Abstract
Splenic infarction is a rare complication of malaria. We report two recent cases of splenic infarction after Plasmodium vivax infection. No systematic review of malaria-induced splenic infarction was available, therefore we conducted a systematic review of the English, French, and Spanish literature in PubMed and KoreaMed for reports of malaria-associated splenic infarction from 1960 to 2012. Of the 40 cases collected on splenic infarction by Plasmodium species, 23 involved P. vivax, 11 Plasmodium falciparum, one Plasmodium ovale, and five a mixed infection of P. vivax and P. falciparum. Of the 40 cases, 2 (5.0%) involved splenectomy and 5 (12.5%) were accompanied by splenic rupture. The median time from symptom onset to diagnosis was 8.5 days (range, 3–90 days). Improved findings after treatment were observed in 8 (88.9%) of 9 patients with splenic infarction on follow-up by computed tomography or ultrasonography. All patients survived after treatment with the exception of one patient with cerebral malaria. Clinicians should consider the possibility of splenic infarction when malaria-infected patients have left upper quadrant pain.
Introduction
Malaria is a serious parasitic infection that affects both residents and travelers in tropical climates. Approximately 300 to 500 million malaria cases and 1.5 to 3.5 million deaths are reported annually.1 However, according to a 2013 World Health Organization (WHO) report, 207 million cases of malaria and an estimated 627,000 deaths occurred in 2012 and the malaria mortality rate has fallen by 42% globally since 2000.2 Malaria in humans is transmitted by the Anopheles mosquito vector and is caused by five species: Plasmodium falciparum (P. falciparum), Plasmodium vivax (P. vivax), Plasmodium ovale (P. ovale), Plasmodium malariae (P. malariae), and Plasmodium knowlesi (P. knowlesi).1,3 Almost all severe forms of malaria are caused by P. falciparum, but serious complications such as severe anemia, respiratory distress, splenic complications, shock, and multiple organ dysfunction also develop with P. vivax infection.4 Splenic complications identified in cases of malaria are splenic infarction, spontaneous splenic rupture, hyperreactive malarial syndrome, hypersplenism, ectopic spleen and splenic torsion, and splenic cysts.5 Splenic infarction is not usually noted and is likely underdiagnosed in many cases of complicated malaria.6 Reports of malaria-associated splenic infarctions are rare, however, more recently, PubMed reports of cases have appeared almost annually. We recently encountered two cases of malaria with splenic infarction caused by P. vivax. However, systematic reviews of the clinical characteristics of splenic infarction resulting from malaria have not been conducted. Therefore, we performed a literature survey of splenic infarction in malaria in addition to summarizing these two cases. Our focus was on clinical outcomes, associated factors, and pathogenesis.
Case Reports
Case 1.
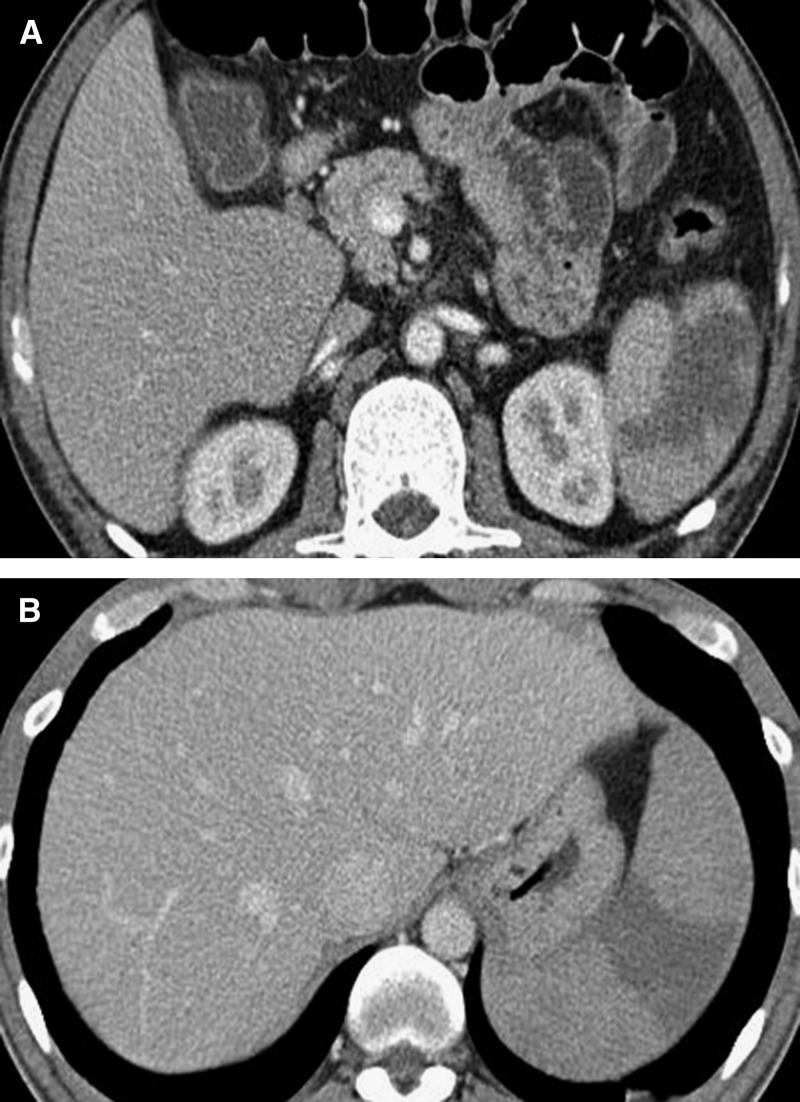
A 46-year-old man visited the emergency room because of a fever that had commenced 8 days previously. On admission, his body temperature was 40.6°C, blood pressure 110/70 mm Hg, heart rate 90/min, and respiratory rate 18/min. Physical examination revealed tenderness in the right upper quadrant of the abdomen, but the liver and spleen were not detectable. His complete blood count was white blood cell count 2.5 × 103/μL, hemoglobin (Hb) 11.2 g/dL, and platelet count of 15 × 103/μL. His chemistry profile was serum aspartate aminotransferase 76 U/L, alanine aminotransferase 24 U/L, total bilirubin 2.5 mg/dL, direct bilirubin 1.6 mg/dL, alkaline phosphatase 328 IU/L, and gamma-glutamyl transpeptidase level 218 IU/L. Features of abdominal computed tomography (CT) were consistent with splenic infarction, including multiple low attenuations in the spleen (Figure 1A). A Wright-Giemsa stain of a peripheral blood smear revealed a ring-form P. vivax trophozoite among the erythrocytes.
Figure 1.
Contrast-enhanced computed tomography of two patients, case 1 (A) and case 2 (B), showing low attenuation density in the spleen.
The patient was given oral chloroquine (25 mg/kg over 48 hours) on the day of admission and his fever subsided after 3 days. Right upper quadrant pain also decreased and additional pain in the left upper quadrant did not appear. At discharge, a 14-day regimen of oral primaquine (15 mg/day) was prescribed. One month later, the patient was stable and symptom free.
Case 2.
A 27-year-old man visited the emergency room because of fever and severe chills that had begun 5 days before admission. The patient was previously admitted to another hospital because of 4 days of fever and chills. However, the patient gradually worsened and he was referred to our hospital for further evaluation and to treat the fever of unknown origin.
Past medical history revealed that the patient had served military duty in a malaria-endemic area of Korea 3 years previously. During mandatory military service, he intermittently used chloroquine and had not experienced a malarial infection. When the patient arrived at our hospital he had a temperature of 39.0°C. The spleen was palpable in the abdomen and the patient complained of abdominal left upper quadrant tenderness. Initial laboratory results included white blood cell count 4.8 × 103/μL, Hb 12.7 g/dL, platelet count 60 × 103/μL, aspartate aminotransferase 47 IU/L, alanine aminotransferase 85 IU/L, and total bilirubin 0.9 mg/dL. Abdominal CT revealed splenomegaly of ∼15 cm and a low-attenuated wedge-shaped region that was consistent with splenic infarction (Figure 1B). A Wright-Giemsa stained blood smear performed within 24 hours of hospitalization revealed erythrocytes infected with P. vivax that ranged from ring-form to merozoites. Oral chloroquine was administered (25 mg/kg over 48 hours) in addition to primaquine (15 mg/day for 14 days). One month later the patient was stable and symptom free.
Methods
Literature search.
We examined relevant literature published between 1960 and 2012 that discussed malaria-associated splenic infarction using the keywords “spleen infarction, malaria” or “spleen rupture, malaria” in a PubMed Medline search. The results were limited to human studies published in English, French, and Spanish. Domestic cases that were not found in PubMed were searched in KoreaMed (http://koreamed.org/SearchBasic.php) using the keywords “spleen infarction, malaria,” “splenic infarction, malaria,” or “spleen rupture, malaria.”7–9 Reports were in Korean or English. Two investigators independently reviewed the articles and discordance was resolved by consensus.
Selection criteria and data extraction.
Diagnostic tools used to diagnose malaria were peripheral blood smear, enzyme-linked immunosorbent assay for antibody detection, nested polymerase chain reaction using 18s ribosomal RNA, and immunochromatography for Plasmodium lactate dehydrogenase. Patients had a positive result for at least one of the tests. After a diagnosis of malaria was confirmed, cases were selected that used at least one of three methods for diagnosing splenic infarction: CT, ultrasonography (US), or gross findings or histopathology. Patients with hemorrhage from splenic rupture accompanied by splenic infarction in examinations were considered eligible. Data collected from each study were age, sex, symptoms (fever, left upper quadrant pain, and right upper quadrant pain), vital signs (hypotension, tachycardia, and respiratory distress), laboratory findings (anemia, thrombocytopenia, and parasitemia), disease state (acute or chronic), type of malaria, order of malarial infection (first or second), chemoprophylaxis (performed or not performed), splenic rupture (presence or absence), splenectomy (undergone or not undergone), and outcome (living or dead). This study was approved and performed according to the guidelines of the Institutional Review Board of Chonbuk National University Hospital (IRB no.: CUH 2014-01-026).
Definition.
Hypotension was defined as systolic pressure ≤ 80 mm Hg, tachycardia as ≥ 100 beats/min, respiratory distress as respiratory rate > 30 breaths/min, severe anemia as Hb < 7.0 g/dL, thrombocytopenia as < 1.5 × 105/μL, and severe thrombocytopenia as < 6.0 × 104/μL.10,11
Results
Selected articles.
Figure 2 shows the process of identifying eligible papers. We discovered 126 references from PubMed (N = 119) and KoreaMed (N = 7) and screened the titles, abstracts, and the full text of these publications. Reasons for exclusion of cases were no case report form, report of only spleen rupture, no malarial cause for spleen infarction or spleen rupture, previous splenectomy and duplication. We included 23 articles and excluded 103.
Figure 2.
Identification and selection of eligible papers.
Clinical characteristics.
A total of 40 patients with malaria-associated splenic infarction were reported from 1960 through 2012. We confirmed the age and sex of 26 patients. The median age was 32 years (range, 3–65 years) with 17 men and 9 women. Left upper quadrant pain developed 2-fold more before antimalarial treatment than after or during antimalarial treatment. Laboratory findings and vital signs are in Table 1. The median time from onset of symptoms to diagnosis of splenic infarction was 8.5 days (range, 3–90 days). Of the 40 patients, 9 had follow-up US or CT and 8 (88.9%) of the 9 with splenic infarction improved. Follow-up periods after antimalarial treatment of the 8 patients that showed improvement were 1, 2, 3, 4, and 10 weeks for a single patient each, two patients that improved after 12 weeks, and one that was not checked during the follow-up period. However, one patient did not have improved findings on follow-up imaging after 1 week of treatment. The reviewed cases had limited details about the clinical situation related to splenic infarction.
Table 1.
Demographic and clinical features in patients with splenic infarction in malaria (N = 40)*
| Characteristics | Malaria type | No. (%) | |||
|---|---|---|---|---|---|
| P. vivax (N = 23) | P. falciparum (N = 11) | Mixed infection in P. vivax and P. falciparum (N = 5) | P. ovale (N = 1) | ||
| Age, y, median (IQR) | 38 (25.5–49.5) | 28 (11–36) | 28 (12.5–53.5) | NA | 32 (21–41.25)† |
| Male sex | 6 | 8 | 2 | 1 | 17 (65.4)‡ |
| Fever | 10/10 | 10/10 | 5/5 | 1/1 | 26/26 (100) |
| LUQ pain | 10/10 | 8/8 | 5/5 | 1/1 | 24/24 (100) |
| Before anti-malarial drug | 6/10 | 7/8 | 3/5 | 16/24 (66.7) | |
| After or during anti-malarial drug | 4/10 | 1/8 | 2/5 | 1/1 | 8/24 (33.3) |
| Hypotension | 0/2 | 0/2 | 3/3 | NA | 3/7 (42.9) |
| Tachycardia | 1/2 | 2/2 | 3/3 | NA | 6/7 (85.7) |
| Respiratory distress | 1/2 | 0/1 | 0/2 | 0/1 | 1/6 (16.7) |
| Severe anemia | 2/9 | 2/6 | 0/4 | 0/1 | 4/20 (20.0) |
| Thrombocytopenia | 7/9 | 4/4 | 3/3 | 1/1 | 15/17 (88.2) |
| Severe thrombocytopenia | 5/9 | 3/4 | 2/3 | 1/1 | 11/17 (64.7) |
| Time from the onset of symptom to diagnosis of splenic infarction, d, median (IQR) | 10 (6–12) | 9 (6–20) | 7 (5.25–7.25) | NA | 8.5 (6.25–10.75)§ |
| Splenic infarction and splenomegaly improvement after anti-malarial treatment | 7/7 | 0/1 | 1/1 | NA | 8/9 (88.9) |
The denominator indicates the available number out of total 40 cases.
Available in 26 cases.
Available in 26 cases.
Available in 24 cases.
IQR = interquartile range; LUQ = left upper quadrant; NA = not available.
Clinical outcomes.
Of the 40 cases, 23 (57.5%) were caused by P. vivax and 11 (27.5%) were caused by P. falciparum. One (2.5%) case was caused by P. ovale and five (12.5%) were caused by mixed infections of P. vivax and P. falciparum. All reported patients survived with the exception of a cerebral malaria patient. Only two (5.0%) patients received a splenectomy and five had cases that were accompanied by splenic rupture (12.5%) (Table 2). All patients were in the acute stage of malaria except for one patient who had concurrent visceral leishmaniasis. Twelve cases had available parasitemia results; of these, four had parasitemia > 105/μL.
Table 2.
Splenic infarction in Plasmodium vivax, Plasmodium falciparum, Plasmodium ovale, and mixed infection with P. vivax and P. falciparum (N = 40)
| Species | Reference (number of patients) | Parasitemia (/μL) | Splenomegaly | Splenic rupture | Splenectomy | Survival |
|---|---|---|---|---|---|---|
| P. vivax | 7 (1) | 1.25 × 105 | Yes | No | No | Live |
| 8 (1) | 2.44 × 103 | Yes | No | No | Live | |
| 9 (1) | NA | NA | No | No | Live | |
| 13 (1) | 1.88 × 103 | Yes | No | No | Live | |
| 48 (1) | NA | Yes | Yes | Yes | Live | |
| 49 (2) | 1.28 × 104 | Yes | No | No | Live | |
| 4.60 × 103 | ||||||
| 50 (13) | NA | NA | No | No | Live | |
| 51 (1) | NA | Yes | Yes | No | Live | |
| 52 (1) | NA | Yes | No | No | Live | |
| 53 (1) | NA | Yes | No | No | Live | |
| P. falciparum | 6 (1) | 2.50 × 104 | Yes | No | No | Live |
| 49 (1) | 1.20 × 105 | Yes | No | No | Live | |
| 54 (2) | NA | Yes | No | No | Live | |
| 55 (1) | NA | Yes | No | Yes | Live | |
| 56 (1) | NA | Yes | No | No | Live | |
| 57 (1) | 1.50 × 104 | NA | No | No | Live | |
| 58 (1) | NA | Yes | No | No | Live | |
| 59 (1) | NA | Yes | No | No | Live | |
| 60 (1) | NA | Yes | Yes | No | Live | |
| 61 (1) | 1.25 × 107 | Yes | No | No | Death | |
| Mixed infection in P. vivax and P. falciparum | 49 (1) | 9.20 × 104* | Yes | No | No | Live |
| 52 (1) | NA | Yes | No | No | Live | |
| 62 (1) | 5.00 × 105† | Yes | No | No | Live | |
| 3.50 × 107‡ | ||||||
| 63 (1) | NA | Yes | Yes | No | Live | |
| 64 (1) | NA | Yes | Yes | No | Live | |
| P. ovale | 12 (1) | 5.00 × 10 | Yes | No | No | Live |
Adding up the parasitemia of P. vivax and P. falciparum.
P. vivax.
P. falciparum.
NA = not available.
Discussion
Frequency of splenic infarction in malaria.
Splenic infarction has been described as a rare complication of malaria, but the exact frequency of malaria-associated splenic infarction remains unclear because of underdiagnosis and underreporting.12,13 Our study found that reports of splenic infarction were primarily from three countries, France, India, and Korea; this finding suggests the possibility of reporting bias by region.
Imaging can usually be performed to evaluate splenic complications in patients that complain of left upper quadrant and when splenic infarction is suspected. However, having clinicians routinely order US or CT images to identify splenic infarction is unreasonable. In addition, US or CT might not be conducted on patients with splenic infarction that is asymptomatic or accompanied by mild symptoms. Even if symptoms develop, splenic infarction might not be diagnosed because US or CT imaging is not available.
Splenic rupture resulting from malaria was reported more frequently with P. vivax than with P. falciparum infection.14 A recent study reported that malaria-associated splenic infarction is primarily caused by P. falciparum in contrast to splenic rupture.6 However, based on our results, P. vivax is as likely to cause splenic infarction as P. falciparum.
Malaria-associated splenic infarction prognosis.
Clinical aspects of splenic rupture can be severe and life-threatening.5 In one study, the case fatality rate of splenic rupture was 18.2%: among 55 patients, 12 died, 10 by splenic rupture and two from pneumonia that developed after splenectomy.14 Of these 55 patients, 33 (60%) underwent splenectomy,14 which was higher than the 5% of splenic infarction patients in our study. In addition, more than 50% of the patients in the previous study presented with hemodynamic collapse at the time of splenic rupture diagnosis, indicating the severity of this complication.14 No patient deaths from malaria-associated splenic infarctions have yet been reported. Almost all patients in our study received conservative management without splenectomy and all survived after treatment, suggesting that splenic infarction in malaria that is not accompanied by complications, such as rupture, can have a benign course and that splenic infarction is not an indication for operation.15
Most splenic infarctions were described during the acute state of malaria infection (Table 2). The median time from the beginning of symptoms to the diagnosis of splenic infarction was 8.5 days (range, 3–90; available in 24 cases), which was longer than the median time from symptoms to splenic rupture, which was 5 days (range, 0–37; available in 49 cases).14 This could be because diagnostic images were performed relatively late and patients' vital signs during splenic infarction were generally more stable than during splenic rupture. The key presentation and management features of splenic infarction and splenic rupture are presented in Table 3. Parasitemia values, the first event of malaria infection, and chemoprophylaxis protocols were not available in most cases.
Table 3.
Comparison of splenic infarction and splenic rupture in clinical presentation and outcome*
| Splenic infarction (N = 35)† | Splenic rupture (N = 53)‡ | Mixed splenic infarction and splenic rupture (N = 5) | |
|---|---|---|---|
| Clinical findings | |||
| LUQ pain or diffuse abdominal tenderness | 19/19 (100%) | 23/52 (44%) | 5/5 (100%) |
| Fever | 21/21 (100%) | 36/52 (69%) | 5/5 (100%) |
| Splenomegaly | 20/20 (100%) | 20/52 (38%) | 5/5 (100%) |
| Median time from fever onset to splenic complications (no. of days, range) | 9 (5–90)§ | 5 (0–37) | 5 (3–11) |
| Management | |||
| Only medical treatment | 34 (97%) | 13 (24%) | 4 (75%) |
| Medical treatment with splenectomy | 1 (3%) | 3 (6%) | 1 (25%) |
| Only splenectomy | 0 | 29 (55%) | 0 |
| None | 0 | 8 (15%) | 0 |
| Death | 1 (3%) | 12 (23%) | 0 |
The denominator indicates the available number.
The number except for 5 cases45,48,57,60,61 with splenic infarction and splenic rupture from a total of 40 patients.
The number except for 2 cases45,60 with splenic infarction and splenic rupture from total 55 patients; see Reference 12.
Available in 19 cases.
LUQ = left lower quadrant.
Splenic infarction in malaria: pathogenesis.
Splenic infarction is a result of parenchymal ischemia from the occlusion of the arterial or venous circulation.15 However, the mechanism by which malaria results in splenic infarction is not clearly understood.6 For our investigation of the literature on malaria, we hypothesized that the following factors would contribute to a hypoxic state attributable to splenic infarction: 1) hypercoagulable state; 2) intrasplenic structural change by adhesion of malaria-infected red blood cells (iRBCs) to endothelial cells, with rosetting of iRBCs and non-iRBCs and splenic cellular hyperplasia; and 3) anemic hypoxia.
Hypercoagulable state.
During the acute stage of malaria, alteration of the coagulation system is observed with P. falciparum and P. vivax. Levels of antithrombin III, protein C, and protein S decrease remarkably in P. falciparum infection.16,17 An increase in von Willebrand factor (vWF) and plasminogen activator inhibitor and a decrease in ADAMTS13 and tissue plasminogen activator were observed in P. vivax and P. falciparum infections.16,18 The reductions in protein C, protein S, and antithrombin III levels strongly suggest the consumption of clotting factors caused by microvascular thrombosis, which is sufficient evidence for the generation of thrombi in malaria.16 Elevated vWF and reduced ADAMTS13 might be associated with intravascular platelet aggregation and microvascular disease.18 These factors are more likely to contribute toward ineffective fibrinolysis and the hypercoagulable state in acute malaria infection by P. falciparum than P. vivax.19
In postmortem studies on patients infected with P. falciparum, however, the formation of thrombin and fibrin, which is a final result of the hypercoagulable state, is not clearly observed in the spleen.20,21 For patients who received antimalarial drugs, which induce a non-natural state during treatment, the rare finding of thrombin and fibrin formation might result from a return to normal condition from a hypercoagulable state after administration of antimalarial drugs.17,22 In contrast with P. falciparum infection, altered thrombostasis and intravascular coagulation have not yet been characterized in P. vivax infection.4,23 However, a study of acute kidney injury that developed with P. vivax infection found that the characteristics of renal biopsies were thrombotic microangiopathy, arterioles filled with an intraluminal fibrin thrombus or platelets, and endothelial injury.24 Local splenic infarction caused by thrombus formation was also observed in splenic rupture caused by P. vivax.25 Therefore, splenic microvascular obstruction by thrombin formation, fibrin deposition, and platelet aggregation, followed by reduction and congestion of local blood flow in the intrasplenic vascular system might partially contribute to local tissue hypoxia in the spleen. Further studies on splenic pathological findings in the absence of antimalarial treatment are needed to identify the role of coagulation disorders in malarial splenic infarction.
Vascular congestion and occlusion: cytoadhesion and rosetting of iRBCs and splenic cellular hyperplasia.
Elevation of cytokines such as tumor necrosis factor (TNF)-α and interferon (INF)-γ, in conjunction with the cytoadhesion of malaria-iRBCs to endothelial cells, can cause endothelial injury and activation, followed by expression of tissue factors and hypercoagulability.22,26,27 Microvascular endothelial adhesion of P. falciparum-iRBCs appears to develop throughout the body, and distribution of iRBCs and extent of sequestration varies by organ system and is not even uniform within the same organ.20,21,28–32 Elevation of TNF-α and IFN-γ/IL-10 and increase in endothelial activation markers such as thrombomodulin, intracellular adhesion molecule (ICAM)-1, vascular adhesion molecule (VCAM)-1, and E-selectin are also observed in P. vivax infection.33,34 Previously, P. vivax was not seen to become sequestered in the deep microvasculature of inner organs.35 However, recent studies suggest the cytoadherence and sequestration of P. vivax-iRBCs.36–39 Further research is required to identify the role of cytoadhesion and sequestration in P. vivax pathogenesis.
In postmortem studies, mature P. falciparum-iRBC sequestration is observed in red pulp, suggesting cytoadherence of iRBCs to the sinusoidal endothelium and mechanical retention.20,21 Malarial parasites express various types of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) that have different adhesive properties and bind to alternative endothelial receptors.40 This determines the organ in which iRBCs are sequestered and the severity of disease.40 The mechanism for PfEMP1 and endothelial receptor ligand-receptor interaction has not yet been identified, particularly in the spleen.40 If mature P. falciparum-iRBCs enter rapidly from a splenic artery into a perifollicular zone, venous sinus and splenic vein where iRBCs are sequestered and adhere to endothelial cells, autoagglutination between other iRBCs, rosetting of iRBCs with non-iRBCs, and platelet-mediated clumping can develop partially or extensively in the fast, closed circulation of the spleen, forming a lesion that obstructs blood flow.21,41 Compared with the closed circulation system that mainly causes congestion of mature-iRBCs, both mature-iRBCs and ring-iRBCs appear to be retained in the splenic cord in slow, open circulation.21,42 In particular, a large amount of ring-iRBCs accumulate upstream of interendothelial slits.42 Macrophage hyperplasia, which occupies about half of the cord volume, and an increasing number of erythrocytes, lymphocytes, plasma cells, polymorphonuclear cells, and mononuclear cells also build up in the splenic cord, contributing to blood congestion.21,41 In P. falciparum malaria, impairment of intrasplenic blood flow by obstructive lesions, increased oxygen requirement and structural change by cellular hyperplasia in both circulation systems are more likely to be attributable to regional ischemia, which might result in splenic infarction.43
In two patients with P. vivax infection who had splenic infarction and splenic rupture, several structures were identified including a hyperplastic splenic cord, multiple erythrocytes, macrophages, and leukocytes that filled ectatic or poorly outlined sinusoids and veins.25,44 Thrombus formation in the vein or sinusoid was also observed.25,44 Recently, in an untreated ruptured spleen that was infected with P. vivax, a diffuse hypercellularity in red pulp that presented with many P. vivax-iRBCs in the cords, massive proliferating plasma cells, and striking intra-sinusoidal histiocytosis were observed by immunohistopathological analysis.45 These findings suggest that intrasplenic structural changes by excessive hyperplastic growth can cause blood flow obstruction, contributing to local splenic hypoxia in P. vivax infection. However, whether sequestration and cytoadhesion of P. vivax-iRBCs in sinusoids are important in pathogenesis remains unclear.35,43
Anemic hypoxia.
Investigating anemia associated with malaria is beyond the scope of this work. The pathogenesis of malarial anemia is multifactorial. The mechanisms of anemia associated with P. vivax and P. falciparum infections vary and are not completely understood.46 As the removal site of extravascular erythrocytes, the spleen is important in the destruction of iRBCs and non-iRBCs.47 Therefore, malarial anemia likely contributes to tissue hypoxia in the spleen.
In summary, malaria-associated splenic infarction is reported as a complication in P. falciparum, P. vivax, and P. ovale infection, but has not yet been identified in P. malariae and P. knowlesi infections. Splenic infarction is primarily detected in the acute phase of infection. Clinicians should consider left upper quadrant pain a predictive sign of splenic infarction and perform imaging such as US or CT if available. In general, the clinical response to medical treatment will likely be sufficient and a splenectomy might not be necessary. However, the pathogenesis of splenic infarction has not been sufficiently elucidated. Based on previous studies, hypercoagulability, intrasplenic structural change directed toward vascular congestion, obstruction caused by hypercellularity and sequestration, and anemia progression likely contribute to the complexity of splenic hypoxia that is followed by splenic infarction in vulnerable areas. Further studies are needed to better understand the pathogenesis of splenic infarction.
Disclaimer: Transparency declarations, none to declare.
Footnotes
Financial support: This paper was supported by research funds from Chonbuk National University in 2013. This paper was supported by Fund of Chonbuk National University Hospital Research Institute of Clinical Medicine.
Authors' addresses: Jeong-Hwan Hwang, Department of Internal Medicine, Chonbuk National University Medical School, Jeonju, Republic of Korea, E-mail: smilehwang77@hanmail.net. Chang-Seop Lee, Department of Internal Medicine, Chonbuk National University Medical School and Research Institute of Clinical Medicine of Chonbuk National University-Chonbuk National University Hospital, Jeonju, Republic of Korea, E-mail: lcsmd@jbnu.ac.kr.
References
- 1.Stanley J. Malaria. Emerg Med Clin North Am. 1997;15:113–155. doi: 10.1016/s0733-8627(05)70288-1. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization World Malaria Report. 2013. http://www.who.int/malaria/publications/world_malaria_report_2013/report/en/ Available at. Accessed May 20, 2014.
- 3.Cox-Singh J, Davis TM, Lee KS, Shamsul SS, Matusop A, Ratnam S, Rahman HA, Conway DJ, Singh B. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis. 2010;46:165–171. doi: 10.1086/524888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anstey NM, Douglas NM, Poespoprodjo JR, Price RN. Plasmodium vivax: clinical spectrum, risk factors and pathogenesis. Adv Parasitol. 2012;80:151–201. doi: 10.1016/B978-0-12-397900-1.00003-7. [DOI] [PubMed] [Google Scholar]
- 5.Zingman BS, Viner BL. Splenic complications in malaria: case report and review. Clin Infect Dis. 1993;16:223–232. doi: 10.1093/clind/16.2.223. [DOI] [PubMed] [Google Scholar]
- 6.Bonnard P, Guiard-Schmid JB, Develoux M, Rozenbaum W, Pialoux G. Splenic infarction during acute malaria. Trans R Soc Trop Med Hyg. 2005;99:82–86. doi: 10.1016/j.trstmh.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Jung EJ, Choo EJ, Kim TH, Jeon MH, Lee EJ, Cho YS, Lee HY, Kim JY. Two cases of vivax malaria accompanied by splenic complications (such as splenic rupture and splenic infarction) Infect Chemother. 2008;40:179–183. [Google Scholar]
- 8.Cho HJ, Kim KH, Kim JI, Ahn CH, Yoo SJ, Lim KW, Kim JS. Splenic infarction caused by vivax malaria. J Korean Surg Soc. 2008;75:213–215. [Google Scholar]
- 9.Jeong SK, Oh YM, Choi SM, Choi KH, Lee WJ, Kim SK. Clinical manifestations of vivax malaria diagnosed patients. J Korean Soc Emerg Med. 2002;13:187–192. [Google Scholar]
- 10.Barber BE, William T, Grigg MJ, Menon J, Auburn S, Marfurt J, Anstey NM, Yeo TW. A prospective comparative study of knowlesi, falciparum, and vivax malaria in Sabah, Malaysia: high proportion with severe disease from Plasmodium knowlesi and Plasmodium vivax but no mortality with early referral and artesunate therapy. Clin Infect Dis. 2013;56:383–397. doi: 10.1093/cid/cis902. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez-Morales AJ, Sánchez E, Vargas M, Piccolo C, Colina R, Arria M, Franco-Paredes C. Occurrence of thrombocytopenia in Plasmodium vivax malaria. Clin Infect Dis. 2005;41:130–131. doi: 10.1086/430837. [DOI] [PubMed] [Google Scholar]
- 12.Cinquetti G, Banal F, Rondel C, Plancade D, de Saint Roman C, Adriamanantena D, Ragot C, Védy S, Graffin B. Splenic infarction during Plasmodium ovale acute malaria: first case reported. Malar J. 2010;9:288. doi: 10.1186/1475-2875-9-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim A, Park YK, Lee JS, Chung MH, Kim ES. A case of symptomatic splenic infarction in vivax malaria. Korean J Parasitol. 2007;45:55–58. doi: 10.3347/kjp.2007.45.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imbert P, Rapp C, Buffet PA. Pathological rupture of the spleen in malaria: analysis of 55 cases (1958–2008) Travel Med Infect Dis. 2009;7:147–159. doi: 10.1016/j.tmaid.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Nores M, Phillips EH, Morgenstern L, Hiatt JR. The clinical spectrum of splenic infarction. Am Surg. 1998;64:182–188. [PubMed] [Google Scholar]
- 16.Mohanty D, Ghosh K, Nandwani SK, Shetty S, Phillips C, Rizvi S, Parmar BD. Fibrinolysis, inhibitors of blood coagulation, and monocyte derived coagulant activity in acute malaria. Am J Hematol. 1997;54:23–29. doi: 10.1002/(sici)1096-8652(199701)54:1<23::aid-ajh4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Vogetseder A, Ospelt C, Reindl M, Schober M, Schmutzhard E. Time course of coagulation parameters, cytokines and adhesion molecules in Plasmodium falciparum malaria. Trop Med Int Health. 2004;9:767–773. doi: 10.1111/j.1365-3156.2004.01265.x. [DOI] [PubMed] [Google Scholar]
- 18.de Mast Q, Groot E, Asih PB, Syafruddin D, Oosting M, Sebastian S, Ferwerda B, Netea MG, de Groot PG, van der Ven AJ, Fijnheer R. ADAMTS13 deficiency with elevated levels of ultra-large and active von Willebrand factor in P. falciparum and P. vivax malaria. Am J Trop Med Hyg. 2009;80:492–498. [PubMed] [Google Scholar]
- 19.Francischetti IM. Does activation of the blood coagulation cascade have a role in malaria pathogenesis? Trends Parasitol. 2008;24:258–263. doi: 10.1016/j.pt.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prommano O, Chaisri U, Turner GD, Wilairatana P, Ferguson DJ, Viriyavejakul P, White NJ, Pongponratn E. A quantitative ultrastructural study of the liver and the spleen in fatal falciparum malaria. Southeast Asian J Trop Med Public Health. 2005;36:1359–1370. [PubMed] [Google Scholar]
- 21.Pongponratn E, Riganti M, Bunnag D, Harinasuta T. Spleen in falciparum malaria: ultrastructural study. Southeast Asian J Trop Med Public Health. 1987;18:491–501. [PubMed] [Google Scholar]
- 22.Hemmer CJ, Kern P, Holst FG, Radtke KP, Egbring R, Bierhaus A, Nawroth PP, Dietrich M. Activation of the host response in human Plasmodium falciparum malaria: relation of parasitemia to tumor necrosis factor/cachectin, thrombin-antithrombin III, and protein C levels. Am J Med. 1991;91:37–44. doi: 10.1016/0002-9343(91)90071-5. [DOI] [PubMed] [Google Scholar]
- 23.Anstey NM, Russell B, Yeo TW, Price RN. The pathophysiology of vivax malaria. Trends Parasitol. 2009;25:220–227. doi: 10.1016/j.pt.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Sinha A, Singh G, Bhat AS, Mohapatra S, Gulati A, Hari P, Samantaray JC, Dinda AK, Agarwal SK, Bagga A. Thrombotic microangiopathy and acute kidney injury following vivax malaria. Clin Exp Nephrol. 2013;17:66–72. doi: 10.1007/s10157-012-0656-9. [DOI] [PubMed] [Google Scholar]
- 25.Lubitz JM. Pathology of the ruptured spleen in acute vivax malaria. Blood. 1949;4:1168–1176. [PubMed] [Google Scholar]
- 26.Francischetti IM, Seydel KB, Monteiro RQ, Whitten RO, Erexson CR, Noronha AL, Ostera GR, Kamiza SB, Molyneux ME, Ward JM, Taylor TE. Plasmodium falciparum-infected erythrocytes induce tissue factor expression in endothelial cells and support the assembly of multimolecular coagulation complexes. J Thromb Haemost. 2007;5:155–165. doi: 10.1111/j.1538-7836.2006.02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner GD, Ly VC, Nguyen TH, Tran TH, Nguyen HP, Bethell D, Wyllie S, Louwrier K, Fox SB, Gatter KC, Day NP, Tran TH, White NJ, Berendt AR. Systemic endothelial activation occurs in both mild and severe malaria. Correlating dermal microvascular endothelial cell phenotype and soluble cell adhesion molecules with disease severity. Am J Pathol. 1998;152:1477–1487. [PMC free article] [PubMed] [Google Scholar]
- 28.Pongponratn E, Riganti M, Punpoowong B, Aikawa M. Microvascular sequestration of parasitized erythrocytes in human falciparum malaria: a pathological study. Am J Trop Med Hyg. 1991;44:168–175. doi: 10.4269/ajtmh.1991.44.168. [DOI] [PubMed] [Google Scholar]
- 29.Pongponratn E, Turner GD, Day NP, Phu NH, Simpson JA, Stepniewska K, Mai NT, Viriyavejakul P, Looareesuwan S, Hien TT, Ferguson DJ, White NJ. An ultrastructural study of the brain in fatal Plasmodium falciparum malaria. Am J Trop Med Hyg. 2003;69:345–359. [PubMed] [Google Scholar]
- 30.Turner GD, Morrison H, Jones M, Davis TM, Looareesuwan S, Buley ID, Gatter KC, Newbold CI, Pukritayakamee S, Nagachinta B. An immunohistochemical study of the pathology of fatal malaria. Am J Pathol. 1994;145:1057–1069. [PMC free article] [PubMed] [Google Scholar]
- 31.Nguansangiam S, Day NP, Hien TT, Mai NT, Chaisri U, Riganti M, Dondorp AM, Lee SJ, Phu NH, Turner GD, White NJ, Ferguson DJ, Pongponratn E. A quantitative ultrastructural study of renal pathology in fatal Plasmodium falciparum malaria. Trop Med Int Health. 2007;12:1037–1050. doi: 10.1111/j.1365-3156.2007.01881.x. [DOI] [PubMed] [Google Scholar]
- 32.Hanson J, Lam SW, Mahanta KC, Pattnaik R, Alam S, Mohanty S, Hasan MU, Hossain A, Charunwatthana P, Chotivanich K, Maude RJ, Kingston H, Day NP, Mishra S, White NJ, Dondorp AM. Relative contributions of macrovascular and microvascular dysfunction to disease severity in falciparum malaria. J Infect Dis. 2012;206:571–579. doi: 10.1093/infdis/jis400. [DOI] [PubMed] [Google Scholar]
- 33.Andrade BB, Reis-Filho A, Souza-Neto SM, Clarêncio J, Camargo LM, Barral A, Barral-Netto M. Severe Plasmodium vivax malaria exhibits marked inflammatory imbalance. Malar J. 2010;9:13. doi: 10.1186/1475-2875-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohnishi K. Serum levels of thrombomodulin, intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin in the acute phase of Plasmodium vivax malaria. Am J Trop Med Hyg. 1999;60:248–250. doi: 10.4269/ajtmh.1999.60.248. [DOI] [PubMed] [Google Scholar]
- 35.Bassat Q, Alonso PL. Defying malaria: fathoming severe Plasmodium vivax disease. Nat Med. 2011;17:48–49. doi: 10.1038/nm0111-48. [DOI] [PubMed] [Google Scholar]
- 36.Carvalho BO, Lopes SC, Nogueira PA, Orlandi PP, Bargieri DY, Blanco YC, Mamoni R, Leite JA, Rodrigues MM, Soares IS, Oliveira TR, Wunderlich G, Lacerda MV, del Portillo HA, Araújo MO, Russell B, Suwanarusk R, Snounou G, Rénia L, Costa FT. On the cytoadhesion of Plasmodium vivax-infected erythrocytes. J Infect Dis. 2010;202:638–647. doi: 10.1086/654815. [DOI] [PubMed] [Google Scholar]
- 37.Chotivanich K, Udomsangpetch R, Suwanarusk R, Pukrittayakamee S, Wilairatana P, Beeson JG, Day NP, White NJ. Plasmodium vivax adherence to placental glycosaminoglycans. PLoS ONE. 2012;7:e34509. doi: 10.1371/journal.pone.0034509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biswas J, Fogla R, Srinivasan P, Narayan S, Haranath K, Badrinath V. Ocular malaria: a clinical and histopathologic study. Ophthalmology. 1996;103:1471–1475. doi: 10.1016/s0161-6420(96)30481-8. [DOI] [PubMed] [Google Scholar]
- 39.Lacerda MV, Fragoso SC, Alecrim MG, Alexandre MA, Magalhães BM, Siqueira AM, Ferreira LC, Araújo JR, Mourão MP, Ferrer M, Castillo P, Martin-Jaular L, Fernandez-Becerra C, del Portillo H, Ordi J, Alonso PL, Bassat Q. Postmortem characterization of patients with clinical diagnosis of Plasmodium vivax malaria: to what extent does this parasite kill? Clin Infect Dis. 2012;55:e67–e74. doi: 10.1093/cid/cis615. [DOI] [PubMed] [Google Scholar]
- 40.Deitsch KW, Chitnis CE. Molecular basis of severe malaria. Proc Natl Acad Sci USA. 2012;109:10130–10131. doi: 10.1073/pnas.1207174109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buffet PA, Safeukui I, Deplaine G, Brousse V, Prendki V, Thellier M, Turner GD, Mercereau-Puijalon O. The pathogenesis of Plasmodium falciparum malaria in humans: insights from splenic physiology. Blood. 2011;117:381–392. doi: 10.1182/blood-2010-04-202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Safeukui I, Correas JM, Brousse V, Hirt D, Deplaine G, Mulé S, Lesurtel M, Goasguen N, Sauvanet A, Couvelard A, Kerneis S, Khun H, Vigan-Womas I, Ottone C, Molina TJ, Tréluyer JM, Mercereau-Puijalon O, Milon G, David PH, Buffet PA. Retention of Plasmodium falciparum ring-infected erythrocytes in the slow, open microcirculation of the human spleen. Blood. 2008;112:2520–2528. doi: 10.1182/blood-2008-03-146779. [DOI] [PubMed] [Google Scholar]
- 43.Rigdon RH. A consideration of the mechanism of splenic infarcts in malaria. Am J Trop Med Hyg. 1944;6:349–354. [Google Scholar]
- 44.Hershey FB, Lubitz JM. Spontaneous rupture of the malarial spleen: case report and analysis of 64 reported cases. Ann Surg. 1948;127:40–57. doi: 10.1097/00000658-194801000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Machado Siqueira A, Lopes Magalhães BM, Cardoso Melo G, Ferrer M, Castillo P, Martin-Jaular L, Fernandez-Becerra C, Ordi J, Martinez A, Lacerda MV, del Portillo HA. Spleen rupture in a case of untreated Plasmodium vivax infection. PLoS Negl Trop Dis. 2012;6:e1934. doi: 10.1371/journal.pntd.0001934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Douglas NM, Anstey NM, Buffet PA, Poespoprodjo JR, Yeo TW, White NJ, Price RN. The anaemia of Plasmodium vivax malaria. Malar J. 2012;11:135. doi: 10.1186/1475-2875-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kai OK, Roberts DJ. The pathophysiology of malarial anaemia: where have all the red cells gone? BMC Med. 2008;6:24. doi: 10.1186/1741-7015-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramos García A, Pérez Avila J, Pérez Ramos E, Valls Martín A, Díaz Hernández A. Splenic rupture in Plasmodium vivax malaria. Rev Cubana Med Trop. 1985;37:187–190. [PubMed] [Google Scholar]
- 49.Gupta BK, Sharma K, Nayak KC, Agrawal TD, Binani A, Purohit VP, Kochar DK. A case series of splenic infarction during acute malaria in northwest Rajasthan, India. Trans R Soc Trop Med Hyg. 2010;104:81–83. doi: 10.1016/j.trstmh.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 50.Kim EM, Cho HJ, Cho CR, Kwak YG, Kim MY, Cho YK. Abdominal computed tomography findings of malaria infection with Plasmodium vivax. Am J Trop Med Hyg. 2010;83:1202–1205. doi: 10.4269/ajtmh.2010.10-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim JS, Hong JS, Park YS, Ahn JY, Seo YH. Spontaneous hemothorax and hemoperitoneum in Plasmodium vivax malaria. Ann Trop Med Parasitol. 2011;105:177–179. doi: 10.1179/136485911X12899838413664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar BG, Shetty MA. Splenic complications in malaria: a case series. Southeast Asian J Trop Med Public Health. 2008;39:791–794. Chakrapani. [PubMed] [Google Scholar]
- 53.Sonkar SK, Uniyal R, Sonkar GK. Three unusual presentations of Plasmodium vivax malaria. Trop Doct. 2011;41:240–241. doi: 10.1258/td.2011.110220. [DOI] [PubMed] [Google Scholar]
- 54.Agarwal VK, Agarwal S, Pathak T. Splenic infarct in falciparum malaria. Indian Pediatr. 1997;34:1050–1501. [PubMed] [Google Scholar]
- 55.Christoforov B, Chiche B, Duflo B, Laffitte M, Fresneau M, Péquignot H. Splenic infarction in primary Plasmodium falciparum infection. Ann Med Interne (Paris) 1976;127:47–49. [PubMed] [Google Scholar]
- 56.Coche G, Estavoyer JM, Leroy J, Costaz R. Splenic infarction in Plasmodium falciparum malarial attack. J Radiol. 1990;71:473–475. [PubMed] [Google Scholar]
- 57.Salord F, Allaouchiche B, Gaussorgues P, Boibieux A, Sirodot M, Gerard-Boncompain M, Biron F, Peyramond D, Robert D. Severe falciparum malaria (21 cases) Intensive Care Med. 1991;17:449–454. doi: 10.1007/BF01690765. [DOI] [PubMed] [Google Scholar]
- 58.Singh BJ, Kumar A. Splenic infarctions in mixed infection with kala azar and falciparum malaria. J Assoc Physicians India. 1991;39:293. [PubMed] [Google Scholar]
- 59.Sur AK, Khawash N, Mitra PK, Ghosh K, Sinharoy D. Splenic infarct in falciparum malaria. Indian Pediatr. 1997;34:72. [PubMed] [Google Scholar]
- 60.Balfe P, Reynolds JV. A rare cause of acute abdomen-Plasmodium falciparum leading to splenic infarction and hemorrhage. Ir Med J. 2008;101:150–151. [PubMed] [Google Scholar]
- 61.Oga A, Sadamitu D, Hattori Y, Nakamura Y, Kohno M, Kawauchi S, Sasaki K. Imported malaria in a Japanese male: an autopsy report. Pathol Int. 2001;51:371–375. doi: 10.1046/j.1440-1827.2001.01206.x. [DOI] [PubMed] [Google Scholar]
- 62.Hovette P, Lecoules S, Boete F, Imbert P, Touze JE, Laroche R. Splenic infarction during P. falciparum and P. vivax malaria. Presse Med. 1994;23:1226. [PubMed] [Google Scholar]
- 63.Choudhury J, Uttam KG, Mukhopadhyay M. Spontaneous rupture of malarial spleen. Indian Pediatr. 2008;45:327–328. [PubMed] [Google Scholar]
- 64.Bansal VK, Krishna A, Misra MC, Khan RN, Noba AL, Kishore N. Spontaneous splenic rupture in complicated malaria: non-operative management. Trop Gastroenterol. 2010;31:233–235. [PubMed] [Google Scholar]