Abstract
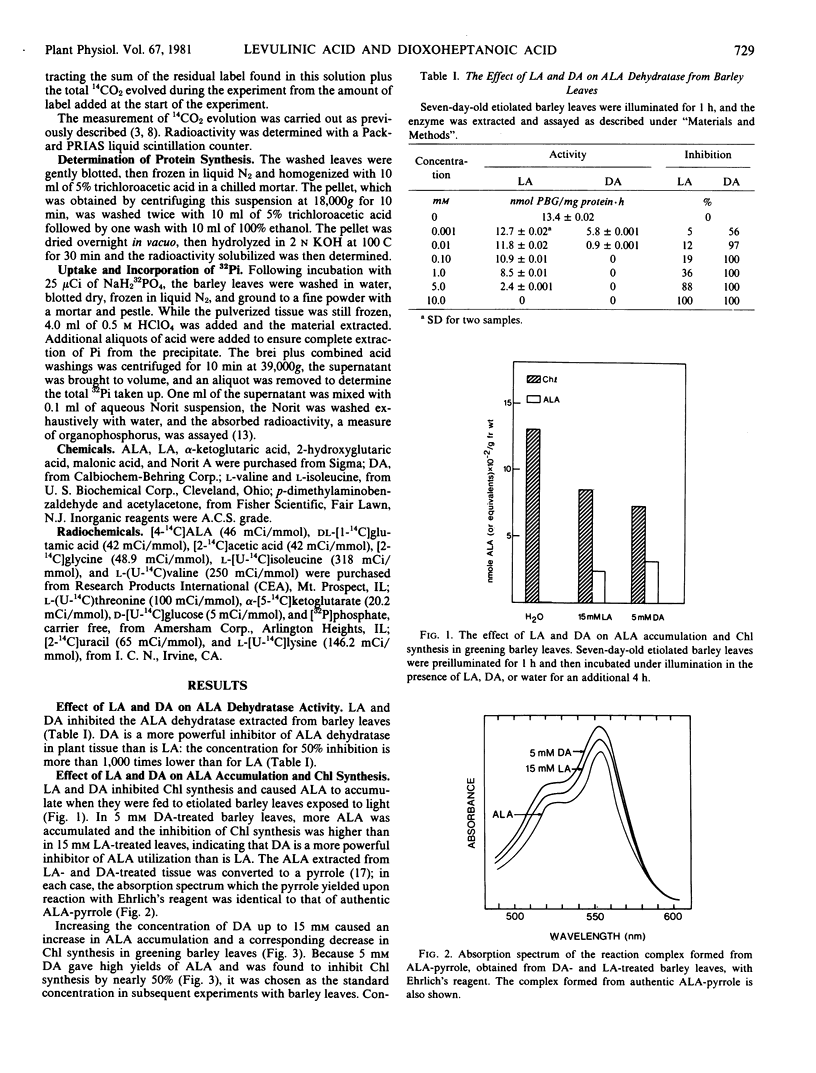
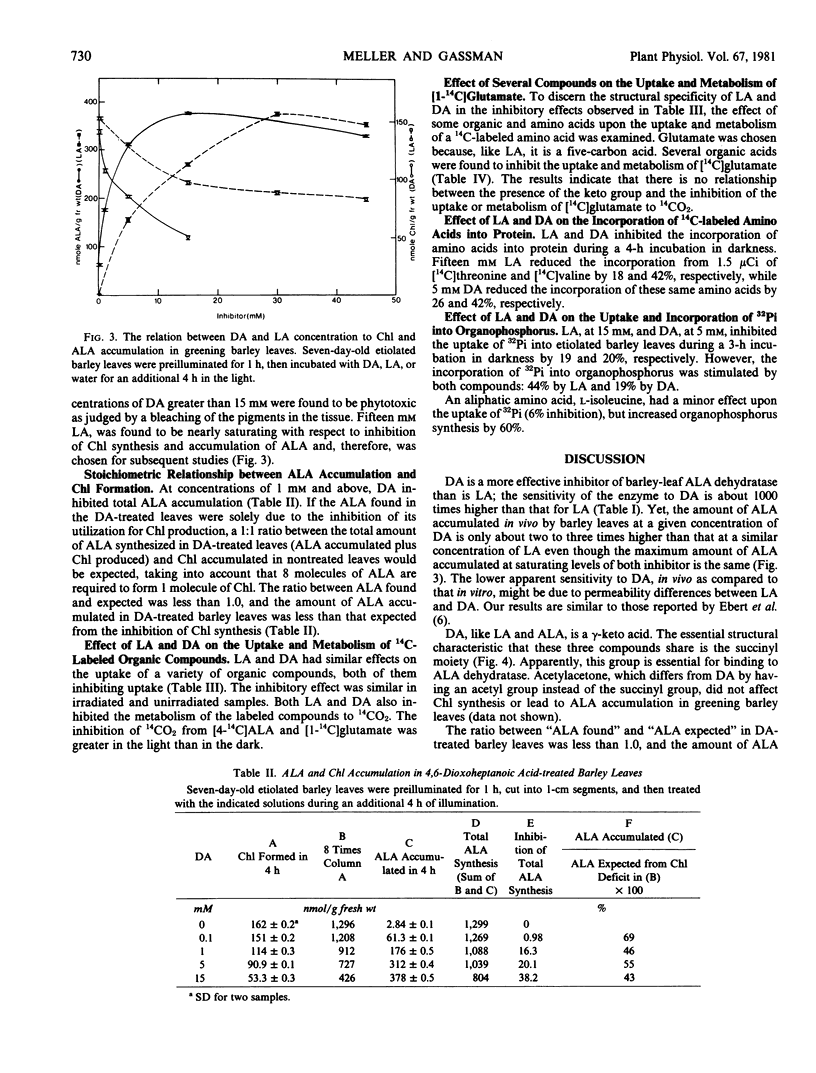
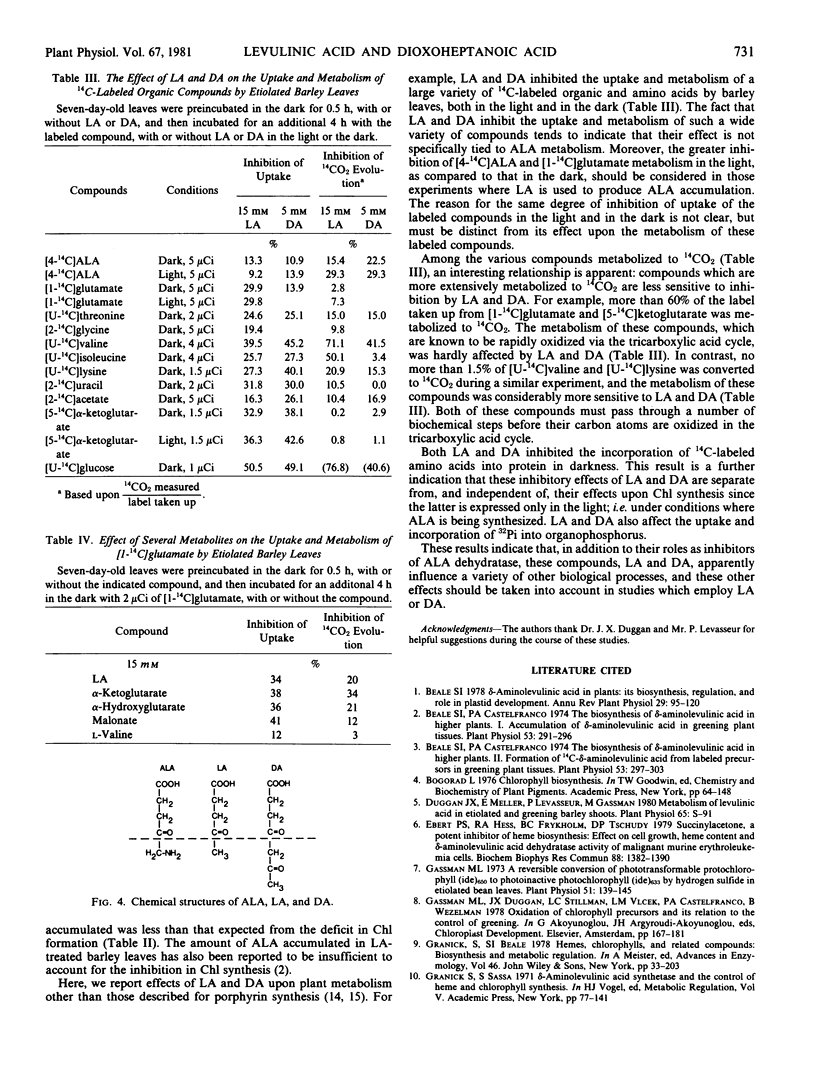
Application of levulinic acid (LA), a competitive inhibitor of δ-aminolevulinic acid (ALA) dehydratase, to greening plant tissues causes ALA to accumulate at the expense of chlorophyll. 4,6-Dioxoheptanoic acid (DA), which has been reported to be an effective inhibitor of this enzyme in animal systems, has a similar but more powerful effect on ALA and chlorophyll metabolism in greening leaves of Hordeum vulgare L. var. Larker. Both LA and DA also inhibit the uptake of [14C]amino acids into etiolated and greening barley leaves and reduce their incorporation into protein. Treatment of etiolated and greening leaves with these compounds results in the inhibition of 14CO2 evolution from labeled precursors, including amino and organic acids. Inhibition of 14CO2 evolution by these compounds is more effective in greening leaves than in etiolated leaves when [4-14C]ALA or [1-14C]glutamate are employed as precursors. Both LA and DA also inhibit the uptake and increase the incorporation of 32Pi into organophosphorus by etiolated barley leaves. These results indicate that LA and DA have more far-reaching effects upon plant metabolism than was previously believed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale S. I., Castelfranco P. A. The Biosynthesis of delta-Aminolevulinic Acid in Higher Plants: I. Accumulation of delta-Aminolevulinic Acid in Greening Plant Tissues. Plant Physiol. 1974 Feb;53(2):291–296. doi: 10.1104/pp.53.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I., Castelfranco P. A. The Biosynthesis of delta-Aminolevulinic Acid in Higher Plants: II. Formation of C-delta-Aminolevulinic Acid from Labeled Precursors in Greening Plant Tissues. Plant Physiol. 1974 Feb;53(2):297–303. doi: 10.1104/pp.53.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert P. S., Hess R. A., Frykholm B. C., Tschudy D. P. Succinylacetone, a potent inhibitor of heme biosynthesis: effect on cell growth, heme content and delta-aminolevulinic acid dehydratase activity of malignant murine erythroleukemia cells. Biochem Biophys Res Commun. 1979 Jun 27;88(4):1382–1390. doi: 10.1016/0006-291x(79)91133-1. [DOI] [PubMed] [Google Scholar]
- Gassman M. L. A Reversible Conversion of Phototransformable Protochlorophyll(ide)(656) to Photoinactive Protochlorophyll(ide)(656) by Hydrogen Sulfide in Etiolated Bean Leaves. Plant Physiol. 1973 Jan;51(1):139–145. doi: 10.1104/pp.51.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granick S., Beale S. I. Hemes, chlorophylls, and related compounds: biosynthesis and metabolic regulation. Adv Enzymol Relat Areas Mol Biol. 1978;46:33–203. doi: 10.1002/9780470122914.ch2. [DOI] [PubMed] [Google Scholar]
- Harel E., Klein S. Light dependent formation of -aminolevulinic acid in etiolated leaves of higher plants. Biochem Biophys Res Commun. 1972 Oct 17;49(2):364–370. doi: 10.1016/0006-291x(72)90419-6. [DOI] [PubMed] [Google Scholar]
- Klein S., Harel E., Ne'eman E., Katz E., Meller E. Accumulation of delta-Aminolevulinic Acid and Its Relation to Chlorophyll Synthesis and Development of Plastid Structure in Greening Leaves. Plant Physiol. 1975 Oct;56(4):486–496. doi: 10.1104/pp.56.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblad B., Lindstedt S., Steen G. On the enzymic defects in hereditary tyrosinemia. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4641–4645. doi: 10.1073/pnas.74.10.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Nandi D. L., Shemin D. Delta-aminolevulinic acid dehydratase of Rhodopseudomonas spheroides. 3. Mechanism of porphobilinogen synthesis. J Biol Chem. 1968 Mar 25;243(6):1236–1242. [PubMed] [Google Scholar]
- Nandi D. L., Waygood E. R. Biosynthesis of porphyrins in wheat leaves. II. 5-aminolaevulinate hydro-lyase. Can J Biochem. 1967 Feb;45(2):327–336. doi: 10.1139/o67-036. [DOI] [PubMed] [Google Scholar]
- Owens T. G., Riper D. M., Falkowski P. G. Studies of Delta-Aminolevulinic Acid Dehydrase from Skeletonema costatum, a Marine Plankton Diatom. Plant Physiol. 1978 Oct;62(4):516–521. doi: 10.1104/pp.62.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzbach S. D., Schiff J. A., Goldstein N. H. Events surrounding the early development of euglena chloroplasts: v. Control of paramylum degradation. Plant Physiol. 1975 Aug;56(2):313–317. doi: 10.1104/pp.56.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withrow R. B., Klein W. H., Price L., Elstad V. Influence of Visible and Near Infrared Radiant Energy on Organ Development and Pigment Synthesis in Bean and Corn. Plant Physiol. 1953 Jan;28(1):1–14. doi: 10.1104/pp.28.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]



