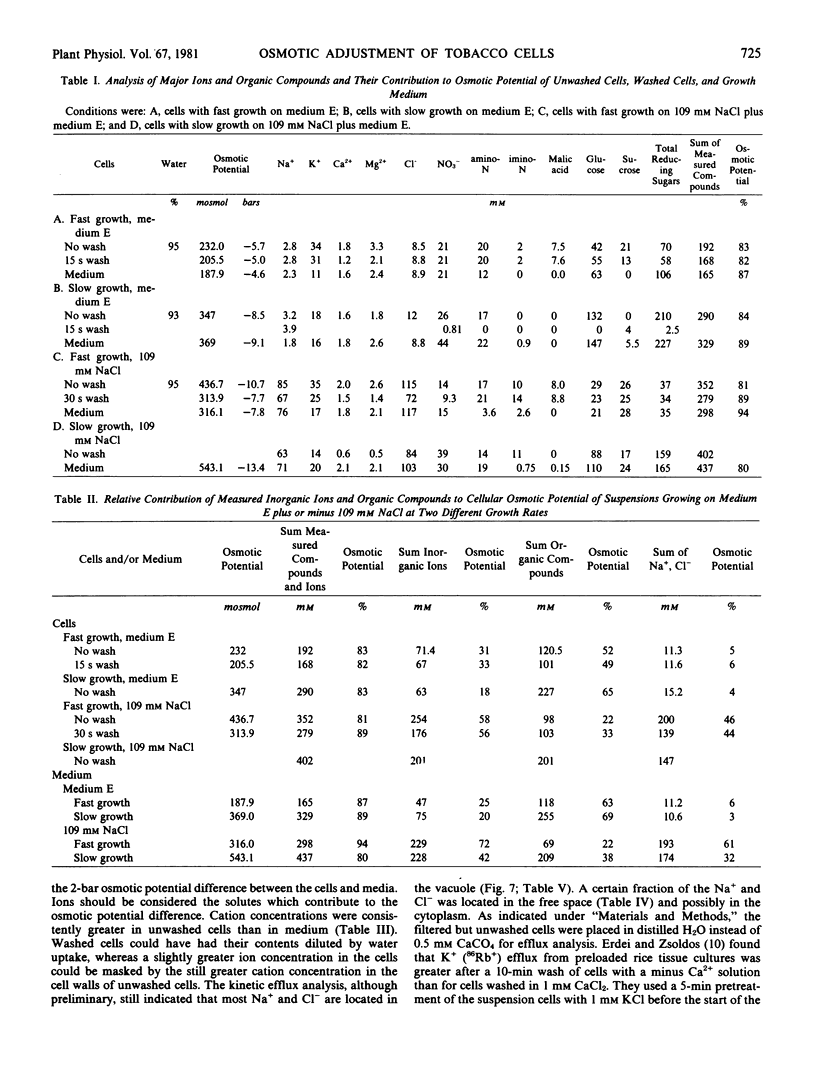
The concentrations of sugars, Na+, Cl−, and NO3− were identical in the cells and medium. Potassium and amino acids were accumulated by the cells. All of the above solutes accounted for 80 to 90% of the osmotic potential for both cell lines when grown on basal medium with or without NaCl. The osmotic potential of growing cells was always 1 to 3 bars more negative than that of the medium. During the first 10 days culture, the cells hydrolyzed the 117 millimolar sucrose present in the fresh media, and the media became more negative by 3 bars. Growing cells absorbed and metabolized the sugars, NH4+, and NO3− during the next 25 days, and the osmotic potential of the media and cells became less negative. The addition of 130 millimolar NaCl made the media and cells osmotically more negative by 6 bars throughout the growth cycle, as compared with cells growing on basal medium.
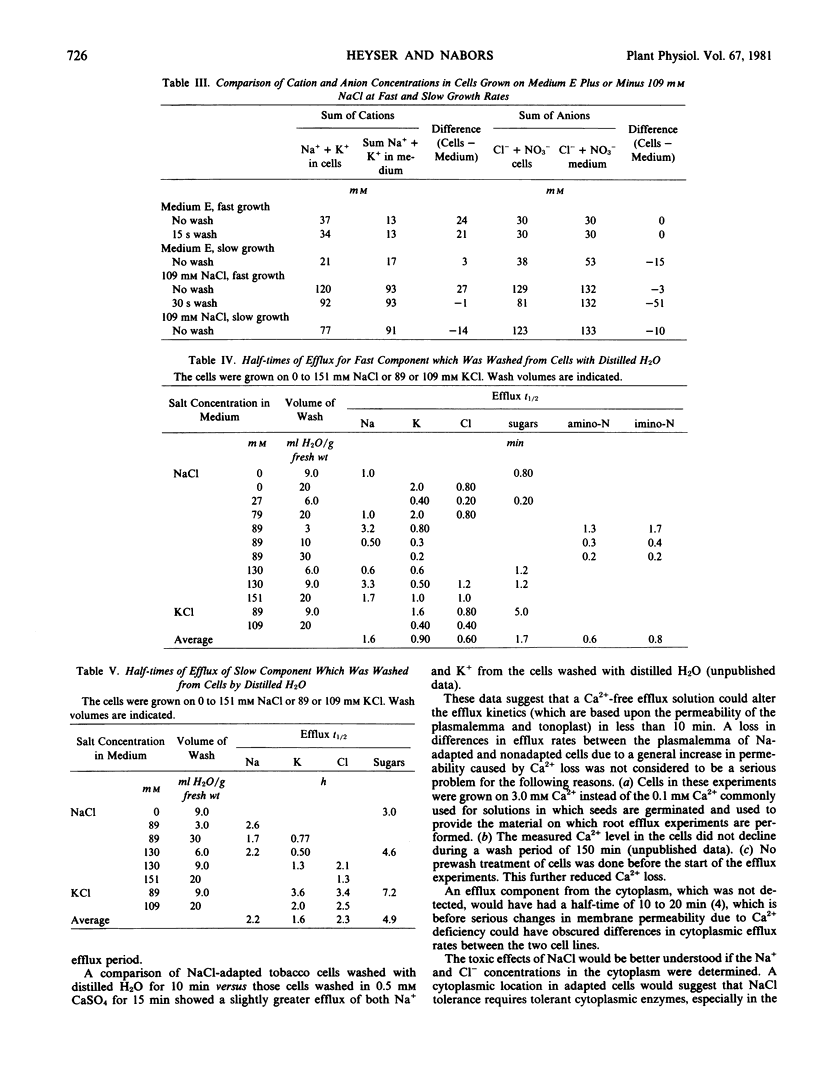
The efflux of cellular solutes during distilled H2O washes was resolved into two components. The fast component (0.6 to 1.7 minutes half-time) included solutes of the free space and cytoplasm, whereas the slow component (1.6 to 4.9 hours half-time) represented the vacuolar solutes. Sodium and Cl− were present in the vacuole. No differences were observed in the solute efflux between the adapted and nonadapted cell lines.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaleff R. S., Parsons M. F. Direct selection in vitro for herbicide-resistant mutants of Nicotiana tabacum. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5104–5107. doi: 10.1073/pnas.75.10.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. The essential role of calcium in selective cation transport by plant cells. Plant Physiol. 1961 Jul;36(4):437–444. doi: 10.1104/pp.36.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote B. D., Hanson J. B. Ion Uptake by Soybean Root Tissue Depleted of Calcium by Ethylenediaminetetraacetic Acid. Plant Physiol. 1964 May;39(3):450–460. doi: 10.1104/pp.39.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACROBBIE E. A., DAINTY J. Ion transport in Nitellopsis obtusa. J Gen Physiol. 1958 Nov 20;42(2):335–353. doi: 10.1085/jgp.42.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manasse R. J. A new culture flask for plant tissue suspension cultures. Experientia. 1972 Jun 15;28(6):723–724. doi: 10.1007/BF01945005. [DOI] [PubMed] [Google Scholar]
- Marx J. L. Plants: can they live in salt water and like it? Science. 1979 Dec 7;206(4423):1168–1169. doi: 10.1126/science.206.4423.1168. [DOI] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Shepard J. F., Bidney D., Shahin E. Potato protoplasts in crop improvement. Science. 1980 Apr 4;208(4439):17–24. doi: 10.1126/science.208.4439.17. [DOI] [PubMed] [Google Scholar]
- Smith I. K. Role of calcium in serine transport into tobacco cells. Plant Physiol. 1978 Dec;62(6):941–948. doi: 10.1104/pp.62.6.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle J. C., Kende H. Ethylene Action and Loss of Membrane Integrity during Petal Senescence in Tradescantia. Plant Physiol. 1980 Jun;65(6):1067–1072. doi: 10.1104/pp.65.6.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANIGUCHI S., ITAGAKI E. Nitrate reductase of nitrate respiration type from E. coli. I. Solubilization and purification from the particulate system with molecular characterization as a metalloprotein. Biochim Biophys Acta. 1960 Nov 4;44:263–279. doi: 10.1016/0006-3002(60)91562-6. [DOI] [PubMed] [Google Scholar]



