Abstract
A randomized, double-blinded, placebo-controlled study was conducted to examine the effect of spatial repellent (SR) in households at risk of malaria in Indonesia. Following presumptive radical cure for malaria in 180 adult men representing sentinels of new infection in four clusters within two villages, all households were given either metofluthrin or placebo mosquito coils. Weekly blood smear screening and human-landing mosquito catches were done throughout the 6 months intervention. Malaria infections occurred in 61 subjects living in placebo households and 31 subjects living in SR coil households, suggesting a 52% protective effect of SR. Likewise, anopheles indoor human landing rates were 32% lower in homes receiving SR coils. Differences in the malaria attack rate between SR- and placebo-treated homes was significant when not accounting for the effects of clustering. When the analysis was adjusted for intra-cluster correlation, the differences between SR- and placebo-treated homes were not statistically significant. The findings provide evidence of SR public health benefit and support a larger trial statistically powered to detect those effects.
Introduction
Malaria continues to be a significant global public health burden despite recent progress in reducing disease rates.1,2 Currently, the recommended tools for malaria control and management from global health authorities, such as the World Health Organization (WHO), include diagnosis, chemotherapy, indoor residual spraying (IRS), and long-lasting insecticide-treated nets (LLINs) to reduce transmission and risk of infection and illness.3 The effectiveness of these tools depend upon many factors such as the quality of diagnostic and treatment services, coverage of homes with IRS or LLINS, relative transmission level, and many social and economic factors. Furthermore, the spread of parasite and anopheline vector resistance to various antimalarial drugs and insecticides, respectively, in combination with the lack of an efficacious malaria vaccine, collectively threaten the effectiveness of current malaria control efforts. This reality emphasizes the need to develop innovative preventive tools that exploit novel mechanisms of action against either the anopheline vector or Plasmodium spp. parasite. Modifying vector behavior through the chemical action of spatial repellency (SR)4 is one such approach. Here, we define SR as the ability of airborne chemicals to reduce human vector contact by eliciting one or more insect behaviors.5 As early as 1953, Muirhead-Thomson6 concluded that chemicals could disrupt contact between humans and malaria-transmitting mosquitoes and thus stop disease transmission without actually killing mosquitoes. Subsequent authors have speculated that spatial repellent products could hold distinct advantages over more traditional vector control tools such as IRS and LLINs.7–11 One key advantage of SR over IRS/LLINs is the ability to create a space with reduced mosquito density without the requirement of the mosquitoes contacting a treated surface. In other words, protection is afforded at a distance and can occur continuously during daytime, early evening, and night—a particular benefit when considering varied anopheline biting patterns and at-risk population lifestyles. Of importance, there is a reduced probability of creating a survival advantage with either behavioral or physiological resistance to the agent inducing this effect.4
Spatial repellency as a means of prevention was considered more than 60 years ago but never seriously pursued. Development algorithms for chemicals aimed against mosquitoes have focused upon mortality effects for setting thresholds of efficacy, and, indeed, SR properties were considered disadvantageous (compromising contact and mortality). Agents of SR as effective tools of malaria prevention remain essentially an unexplored chemical universe. Spatial repellency has nonetheless been well documented under experimental conditions.12–16 Adopting SR as a broad prevention strategy, however, requires more practical demonstrations of impact. The current study aimed to provide limited proof-of-concept evidence of SR-mediated reduction of malaria transmission in communities naturally exposed to the pathogen.
Materials and Methods
Ethics statement.
Ethical review and approval for this study was granted by the Ethics Committee (EC) of the Faculty of Medicine, Hasanuddin University and endorsed by the Eijkman Institute Research Ethics Committee, Jakarta, Indonesia. Informed consent was obtained by subjects following EC guidelines to include descriptions of the study risks, benefits, and procedures of radical cure and follow-up. All adverse events were captured during participant follow-up and reported to monitoring authorities after approved protocol.
Study site.
The study was conducted in Southwest Sumba District, East Nusa Tenggara Province, Indonesia (Figure 1). The 382,268 residents of the district live in 94 villages. Cross-sectional surveys during wet and dry seasons at 45 sites in this district during 2007 indicated a seasonal pattern of hypo- to meso-endemic malaria transmission with prevalence of microscopically patent parasitemia ranging from 0% to 34%, with a median prevalence of 6%.17 The higher prevalence among these sampled sites is typically nearer the coast. Two such villages, Umbungedo and Wainyapu, with populations of 2,678 and 2,576, respectively, served as the study sites. The prevalence of parasitemia in two mass blood surveys of the villages employing random sampling (50% of residents) was 3.5% at Umbungedo and 24.7% at Wainyapu 3 and 10 months before the start of the intervention, respectively (Table 1). Although very little is known of the malaria vector bionomics in this area, one entomologic survey documented 11 species of anophelines occurring in this district: Anopheles sundaicus, Anopheles subpictus, Anopheles barbirostris, Anopheles hyrcanus, Anopheles aconitus, Anopheles flavirostris, Anopheles annularis, Anopheles maculatus, Anopheles tessellatus, Anopheles vagus, and Anopheles kochi.18 These species occurred in relative abundance in accordance with their respective preferred habitats including coastal marshes and ponds, seasonal rice paddies, and forested hillsides. This setting is typical for many rural malaria-endemic areas in Indonesia, particularly high risk coastal zones.19 Residents of the study village work principally in agriculture, have no public electricity or water, and reside overwhelmingly in traditional large thatch and bamboo homes averaging 80 m3 that offer little protection from mosquito entry. Thus, the site maintained relatively high malaria attack rates, primarily from An. sundaicus, which is typically found in coastal settings and has been confirmed to be an important vector species in the region.
Figure 1.
Map of the study site (box) in the Southwest Sumba District and its location in the Indonesian archipelago (map not to scale). The District is located in the western part of Sumba Island (insert).
Table 1.
Parasitologic baseline data of the study sites
| Study location* | Number of samples collected in MBS† | Number of malaria positive cases | SPR‡ (%) |
|---|---|---|---|
| W1 | 180 | 57 | 31.7 |
| W2 | 197 | 75 | 38.1 |
| U1 | 404 | 23 | 5.7 |
| U2 | 449 | 15 | 3.3 |
A total of four clusters were designated from two study villages; Wainyapu 1 (W1), Wainyapu 2 (W2); Umbungedo 1 (U1), Umbegedo 2 (U2).
Mass blood survey (MBS) conducted at 3 (U1 and U2) and 10 months (W1 and W2) before intervention.
Slide positivity rate (SPR).
Sample size.
Previous malaria incidence rate surveys in other locations of Indonesia20 were used to predict a likely malaria attack rate in the current study villages ranging between 0.2 and 2.0 infections/person-year. Assuming a 6-month exposure risk period (i.e., approximate typical high malaria transmission period at this location), it was anticipated that between 0.1 and 1.0 infections would occur per person or that the proportion of subjects becoming infected during the intervention would fall between 10% and 100%. Sample size requirements were estimated based on the ability to detect a difference between treatment arms with standard alpha = 0.05 and beta = 0.80, and powered to permit detection of a 25% intervention effect size with a relatively low attack rate (20%).21–23
Study design.
The study was a split cluster-randomized, double-blinded, and placebo-controlled longitudinal cohort design. The selection of cluster randomization was based on the distribution and movement of mosquitoes and chemicals eliciting SR; both of these could move from house to house within a village, therefore the spatial unit of potential impact was the cluster. The study was designed and powered to develop evidence of sufficient efficacy for SR to justify a much larger and adequately statistically powered cluster randomized SR trial. The SR agent used in this study was metofluthrin, a commonly used compound in commercially available mosquito coils, and with demonstrated repellency effects against anopheline mosquitoes. 7–9
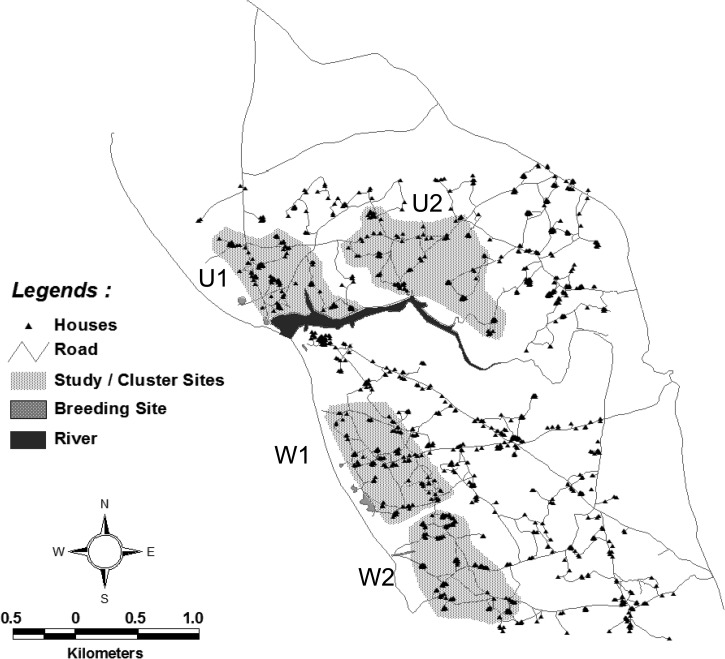
After baseline entomologic and parasitologic (Table 1) site surveys, households from both Wainyapu and Umbungedo villages were stratified into four clusters: Wainyapu 1 (W1, pop. 502, houses 92) and Wainyapu 2 (W2, pop. 523, houses 102), Umbungedo 1 (U1, pop. 596, houses 93) and Umbungedo 2 (U2, pop. 651, houses 98) (Figure 2). The areas of each cluster were chosen with the intent of roughly dividing each village into equal halves. The clusters in each village were randomized to receive either treatment mosquito coils (containing 0.00975% metofluthrin) or placebo mosquito coils (containing inert ingredients only and no metofluthrin). The randomization process ensured that each village had both active and placebo treatments. The study administrator obtained a list of lot manufacturing codes from the coil manufacturer (S.C. Johnson Co., Ho Chi Minh, Vietnam) that identified coils as either active or placebo. The administrator then assigned a code specific to each home and labeled packages of coils corresponding to cluster assignment to active or placebo coil treatment. These assignments were kept in a sealed envelope in a secure location within the managing center of the research program (Jakarta). Thus, the investigators, research team, study subjects, and residents were blinded as to which cluster received active versus placebo coils until after completion of the study.
Figure 2.
Map of the household clusters in the Umbungedo and Wainyapu villages. Four clusters: U1, U2, W1, and W2 (grayed) were selected, each consisted of ca. 100 households and 500 people each.
The primary endpoint for estimating the protective efficacy of this spatial repellent intervention was malaria incidence among 45 sentinel subjects resident (study participants) in each of the four clusters, i.e., 180 subjects in all. These sentinel subjects, called the attack rate cohort, were all men living in separate households, which received a directly observed, presumptive radical cure to clear them of any standing (patent, sub-patent, or latent) malaria infections of blood and liver. Weekly blood film exams were conducted for the duration of the study. This provided an essential analytical advantage, i.e., all new infections occurring in these subjects could only have originated from mosquito-borne sporozoite inoculation rather than recrudescence or relapse stemming from infection before intervention.
Enrollment for attack rate cohort and radical cure.
Men 18 to 60 years of age representing single households among study clusters were provided the opportunity to enroll in the study. Following informed consent, screening consisted of physical examination by a study physician and a qualitative test for G6PD deficiency (Trinity Biotech qualitative G6PD assayTM, ref 345-UV, Trinity Biotech, St. Louis, MO). In addition to G6PD normal status, eligibility requirements included a bodyweight ≥ 40 kg, hemoglobin > 8 mg/dL (Hb201+, HemoCue AB, Angelholm, Sweden) no significant chronic illness, participant must sleep in the village > 90% of nights, and no plans for extended travel during the study. A total of 180 subjects (75 men per village plus an additional 30 to account for anticipated losses to follow-up) were treated using a fixed combination formulation of dihydroartemisinin (DHA) 6.4 mg/kg and piperaquine (P) 51.2 mg/kg body weight for 3 days (Arterakin™, PHARBACO Central Pharmaceutical JSC No. 1, Hanoi, Vietnam) and 0.5 mg/kg body weight primaquine (Kimia Farma, Semarang, Indonesia) for the 28 days immediately before starting the coil intervention. The DHA+P combination is currently the first-line antimalarial drug for malaria treatment in Indonesia.24 Although primaquine treatment policy in Indonesia calls for 0.25 mg/kg for 14 days, we administered 0.5 mg/kg for 28 days to ensure a greater probability of complete efficacy against relapse, and knew this dose to be safe and well tolerated in Indonesians.25,26 The same dose of primaquine for 14 days and administered with DHA-P was 98% efficacious in Indonesian soldiers infected in Papua.27 New malaria infections among the 180 participants were monitored with weekly microscopic examination of Giemsa-stained blood films at project-dedicated field clinics and laboratories located in each of the two study villages.28 Participants found positive for malaria parasites were immediately treated with DHA+P and removed from the study, thereby ending their contribution to person-time at risk of infection.
Experimental intervention.
Immediately after completion of radical cure in the attack rate cohort, intervention was simultaneously initiated in all households. Blank (metofluthrin-free) or 0.00975% metofluthrin-treated coils of identical packaging and color were randomly assigned to houses using a 90:10 distribution ratio of each treatment within a single study cluster (W1, W2, U1, and U2). The 90:10 distribution ratio effectively provided a 90% coverage rate for treatments within clusters, and the minority treatment could reveal trends in village level effects (but without the power to ascertain quantitative effects). All houses were provided four coils each night by project personnel and were ignited at 1800 h. One coil was positioned at each of four corners of the house (virtually all were single room dwellings) and placed on standard metal stands fixed within 20 × 20 × 6 cm metal pans. The pans facilitated stabilization of the coil on the bamboo flooring and provided protection to the coil from excessive wind currents resulting in more even burning. The ∼288,000 active and placebo coils used were manufactured by S.C. Johnson, Inc., to the specifications of this trial. They were designed to provide a 12-hour burn and homeowners were asked to relight coils if they burned out prematurely. Research team members regularly surveyed coil pans at randomly selected homes each morning to ascertain successful burn rates (i.e., cm length of coil remaining at dawn). They also routinely surveyed randomly selected homes for adverse health effects conceivably related to coil burning.
Entomologic parameters.
Adult mosquito densities were measured using human-landing catches (HLC). Five sentinel houses within each study cluster were selected for sampling. Collections were conducted weekly from all sentinel houses within a given village simultaneously. Sentinel houses were blindly selected to include two houses with metofluthrin active coils, two with blank coil treatments, and one house without coil intervention during the given sampling night. This ensured comparison between active and placebo and to “natural” conditions. The sentinel house without the coil was provided a blank coil (the lone exception to coil treatment blinding) on all nights other than when the HLC was being performed. Teams of two collectors were assigned per house, one positioned indoors at the center of the house and one located outside on the verandah ∼1 m from the exterior wall. Collectors removed all mosquitoes landing on their exposed lower legs using a mouth aspirator. Collections were conducted from 1800 to 0600 h for 50 min every hour. Collectors rotated the indoor and outdoor position every hour. Samples were placed into individual holding containers labeled by hour of collection, unique house code (that corresponded to treatment), and collection location (indoor or outside). Mosquitoes were immediately killed by chloroform vapor in the field and identified to species (or species complex) using morphological characteristics.29 All specimens were transported to the project laboratory upon completion of the 12 h HLC and a representative random sample of anophelines were dissected for parity and scored as either gravid/parous or nulliparous.30 Partial (head-thorax) and whole anopheline specimens were placed singly into individual vials and stored with silica gel desiccant until further processing at the Eijkman Institute for Molecular Biology, Jakarta, for detection of malaria sporozoites and molecular-based species identification, where applicable.
Mosquito samples were evaluated for Plasmodium spp. infection using a circumsporozoite protein (CSP) enzyme-linked immunosorbent assay (ELISA)31 and polymerase chain reaction (PCR) methodologies32 to derive corresponding malaria sporozoite rates by parasite (P. falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale) and vector species. Together with time-adjusted HLC densities (anophelines/person-night), matched sporozoite rates were used to derive the entomological inoculation rates (EIRs) for each treatment arm.33
Indoor and outdoor resting collections were conducted weekly from the five sentinel houses used for HLC in each cluster. During daylight early morning hours, a team of three persons per house systematically sampled each house for a total of 60 min from four locations (inside, under the house, on the outside house veranda, and within the peridomestic area (10 m circumference from the house). Sampling was conducted using a modified Prokopack handheld aspirator.34 Additionally, to capture blood-fed mosquitoes, a wooden “resting box” fitted with a black cloth lining was placed outdoors of each sampled house within a standard 10 m distance from the exterior walls and in a location with high probability for vector refuge. All captured resting mosquitoes were placed in labeled containers and returned to the laboratory for identification and processing for blood-meal analysis.35
Statistical analyses.
The impact of SR on risk of malaria was estimated by measuring incidence density of new cases of malaria among cohorts of 45 sentinel subject men in each of the study clusters. The primary estimate of impact was determined by calculating the protective efficacy of the intervention based on incidence density (number of infections per person year at risk) of new parasitemias among the malaria attack cohort as follows22,23,36:
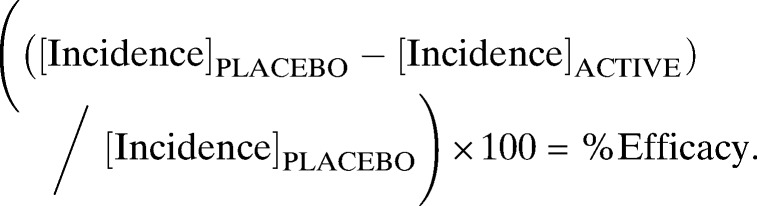 |
Risk rate (RR) was calculated from the ratio of overall incidence rate in the active and placebo groups. To adjust for possible clustering effect, geometric mean of the cluster incidence rate (RRGM) was used to estimate of intervention effect when the incidence rates in each group were highly skewed.22 One approach to cumulative incidence analysis involved direct adjustment of the χ2 statistic, which depends on clustering effects for each intervention group.
The secondary endpoint, anopheline vector human landing rates during the study period, was analyzed by the nonparametric Wilcoxon rank-sum statistical test to compare cumulative indoor catch densities between treatment arms by week of collection with 2 × 2 contingency tables used to generate and compare risk rates over time. The EIR by matched time and place were calculated combining the mean human-landing density with proportion of anophelines deemed “infective” with sporozoites.33 All hourly HLC rates were adjusted for 60 min before calculating the EIR.
Results
Intervention.
The morning observation of coil remnants showed high success “burn” rates. Of the 263,520 coils observed during the intervention period, 97.82% had no coil material remaining the following morning. Of the 2.18% coils that failed, the average coil remnant was 34 cm (95% confidence interval [CI]: 26.7–42.2) of a total starting coil length of 125 cm. There was no instance of all four coils failing in the same house on the same night. Full exposure to coil smoke/active ingredient occurred during the 6 months of intervention. No coil-related serious adverse events were reported, and reported adverse event rates did not differ between active and placebo coil homes. No burn injuries occurred, nor did any home fire incidents, and no household requested cessation of coil burning.
Malaria attack and protective efficacy.
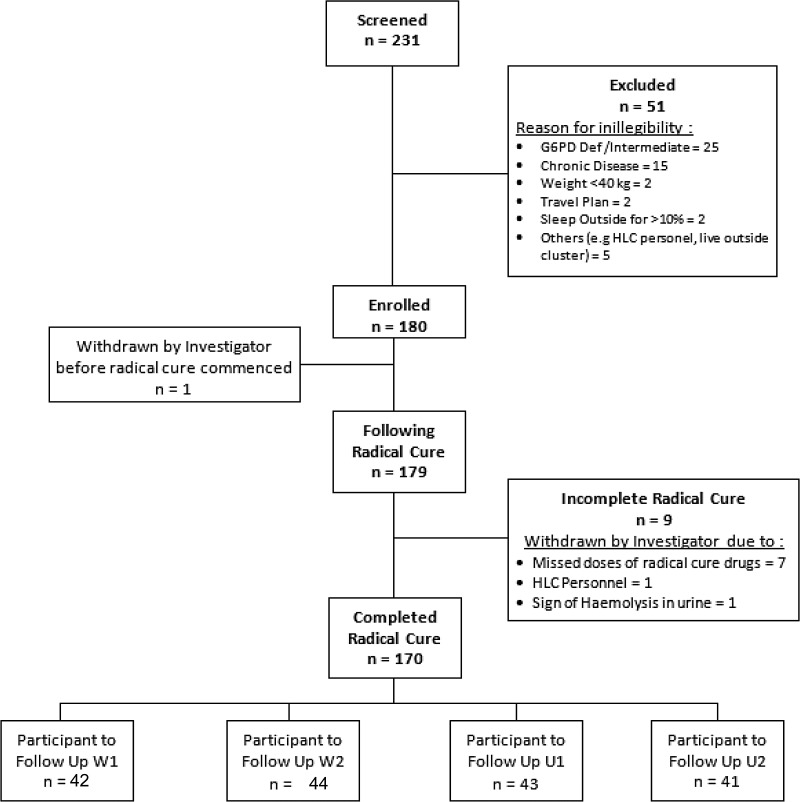
Figure 3 summarizes screening, enrollment, and follow-up of the 180 subjects of the incidence density cohorts among the four clusters. Almost all subjects completed radical cure and the 6 months of follow-up. Analytical results for incidence rate, cumulative incidence analysis, and clustering effect adjustment are presented in Table 2. A total of 61 malaria infections within 1,468 person-weeks at risk were seen in participants whose households were given blank coils (clusters W2 and U1), with a calculated incidence density of 2.184 infections/person-year. In contrast, 31 malaria attacks occurred among participants in metofluthrin coil-treated households among the 1,540 person-weeks at risk resulting in a calculated 1.04 infections/person-year. The protective efficacy of metofluthrin coils was thus estimated at 51.6% (95% CI 25.4–68.6%). The relative risk (RR [95% CI] of infection among active versus placebo coils was 0.48 [0.31–0.75]).
Figure 3.
Flowchart of the screening and enrollment of study volunteers. Two hundred and thirty-one subjects were screened for G6PD deficiency and 180 consented to be enrolled and provided radical cure for malaria of which 170 completed the treatment and subsequently followed up for 6 months during the intervention.
Table 2.
Incidence rate and cumulative incidence of malaria in clusters treated with metofluthrin (active)- and placebo coils
| Wainyapu | Umbungedo | All villages | ||||
|---|---|---|---|---|---|---|
| Cluster 1 (W1) | Cluster 2 (W2) | Cluster 3 (U1) | Cluster 4 (U2) | Cluster 1 + 4 | Cluster 2 + 3 | |
| 90% Active+ 10% placebo | 10% Active+ 90% placebo | 10% Active+ 90% placebo | 90% Active+ 10% placebo | Active clusters | Placebo clusters | |
| Household active:placebo | 108 (98:10) | 114 (11:103) | 115 (12:103) | 108 (98:10) | 216 (196:20) | 229 (23:206) |
| Population | 368 | 523 | 596 | 633 | 1001 | 1119 |
| Samples | 42 | 44 | 43 | 41 | 83 | 87 |
| Malaria incident | 26 | 40 | 21 | 5 | 31 | 61 |
| Incidence density | ||||||
| Person-week | 652 | 602 | 866 | 888 | 1540 | 1468 |
| Incidence rate | 0.040 | 0.066 | 0.024 | 0.006 | 0.020 | 0.042 |
| Without clustering effect | ||||||
| RR (95% CI) | 0.484 (0.314–0.746) | |||||
| With clustering effect | ||||||
| RRGM (95% CI) | 0.652 (0.088–4.802) | |||||
| Cumulative incidence | ||||||
| Proportion of incidence | 0.619 | 0.909 | 0.488 | 0.122 | 0.373 | |
| Without clustering effect | ||||||
| RR (95% CI) | 0.533 (0.390–0.727) | |||||
| With clustering effect | ||||||
| Cluster-specific adjusted χ2 (P-value) | 2.356 (P = 0.124) | |||||
RR = rate ratio; CI = confidence interval.
Because Wainyapu and Umbungedo were less similar than anticipated, a second analysis based on cumulative incidence was performed to account for possible clustering effects; the RR became 0.65 (0.09–4.8), i.e., statistically insignificant and protective efficacy was reduced 46.8% (95% CI 27.3–61%). The RR without clustering effects was 0.53 (0.39–0.73), but a cluster-specific adjusted χ2 value was 2.356 (P = 0.124).
Anopheline landing rates.
A total of 26 weeks of HLC were performed within each cluster during the intervention trial. From these collections, An. sundaicus species E was the predominant anopheline captured representing 86.6% (N = 1,603) and 82.2% (N = 74) of the total collections from Wainyapu and Umbungedo villages, respectively. Distribution of other anopheline species included: 11.2% (264) An. subpictus sensu lato, 0.3% (7) An. indefinitus, 0.2% (5) An. vagus, 0.89% (21)An. barbirostris, 0.04% (1) An. annularis and An. maculatus, 0.2% (5) An. aconitus, 0.08% (2) An. kochi and 0.2% (5) An. tessellatus. The majority of An. sundaicus were collected outdoors to give an indoor to outdoor biting ratio of 1:1.74.
A total of 2,345 anophelines were processed for CSP detection using ELISA and PCR analyses. Of these, only An. sundaicus were detected CSP positive (15 of 1,825) and only from the W1 and W2 (Wainyapu) clusters. Molecular identification examining the mtDNA of samples of An. sundaicus found all specimens assayed to be An. sundaicus E, as yet an undescribed formal species in the complex.37
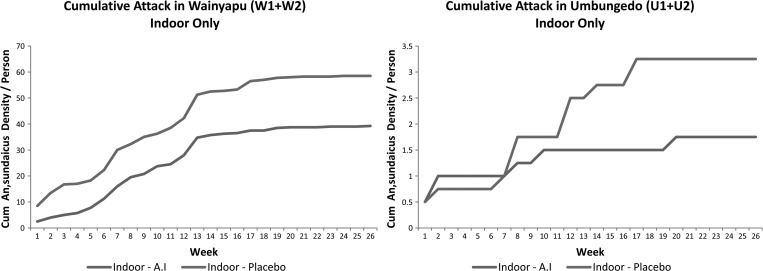
The cumulative indoor An. sundaicus landing rates from Wainyapu and Umbungedo villages are shown in Figure 4. Overall, there was a significantly reduced landing density from collections performed at sentinel households containing metofluthrin coils as compared with those assigned to blanks (P = 0.0342). This difference resulted in a combined 32.9% reduction in An. sundaicus attack rate on collectors at sentinel households with active coils compared with blank houses in Wainyapu (W1 and W2) (P = 0.04388). Similar attack rate ratios could not be performed in the Umbungedo clusters as overall HLC densities of An. sundaicus mosquitoes were too low.
Figure 4.
Cumulative weekly indoor attack rates of Anopheles sundaicus, pooled by village cluster in Wainyapu and Umbungedo, respectively. Both villages showed significantly different indoor Anopheles biting densities between active and placebo houses during 26 weeks of observation.
Age-grading.
The proportion of sampled females categorized as “older,” combining parous and gravid (those with developing ovarian follicles as evidence of recent blood meal), and those “younger” as nulliparous and recently emerged were compared between the four sub-cluster sentinel HLC sites. Sufficient HLC numbers were only present in W1 and W2 areas to allow comparisons. Overall older: younger ratio between “active,” “blank,” and “no coil” homes were not statistically different over the entire study, therefore indicating sites remained comparable regarding age structure throughout the sampling.
Adult resting collections.
Despite many hours of effort each week, the total number of anophelines captured indoors and outside of the sentinel houses was extremely low, only 88 (0.17 Anopheles per house sampling week) were recorded of which 60% were collected indoors. The remaining 35 mosquitoes were found either underneath the house, on the veranda area, or in the immediate surroundings. Collection attempts in outdoor locations produced only 10% of all mosquitoes captured. The predominant species (75%) was An. sundaicus, 64% of which were found indoors. This is further evidence confirming its status as the primary malaria vector in the study area. Anopheles subpictus s.l. represented the second most common species (17%). Only 22 (29%) of 74 tested contained evidence of a blood meal, 77% were captured resting indoors. Human blood was only detected in four samples (three An. sundaicus), others included single or mixed dog, goat, pig, bovine, and avian (chicken) blood proteins.
Discussion
This study focused on two primary challenges: 1) establish a proof-of-concept regarding spatial repellents for a reduction in malaria transmission and 2) determine what entomological measures might be predictive of an SR impact. Incident infection, pooled according to intervention treatment (i.e., active versus placebo coils), served as the indicator of transmission and the primary parasitological impact outcome. The two villages were selected with the assumption that clusters could be pooled but calculated incidence rates following intervention indicated this would be inappropriate. For that reason we applied an additional statistical analysis to allow for possible cluster effects. The authors acknowledge that conventional cluster-randomized study designs typically incorporate more clusters to reconcile the intra-cluster variation; however, it is important to note this work was never intended to be a robust proof of concept, which necessarily will require multiple replicates of each cluster.
The findings in this study offer preliminary evidence of reduced transmission in clusters of homes treated with a spatial repellent product containing metofluthrin. Such evidence of human health impact is a fundamental and essential component in the critical path of development of any vector control tool,38–41 especially new paradigms such as SR.4 If the crude estimate of protective efficacy shown here, about 52%, is verified in statistically robust cluster-randomized trials, this instrument of control would likely approximate that benefit associated with LLINs.42
It is important to note this study was not intended to assess a practical method of delivery of a spatial repellent product in malaria control. Using four coils per single-room home every night under direct supervision and monitoring is of course neither practical nor feasible as a long-term intervention practice. A flameless and passive means of distributing an active ingredient vapor that elicits SR would offer far greater use. However, the burning coil system was deemed an expedient means of testing the concept of SR reduction of risk of malaria infection because this format provides a nearly constant concentration of repellent chemical throughout the night (Johnson SC, personal communication). The multiple coils per home, direct supervision of use, and clustering to capture possible village protective effects were all deliberate means of maximizing protective efficacy as integral to proof of concept.
Analyses of HLCs indicated a significant reduction in vector landing rates of the primary malaria vector in the study area, An. sundaicus, in those houses that contained metofluthrin active coils compared with blank coils. As this was the primary attacking species and the only anopheline to be found positive for Plasmodium sporozoites, there is a high probability that the reduction in malaria incidence among study participants was directly associated with the reduction in human-vector contact by this species. For An. sundaicus in western Sumba, only HLC and sporozoite infections were found useful as correlates for coil effectiveness, i.e., significantly reducing indoor vector contact with humans. Other monitoring such as longitudinal age determination (parity), indoor/outdoor vector resting collections, and blood meal analysis, proved to be imprecise measures of potential impact with this vector species and epidemiological setting.
The authors recognize that chemicals such as metofluthrin exert a number of actions on mosquitoes, which may ultimately result in a vector free space. These actions are dose-dependent and include vapor phase repellency (at concentrations below toxic thresholds) and vapor phase toxicity (at higher doses). Although the current protocol could not differentiate the contribution of these two actions to the success of reduced HLC, we are confident that the concentration encountered in the house was well below the toxic level for metofluthrin based on air sampling conducted inside and outdoors of experimental huts using same dose metofluthrin coils4. Nevertheless, this remains a critical missing piece of the equation and will require a more detailed, integrated investigative approach involving other tools such as laboratory-based excito-repellency assays (e.g., HITSS) and field-based experimental huts (e.g., entry and exit traps).4
The current study also begs the questions of mosquito diversion and village-level protective effects. The minority treatments within clusters (i.e., the 10 of the 90:10 randomization in each cluster) were inadequately powered for observation of definitive findings. However, the cumulative incidence of malaria infection among homes receiving placebo and being surrounded by homes receiving active coils was similar to that among homes in clusters with a placebo majority.
Another possible weakness in this study is the potential confounding effect by variable risk of exposure among the few clusters used. In other words, the 52% protective efficacy could be less an effect of SR and more geographic variance in risk of infection that happened to align by chance with randomized SR assignment. The more clusters examined, the less likely chance observations will confound intervention impact estimates. In this study we used a split-cluster design that renders only two clusters per treatment, thus resulting in a sample size of just two paired treatment clusters. Randomization to placebo treatment at these two paired cluster sites need only have favored the more heavily malarious cluster twice in a row to explain the observed protective effects. However, such an explanation requires invoking a degree of heterogeneity in transmission dynamics between the clusters within the same villages that is unlikely to have occurred during this 26-week trial.
In conclusion, this study has added further evidence that a vector control strategy, which reduces mosquito attack rates without requiring direct vector contact on treated surfaces, can reduce malaria transmission in endemic settings.43 The results presented here have encouraged the substantial investment required to validate SR as a means of risk and harm reduction using larger cluster-randomized trials, as was done with insecticide-treated nets,38,44, including the investigation of possible risk/infection diversion effects of SR.
ACKNOWLEDGMENTS
We thank Christina Nixon who provided much appreciated valuable technical assistance and strenuous work in the field. We also express our gratitude to the tireless efforts of Fitria Wulandari, our study administrator, at the Eijkman-Oxford Clinical Research Unit in Jakarta. Dede Sudiana of the same laboratory provided expert logistical and administrative support of this endeavor. We gratefully thank the residents in the study area for the time and patience to participate in this trial. Special gratitude is expended to the Southwest Sumba District Health Department for their kind support, the industrious and unflagging energy of the parasitology and entomology teams, local field workers, data entry clerks, and local volunteers for their dedication to meeting the project goals.
Disclaimer: The contents are the responsibility of the authors and do not necessarily reflect the views of the United States Government.
Footnotes
Financial support: This study was funded by an award from the Bill & Melinda Gates Foundation (BMGF) to ALERTAsia Foundation in Jakarta on behalf of the Hasanuddin University and Eijkman Institute for Molecular Biology. We express our gratitude to both Foundations for their generosity and vigorous support of this endeavor, especially Kate Aultman at the BMGF, and to Claudia Surjadjaja, Shanti Gayatri, and Manihar Panjaitan. We are deeply grateful to Daniel Lawson and Maude Maier of the S.C. Johnson and Son, Corp., USA for providing the mosquito coils and for expert assistance in the study design. JKB is supported by the Wellcome Trust (grant No. B9RJIXO).
Authors' addresses: Din Syafruddin, Eijkman Institute for Molecular Biology, Jakarta, Indonesia, and Department of Parasitology, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia, E-mail: din@eijkman.go.id. Michael J. Bangs, Public Health and Malaria Control, International SOS, Kuala Kencana, Papua Indonesia, E-mail: bang_michael@fmi.com. Dian Sidik, Department of Epidemiology, Faculty of Public Health, Hasanuddin University, Makassar, E-mail: dian_sidiq@yahoo.com. Iqbal Elyazar and Siti Nurleila, Eijkman-Oxford Clinical Research Unit, Jakarta, Indonesia, E-mails: iqbal.elyazar@gmail.com and snurleila@eocru.org. Puji BS Asih, Krisin Chan, and Christian Nixon, Eijkman Institute for Molecular Biology, Jakarta, Indonesia, E-mails: puji@eijkman.go.id, sanariliskris@yahoo.com, and cnixon@lifespan.org. Joko Hendarto, Isra Wahid, and Hasanuddin Ishak, Department of Parasitology, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia, E-mails: jokohendarto@gmail.com, israwahid@gmail.com, and hasanuddin.ishak@gmail.com. Claus Bøgh, The Sumba Foundation, Bali, Indonesia, E-mail: cbogh@cbn.net.id. John P. Grieco, Department of Preventive Medicine and Biometrics, Uniformed Services, University of the Health Sciences, Bethesda, MD, E-mail: jgrieco@usuhs.mil. Nicole L. Achee, Department of Biological Sciences, Eck Institute for Global Health, University of Notre Dame, Notre Dame, IN, E-mail: nachee@nd.edu. J. Kevin Baird, Eijkman-Oxford Clinical Research Unit, Jakarta, Indonesia, and Centre for Tropical Medicine, Nuffield Department of Medicine, University of Oxford, Oxford, UK, E-mail: jkevinbaird@yahoo.com.
References
- 1.World Health Organization . World Malaria Report. Geneva: WHO; 2011. [Google Scholar]
- 2.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KG, Haring D, Fullman N, Naghavi M, Lozano M, Lopez AD. Global malaria mortality between 1980 and 2010: a systematic review. Lancet. 2011;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 3.Hill J, Rowland M. Insecticide-treated nets. Adv Parasitol. 2006;61:77–128. doi: 10.1016/S0065-308X(05)61003-2. [DOI] [PubMed] [Google Scholar]
- 4.Achee NL, Bangs MJ, Farlow R, Killeen GF, Lindsay S, Logan JG, Moore SJ, Rowland M, Sweeney K, Torr SJ, Zwiebel LJ, Grieco JP. Spatial repellents: from discovery and development to evidence-based validation. Malar J. 2012;11:164. doi: 10.1186/1475-2875-11-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . Guidelines for Efficacy Testing of Spatial Repellents. Geneva: WHO; 2013. [Google Scholar]
- 6.Muirhead-Thomson RC. Mosquito Behavior in Relation to Malaria Transmission and Control in the Tropics. London, UK: Edward Arnold and Company; 1953. [Google Scholar]
- 7.Kawada H, Maekawa Y, Tsuda Y, Takagi M. Laboratory and field evaluation of spatial repellency with metofluthrin-impregnated paper strip against mosquitoes in Lombok Island, Indonesia. J Am Mosq Control Assoc. 2004;20:292–298. [PubMed] [Google Scholar]
- 8.Kawada H, Maekawa Y, Takagi M. Field trial on the spatial repellency of metofluthrin-impregnated plastic strips for mosquitoes in shelters without walls (beruga) in Lombok, Indonesia. J Vector Ecol. 2005;30:181–185. [PubMed] [Google Scholar]
- 9.Ujihara K, Mori T, Iwasaki T, Sugano M, Shono Y, Matsuo N. Metofluthrin: a potent new synthetic pyrethroid with high vapor activity against mosquitoes. Biosci Biotechnol Biochem. 2004;68:170–174. doi: 10.1271/bbb.68.170. [DOI] [PubMed] [Google Scholar]
- 10.Kawada H, Maekawa Y, Tsuda Y, Takagi M. Trial of spatial repellency of metofluthrin-impregnated paper strip against Anopheles and Culex in shelters without walls in Lombok, Indonesia. J Am Mosq Control Assoc. 2004;20:434–437. Erratum in J Am Mosq Control Assoc 21: 105. [PubMed] [Google Scholar]
- 11.Kennedy JS. The excitant and repellent effects on mosquito of sublethal contact with DDT. Bull Entomol Res. 1947;37:593–607. doi: 10.1017/s0007485300030091. [DOI] [PubMed] [Google Scholar]
- 12.De Zulueta J, Cullen JR. Deterrent effect of insecticides on malaria vectors. Nature. 1963;200:860–861. doi: 10.1038/200860a0. [DOI] [PubMed] [Google Scholar]
- 13.White GW. Terminology of insect repellents. In: Debboun M, Frances SP, Strickman D, editors. Insect Repellents. Boca Rotan, FL: CRC Press; 2007. pp. 31–46. [Google Scholar]
- 14.Grieco JP, Achee NL, Andre RG, Roberts DR. A comparison study of house entering and exiting behavior of Anopheles vestitipennis using experimental hut sprayed with DDT or deltramethrin in the southern district of Toledo, Belize, CA. J Vector Ecol. 2000;25:62–73. [PubMed] [Google Scholar]
- 15.Ogoma SB, Lweitoijera DW, Ngonyani H, Furer B, Russell TL, Mukabana TR, Killeen GF, Moore SJ. Screening mosquito house entry points as a potential method for integrated control of endophagic filariasis, arbovirus and malaria vectors. PLoS Negl Dis. 2010;4:e773. doi: 10.1371/journal.pntd.0000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill N, Lenglet A, Arnez AM, Carneiro I. Plant-based insect repellent and insecticide treated nets to protect against malaria in areas of early evening biting vectors: double blind randomized placebo controlled trial in the Bolivian Amazon. BMJ. 2007;335:1023. doi: 10.1136/bmj.39356.574641.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Syafruddin D, Krisin, Asih P, Sekartuti, Dewi RM, Coutrier F, Rozy IE, Susanti AI, Elyazar IR, Sutamihardja A, Rahmat A, Kinzer M, Rogers WO. Seasonal prevalence of malaria in West Sumba District, Indonesia. Malar J. 2009;8:8. doi: 10.1186/1475-2875-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asih PB, Dewi RM, Tuti S, Sadikin M, Sumarto W, Sinaga B, van der Ven AJ, Sauerwein RW, Syafruddin D. Efficacy of artemisinin-based combination therapy for uncomplicated falciparum malaria in West Sumba district, East Nusa Tenggara Province, Indonesia and the genotypic profiles of the parasite. Am J Trop Med Hyg. 2009;80:914–918. [PubMed] [Google Scholar]
- 19.Elyazar IR, Hay SI, Baird JK. Malaria distribution, prevalence, drug resistance and control in Indonesia. Adv Parasitol. 2011;74:41–174. doi: 10.1016/B978-0-12-385897-9.00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling J, Baird JK, Fryauff DJ, Sismadi P, Bangs MJ, Lacy M, Barcus MJ, Gramzinski R, Maguire JD, Kumusumangsih M, Miller GB, Jones TR, Chulay JD, Hoffman SL. Randomized, placebo-controlled trial of atovaquone/proguanil for the prevention of Plasmodium falciparum and Plasmodium vivax malaria among migrants to Papua, Indonesia. Clin Infect Dis. 2002;35:825–833. doi: 10.1086/342578. [DOI] [PubMed] [Google Scholar]
- 21.Bennett S, Parpia T, Hayes R, Cousens S. Methods for the analysis of incidence rates in cluster randomized trials. Int J Epidemiol. 2002;31:839–846. doi: 10.1093/ije/31.4.839. [DOI] [PubMed] [Google Scholar]
- 22.Dean AG, Sullivan KM, Soe MM. Open epi: Open source epidemiologic statistic for Public Health. 2010. www.OpenEpi.com Version 2.3.1. Available at. Updated 2010. Accessed September 22, 2012.
- 23.Brookmeyer R, Chen YQ. Person-time analysis of paired community intervention trials when the number of communities is small. Stat Med. 1998;71:2121–2132. doi: 10.1002/(sici)1097-0258(19980930)17:18<2121::aid-sim907>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 24.Directorate General for Diseases Control and Environmental Health . Guide to Malaria Case Management (Pedoman Tatalaksana Malaria) Jakarta, Indonesia: Ministry of Health; 2011. [Google Scholar]
- 25.Baird JK, Lacy MD, Basri H, Barcus MJ, Maguire JD, Bangs MJ, Gramzinski R, Sismadi P, Krisin, Ling J, Wiady I, Kusumaningsih M, Jones TR, Fryauff DJ, Hoffman SL. Randomized, parallel placebo-controlled trial of primaquine for malaria prophylaxis in Papua, Indonesia. Clin Infect Dis. 2001;33:1990–1997. doi: 10.1086/324085. [DOI] [PubMed] [Google Scholar]
- 26.Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39:1336–1345. doi: 10.1086/424663. [DOI] [PubMed] [Google Scholar]
- 27.Sutanto I, Tjahyono B, Barsi H, Taylor WB, Putti FA, Meilia RA, Setiabudy R, Nurleila S, Ekawaty LL, Elyazar I, Farrar J, Sudoyo H, Baird JK. Randomized, open-label trial of primaquine against vivax malaria relapse in Indonesia. Antimicrob Agents Chemother. 2013;57:1128–1135. doi: 10.1128/AAC.01879-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization . Basic Malaria Microscopy Part 1. Learner's Guide. Second edition. Geneva: WHO; 2010. [Google Scholar]
- 29.O?Connors CT, Soepanto A. Jakarta, Indonesia: The Ministry of Health; 1979. Kunci kunci bergambar untuk Anopheles betina dari Indonesia. Translated and revised by Atmosoedjono S, Bangs, MJ, 1989. Illustrated key to the Anopheles of Indonesia; pp. 1–40. [Google Scholar]
- 30.Detinova TS. Geneva: World Health Organization; 1962. Age-grouping methods in Diptera of medical important with special reference to some vectors of malaria; p. 216. [PubMed] [Google Scholar]
- 31.Burkot TR, Williams JL, Schneider I. Identification of Plasmodium falciparum infected mosquitoes by double antibody enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1984;33:783–788. doi: 10.4269/ajtmh.1984.33.783. [DOI] [PubMed] [Google Scholar]
- 32.Singh B, Bobogare A, Cox-Singh J, Snounou G, Abdullah MS, Rahman HA. A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am J Trop Med Hyg. 1999;60:687–692. doi: 10.4269/ajtmh.1999.60.687. [DOI] [PubMed] [Google Scholar]
- 33.Beier JC. Vector incrimination and entomological inoculation rates. In: Doolan DL, editor. Malaria Methods and Protocols. Totowa, NJ: Humana Press; 2002. pp. 3–11. [DOI] [PubMed] [Google Scholar]
- 34.Vazquez-Prokopec GM, Galvin WA, Kelly R, Kitron U. A new, cost-effective, battery-powered aspirator for adult mosquito collections. J Med Entomol. 2009;46:1256–1259. doi: 10.1603/033.046.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zimmerman RH, Galardo AK, Lounibos LP, Arruda M, Wirtz R. Bloodmeal hosts of Anopheles species (Diptera: Culicidae) in a malaria-endemic area of the Brazilian Amazon. J Med Entomol. 2006;43:947–956. doi: 10.1603/0022-2585(2006)43[947:bhoasd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 36.Donner A, Klar N, Zou G. Methods for the statistical analysis of binary data in split-cluster designs. Biometrics. 2004;60:919–925. doi: 10.1111/j.0006-341X.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- 37.Dusfour I, Blondeau J, Harbach RE, Vythilingham I, Baimai V, Trung HD, Sochanta T, Bangs MJ, Manguin S. Polymerase chain reaction identification of three members of the Anopheles sundaicus (Diptera: Culicidae) complex, malaria vectors in Southeast Asia. J Med Entomol. 2007;44:723–731. doi: 10.1603/0022-2585(2007)44[723:pcriot]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 38.Grieco JP, Achee NL, Chareonviriyaphap T, Suwonkerd W, Chauhan K, Sardelis MR, Roberts DR. A new classification system for the actions of IRS chemicals traditionally used for malaria control. PLoS ONE. 2007;2:e716. doi: 10.1371/journal.pone.0000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Said SH, Grieco JP, Achee NL. Evaluation of contact irritant and spatial repellent behavioral responses of male Aedes aegypti to vector control compounds. J Mosq Contr Assoc. 2009;25:436–441. doi: 10.2987/09-5895.1. [DOI] [PubMed] [Google Scholar]
- 40.Hawley WA, Phillips-Howard PA, ter Kuile FO, Terlouw DJ, Vulule JM, Ombok M, Nahlen BL, Gimnig JE, Kariuki SK, Kolczak MS, Hightower AW. Community-wide effects permethrin-treated bed nets on child mortality and malaria morbidity in western Kenya. Am J Trop Med Hyg. 2003;68:121–127. [PubMed] [Google Scholar]
- 41.Phillips-Howard PA, ter Kuile FO, Nahlen BL, Alaii JA, Gimnig JE, Kolczak MS, Terlouw DJ, Kariuki SK, Shi YP, Kachur SP, Hightower AW, Vulule JM, Hawley WA. The efficacy of permethrin-treated bed nets on child mortality and morbidity in western Kenya II. Study design and methods. Am J Trop Med Hyg. 2003;68:10–15. [PubMed] [Google Scholar]
- 42.Lengeler C. Insecticide treated bed net and curtain for preventing malaria (review) The Cochrane Library; 2009. http://www.thecochranelibrary.com/userfiles/ccoch/file/CD000363.pdf Available at. Accessed September 25, 2014. [DOI] [PubMed] [Google Scholar]
- 43.Hill N, Zho HN, Wang P, Guo X, Carneiro I, Moore SJ. A household randomized, controlled trial of the efficacy of 0.03% transfluthrin coils alone and in combination with long-lasting insecticidal nets on the incidence of Plasmodium falciparum and Plasmodium vivax malaria in Western Yunnan Province, China. Malar J. 2014;13:208. doi: 10.1186/1475-2875-13-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hemingway J, Beaty BJ, Rowland M, Scott TW, Sharp BL. The innovative vector control consortium: improved control of mosquito-borne diseases. Trends Parasitol. 2006;22:308–312. doi: 10.1016/j.pt.2006.05.003. [DOI] [PubMed] [Google Scholar]