Abstract
Implementation of mass drug administration for lymphatic filariasis (LF) has been delayed in central Africa because of incomplete mapping and coendemic loiasis. We mapped two regions in eastern Democratic Republic of Congo that were suspected to have LF. Night blood samples were collected from 2,724 subjects in 30 villages. Filarial antigenemia rates by card test exceeded 1% in 28 villages (range = 0–14%). Prevalence rates for large sheathed microfilariae (Mf) ranged from 4% to 40%; Mansonella perstans rates ranged from 22% to 98%. Large Mf were exclusively Loa loa by microscopy, and only 1 of 337 samples tested by quantitative polymerase chain reaction (qPCR) was positive for Wuchereria bancrofti DNA. Filarial antigen positivity was strongly associated with high L. loa Mf counts. Periodicity studies revealed atypical patterns, with no significant diurnal periodicity in some individuals. Thus, methods routinely used for LF mapping may not be reliable in areas in central Africa that are highly endemic for loiasis.
Introduction
Lymphatic filariasis (LF) affects some 120 million people in 73 countries, and approximately 1.3 billion people are at risk of becoming infected with the nematode parasites (Wuchereria bancrofti and Brugia species) that cause this disease.1 The Global Program to Eliminate Lymphatic Filariasis (GPELF) is using mass drug administration (MDA) to reduce filarial infection rates below those required for sustained transmission with the goal of permanently eliminating LF in all endemic countries by the year 2020. The progress of GPELF is variable: some countries have already approached the elimination target, whereas others have not even started with MDA. Accurate mapping of the distribution of LF is a crucial first step for LF elimination programs. This is especially important for regions with loiasis, because ivermectin used in LF elimination programs can cause serious adverse events (including death) in persons with heavy Loa loa infections. Two tests that are widely used for mapping LF are microfilaria (Mf) detection (by microscopic examination of stained thick smears prepared with blood collected at night) and detection of filarial antigenemia (immunological detection of soluble W. bancrofti antigens in peripheral blood) by immunochromatographic card test (ICT). Because W. bancrofti Mf in Africa exhibit nocturnal periodicity and because L. loa Mf exhibit diurnal periodicity, large sheathed Mf in night blood are generally assumed to be W. bancrofti, and Mf present in blood collected during the day are assumed to be L. loa.2 Mansonella perstans is a third filarial species that infects humans in many areas of Africa, but M. perstans Mf can easily be distinguished from those of L. loa and W. bancrofti based on their smaller size and lack of a sheath.
The circulating W. bancrofti antigen that is detected by the ICT card test is present in blood collected during the day or night. For convenience reasons, the test is often performed with blood samples collected during the day. Furthermore, this test has be extensively evaluated and used in many parts of the world for mapping and monitoring LF elimination programs. However, the ICT card test has not been widely used in the past in central Africa, because large-scale LF elimination programs have not been started in most countries in this region. This paper shows that tests that are routinely used to map LF may not provide accurate results in areas of central Africa that are highly endemic for loiasis.
Materials and Methods
Study area.
In total, 14 villages in the Ituri region (Mambasa Territory) and 16 villages in the Haut Uele region (Watsa Territory) of the Orientale Province in the eastern Democratic Republic of Congo (DRC) (Figure 1 and Supplemental Table 1) were screened for Mf and filarial antigenemia. Most villages were located in remote forested areas east of the Okapi game reserve. The surveys were conducted in July of 2011 and January of 2013. No community-directed treatment with ivermectin had been performed in the study areas before these surveys. Historically the Ituri region has been reported to be endemic for L. loa, W. bancrofti, and M. perstans (with Mf in the blood) as well as Onchocerca volvulus and M. streptocerca (with Mf in skin).3 More recent rapid epidemiological mapping of onchocerciasis indicated mostly meso- and hyperendemic villages in Mambasa and lower endemicity in Watsa.4
Figure 1.
Map of (left panel) the study area in the northeastern DRC showing (right panel) the examined villages in the Mambasa and Watsa Territories.
Sample collection.
A convenience sample of 50–100 individuals ages ≥ 14 years old was tested in each village between 21:00 and 01:00 hours to assess filarial antigenemia and Mf rates; 200 μL finger-prick blood was collected in (ethylenedinitrilo)tetraacetic acid (EDTA) -coated tubes.
Antigen and Mf testing.
The ICT test for circulating W. bancrofti antigen (Binax Filariasis Now Card Test; Alere, Scarborough, ME) was performed according to the manufacturer's instructions and read strictly at 10 minutes after applying the blood sample. Positive results were documented by photography (Figure 2). Mf testing was performed using three-line blood smears (60 μL blood total on the slide) as previously described.5 Giemsa-stained slides were read at 400× magnification, and numbers of small/thin (M. perstans) and large (L. loa or W. bancrofti) Mf were recorded. For each slide positive for large Mf, 10 large Mf were examined at a 1,000× magnification for differentiation of L. loa and W. bancrofti. Mf with a single nucleus in the tip of the tail were identified as L. loa. Slides positive for Mf were sent to Washington University in St. Louis, MO for reexamination by microscopy and DNA testing. Some positive and negative ICT cards were also sent to Washington University for DNA testing.
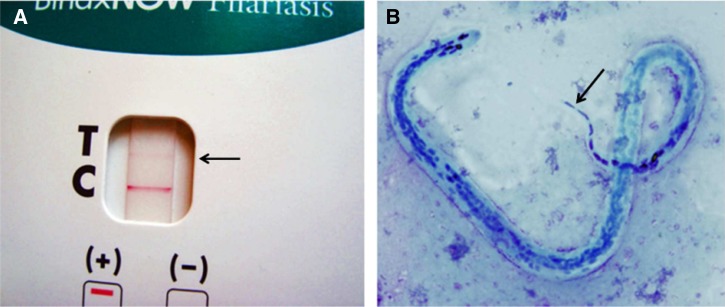
Figure 2.
A shows a weakly positive ICT card test from a subject from the Ituri region with L. loa Mf. Note that the test line (T; marked with an arrow) is the same color as the control (C) line. B shows a Giemsa-stained L. loa Mf in a night blood smear from the same subject. Note the characteristic elongated nuclei in the tip of the tail (arrow).
Periodicity of L. loa.
The periodicity of L. loa Mf was studied in seven adults (four men and three women; age range = 28–78 years) in July of 2012. These individuals had been noted to have high L. loa Mf counts (> 2,000/mL) in night blood in 2011. Periodicity was assessed by counting Mf in 60-μL thick smears prepared with blood collected by finger prick at 05:00, 13:00, and 21:00 hours.
Differentiation of L. loa and W. bancrofti by quantitative polymerase chain reaction.
High Mf densities in many slides made it impossible to examine all large Mf by microscopy at 1,000× magnification to exclude the presence of W. bancrofti Mf. Therefore, we used probe-based quantitative real-time polymerase chain reaction (qPCR) assays to detect filarial DNA. DNA extraction was performed 3–6 months after preparation of the Giemsa-stained blood smears. Sterile razor blades were used to scrape dried blood from stained blood smears (two lines; equivalent to 40 μL). DNA was isolated from dried blood using the QIAamp DNA Extraction Kit for Blood (QIAGEN, Valencia, CA). Alternatively, DNA was extracted from dried blood on one-half of an ICT card sample application pad as previously described.6 Blood samples were tested with two separate qPCR reactions for detection of W. bancrofti and L. loa DNA as previously described.6,7 Giemsa-stained slides from Côte d'Ivoire that contained W. bancrofti Mf but no L. loa Mf were used as positive controls for the W. bancrofti qPCR assay and negative controls for the L. loa qPCR assay.
Statistics.
R 3.0.1 (http://www.r-project.org/)8 was used to develop the generalized linear model (GLM)9 and design the map.10,11 A GLM with a binomial function was developed to identify significant predictors of positive ICT test results. Predictor variables in this model included age, sex, number of L. loa Mf per milliliter, and number of M. perstans Mf per milliliter. Maps were drawn using the R3.0.1 packages Maps (http://cran.r-project.org/web/packages/maps/index.html) and Mapdata (http://cran.r-project.org/web/packages/mapdata/index.html).10,11 Global positioning system (GPS) coordinates of the villages were recorded during the study period, and data detailing the administrative areas were taken from DIVA-GIS (www.diva-gis.org).
Ethical approval.
This mapping was performed as part of the National Program to Eliminate LF in the DRC. Collection of blood samples for the periodicity study was approved by the Ethical Committee of the School of Public Health, Kinshasa University, DRC.
Results
Prevalence rates for positive ICT tests and Mf.
In total, 2,724 individuals were tested in 30 villages in the Ituri and Haut Uele regions. Overall prevalence rates for filarial antigenemia and Mf in night blood with large sheathed Mf were 6% and 22%, respectively (Table 1). Positive ICT tests were observed in 28 of 30 villages, and 7 villages had ICT rates of at least 10%. Positive ICT tests had clearly visible T lines that were the same color as the control or C lines (Figure 2A). This result distinguishes these test results from false-positive tests that occur when tests are read well after the recommended read time of 10 minutes.12
Table 1.
Summary of filarial antigenemia and Mf test results from night blood surveys in the Ituri and Haut Uele regions in the eastern DRC by village
| Village name | N tested | ICT positive (%) | Rate of L. loa Mf (%) | Geometric mean* of L. loa (Mf/mL) | Rate of Mp Mf (%) | Geometric mean* of Mp Mf (Mf/mL) |
|---|---|---|---|---|---|---|
| Ituri | ||||||
| Memekidele | 71 | 10 | 27 | 160.3 | 97 | 2,605.3 |
| Aluta | 100 | 10 | 19 | 241.2 | 98 | 547.7 |
| KeroZanzibar | 90 | 7 | 21 | 168.1 | 85 | 877.4 |
| Digbo | 77 | 6 | 27 | 325.4 | 97 | 2,033.9 |
| Ekwe | 100 | 5 | 13 | 342.1 | 94 | 1,481.3 |
| Epulu | 98 | 5 | 17 | 155.6 | 44 | 379.3 |
| Salate | 89 | 4 | 10 | 567.7 | 62 | 413.4 |
| Saiyo | 53 | 4 | 19 | 545.0 | 83 | 2,018.0 |
| Nduye | 100 | 3 | 26 | 257.8 | 78 | 276.7 |
| Komboni | 100 | 3 | 21 | 309.5 | 92 | 1,194.1 |
| Molokayi | 100 | 3 | 10 | 302.2 | 61 | 449.9 |
| Malembi | 100 | 2 | 13 | 290.1 | 96 | 1,048.9 |
| Bapukeli | 98 | 2 | 19 | 147.2 | 70 | 913.4 |
| Butiaba 2 | 50 | 0 | 16 | 180.5 | 86 | 1,125.5 |
| Haut Uele | ||||||
| Bayitebi | 100 | 14 | 37 | 150.3 | 76 | 410.5 |
| Obo II | 100 | 13 | 30 | 161.3 | 75 | 221.6 |
| Kossia | 100 | 12 | 29 | 203.4 | 79 | 648.6 |
| Obo I | 100 | 10 | 37 | 314.1 | 76 | 288.1 |
| Luwi | 100 | 10 | 27 | 285.6 | 79 | 547.8 |
| Apodo | 100 | 8 | 40 | 451.2 | 73 | 429.7 |
| Netiti-Gombari | 100 | 8 | 25 | 278.1 | 67 | 562.3 |
| Tibodri | 100 | 8 | 26 | 137.8 | 65 | 506.9 |
| Bakiri | 100 | 7 | 35 | 267.3 | 59 | 832.8 |
| Dodi | 100 | 7 | 14 | 270.7 | 91 | 1,205.5 |
| Andekofu | 88 | 6 | 20 | 324.1 | 61 | 499.1 |
| Osso I | 100 | 5 | 24 | 212.5 | 63 | 607.1 |
| Kadungu | 87 | 3 | 21 | 109.1 | 50 | 351.1 |
| Ngili-ngili | 94 | 2 | 4 | 139.9 | 22 | 616.8 |
| Andra | 76 | 1 | 20 | 113.8 | 30 | 1,367.3 |
| Toli | 53 | 0 | 11 | 92.6 | 43 | 594.9 |
| Total | 2,724 | 6 | 22 | 231.2 | 72 | 582.3 |
Large sheathed Mf were identified by morphology as L. loa, whereas small unsheathed Mf were identified as M. perstans (Mp). Mf densities were not available for all Mp-positive samples from some villages.
The geometric mean number of Mf was calculated for Mf-positive subjects.
Prevalence rates by village for large sheathed Mf in night blood ranged between 4% and 40% (Table 1). Geometric mean Mf densities in infected individuals ranged from village to village from 92.6 to 567.7 Mf/mL, and some individuals had Mf counts of > 20,000/mL. The maximum number of large Mf of 47,448 Mf/mL was detected in an 85-year-old woman who had lived in Apodo for the last 35 years. She also had M. perstans (1,549 Mf/mL). Many of the large sheathed Mf were unambiguously identified as L. loa by morphology (Figure 2B), and no W. bancrofti Mf were identified. However, given the high number of large Mf in the slides (12% of the samples with large Mf had > 120 Mf per 60 μL), it was impossible to exclude the presence of W. bancrofti Mf by microscopy alone.
M. perstans Mf are much smaller than Mf of L. loa or W. bancrofti, and they were easily differentiated by microscopy. M. perstans prevalence rates by village ranged from 22% to 98%. Eighteen (60%) of the villages surveyed had M. perstans Mf prevalence rates of at least 70%. Geometric mean Mf counts for individuals with M. perstans by village ranged between 221.6 and 2,605.3 Mf/mL, with a geometric mean density of 582.3 Mf/mL. This number is more than two times as high as the mean density of L. loa Mf.
Species identification of Mf in night blood by qPCR.
qPCR testing was performed on blood from 337 slides that were positive for large sheathed Mf by microscopy (Table 2). L. loa DNA was detected in 294 (87%) samples. Because some blood samples contained very few Mf and recovery of DNA from stained smears is incomplete, it is likely that all tested slides with large Mf contained L. loa DNA. In contrast, W. bancrofti DNA was detected in only 1 of 337 blood samples tested (0.3%). This sample was from a 66-year-old woman who had lived in Ekwe village for the past 5 years and also had loiasis. We do not have information on where she lived before that time. We also tested sample application pads from 52 positive and 60 negative ICT cards for the presence of L. loa and W. bancrofti DNA; 36 of 52 subjects with positive ICT card results had large sheathed Mf in their thick blood smears, and 33 of the sample application pads from these ICT cards were positive for L. loa DNA by qPCR. Blood from the same female patient mentioned above from Ekwe was positive for DNA of both L. loa and W. bancrofti, but none of the other 111 card test application pads were positive for W. bancrofti DNA; 1 of 16 ICT positive blood samples from people with blood smears that did not contain large Mf was positive for L. loa DNA by qPCR. Also, 10 of 60 ICT negative card tests were from people with large Mf in night blood smears, and 8 of them were positive for L. loa DNA by qPCR. The other 50 ICT negative samples had no large Mf in night blood, and 1 of these samples was positive for L. loa DNA by qPCR. These results show that (with one exception) the large Mf present in night blood samples collected in this study were L. loa.
Table 2.
Detection of filarial antigenemia (ICT) and parasite DNA in night blood samples from subjects with large sheathed Mf in night blood smears
| Village | N tested by qPCR | N positive by L. loa DNA by qPCR | N positive for W. bancrofti DNA by qPCR | |
|---|---|---|---|---|
| ICT positive | ICT negative | |||
| Ituri | ||||
| Memekidele | 5 | 15 | 19 | 0 |
| Aluta | 8 | 12 | 18 | 0 |
| KeroZanzibar | 3 | 16 | 18 | 0 |
| Digbo | 4 | 16 | 12 | 0 |
| Ekwe | 4 | 9 | 12 | 1 |
| Epulu | 1 | 16 | 14 | 0 |
| Salate | 2 | 7 | 8 | 0 |
| Saiyo | 2 | 8 | 10 | 0 |
| Nduye | 3 | 23 | 23 | 0 |
| Komboni | 4 | 18 | 17 | 0 |
| Molokayi | 2 | 8 | 9 | 0 |
| Malembi | 3 | 11 | 13 | 0 |
| Bapukeli | 1 | 18 | 16 | 0 |
| Butiaba 2 | 0 | 8 | 8 | 0 |
| Haut Uele | ||||
| Bayitebi | 14 | 0 | 13 | 0 |
| Obo II | 13 | 0 | 11 | 0 |
| Kossia | 12 | 0 | 8 | 0 |
| Obo I | 10 | 0 | 9 | 0 |
| Luwi | 10 | 0 | 7 | 0 |
| Apodo | 8 | 3 | 11 | 0 |
| Netiti-Gombari | 7 | 0 | 7 | 0 |
| Tibodri | 6 | 0 | 6 | 0 |
| Bakiri | 7 | 0 | 7 | 0 |
| Dodi | 7 | 0 | 7 | 0 |
| Andekofu | 4 | 0 | 4 | 0 |
| Osso I | 5 | 2 | 5 | 0 |
| Kadungu | 2 | 0 | 2 | 0 |
| Ngili-ngili | 0 | 0 | – | – |
| Andra | 0 | 0 | – | – |
| Toli | 0 | 0 | – | – |
| Total | 147 | 190 | 294 | 1 |
Relationship between ICT card positivity and L. loa Mf density.
ICT card test positivity was clearly linked to the presence and number of L. loa Mf in night blood smears (Table 3). In total, 131 of 577 (22.7%) blood samples from people with L. loa Mf had positive ICT tests. Although only 2% of individuals without L. loa Mf in night blood had positive antigen tests, almost 60% of those with Mf counts > 2,000/mL had positive ICT tests; 7 of 15 subjects with Mf counts between 8,000 and 30,000 Mf/mL had positive ICT tests. The only person with > 30,000 Mf/mL in the night blood was the elderly woman mentioned above who had a negative ICT test.
Table 3.
Relation of positive filarial antigen test results (ICT) with L. loa and M. perstans Mf count
| Mf density (Mf/mL) | ICT negative | ICT positive | ICT invalid | Total N | |||
|---|---|---|---|---|---|---|---|
| N | Percent | N | Percent | N | Percent | ||
| L. loa | |||||||
| 0 | 2,065 | 97.59 | 37 | 1.75 | 14 | 0.66 | 2,116 |
| 1–100 | 206 | 91.56 | 18 | 8.00 | 1 | 0.44 | 225 |
| 101–2,000 | 240 | 76.68 | 72 | 23.00 | 1 | 0.32 | 313 |
| > 2,000 | 29 | 41.43 | 41 | 58.57 | 0 | 0 | 70 |
| Total | 2,540 | 93.25 | 168 | 6.17 | 16 | 0.58 | 2,724 |
| M. perstans | |||||||
| 0 | 673 | 95.33 | 29 | 4.10 | 4 | 0.57 | 706 |
| 1–100 | 270 | 94.07 | 17 | 5.92 | 0 | 0 | 287 |
| 101–2,000 | 740 | 90.13 | 77 | 9.38 | 4 | 0.49 | 821 |
| > 2,000 | 282 | 88.96 | 33 | 10.41 | 2 | 0.63 | 317 |
| Total | 1,965* | 92.21 | 156* | 7.32 | 10* | 0.47 | 2,131* |
Mf were counted on a single 60-μL night blood smear, and counts were converted to Mf per milliliter.
M. perstans Mf counts were not available for all M. perstans Mf-positive individuals. Slides from 593 M. perstans Mf-positive and L. loa Mf-negative subjects from six villages were not counted.
The ICT positivity rate for blood samples from persons who were negative for M. perstans Mf and persons with low (≤ 100 Mf/mL), medium (101–1,999 Mf/mL), or high M. perstans Mf counts (> 2,000 Mf/mL) ranged between 4% and 10%. Mf counts between 8,000 and 30,000 Mf/mL were detected in 65 individuals, and only 8 of these people had positive ICT test results. Higher M. perstans Mf counts were observed in only four subjects, and all of these subjects had negative ICT test results. A GLM comparing the differences in ICT test results with M. perstans Mf counts found no relationship between these two variables, whereas high L. loa Mf counts were strongly associated with positive ICT test results (Table 4).
Table 4.
Results from a GLM that assessed associations between filarial antigen test results and age, sex, and Mf counts for L. loa and M. perstans
| Variable | Estimate | SE | Z score | P value |
|---|---|---|---|---|
| Intercept | −3.176 | 1.043 | −11.208 | < 0.0001 |
| Age | 0.174 | 0.302 | 2.114 | 0.0345 |
| Sex (males) | 0.697 | 0.766 | 0.910 | 0.363 |
| L. loa (Mf/mL) | 0.089 | 0.066 | 4.958 | < 0.0001 |
| M. perstans (Mf/mL) | −0.045 | 0.036 | −1.276 | 0.202 |
Periodicity of L. loa Mf.
Periodicity studies were performed because of the unexpected finding that L. loa Mf were commonly seen in night blood. Finger prick blood was collected at 21:00, 05:00, and 13:00 hours from seven people that had L. loa Mf present in night blood samples during the first survey in 2011. All of these individuals were coinfected with M. perstans at that time, with Mf densities between 333 and 8,413 Mf/mL. At the time of reexamination in 2012, one man was Mf-negative, and the others had night blood L. loa Mf counts between 608 and 31,728 Mf/mL. Two individuals (subjects 2 and 3) had a diurnally periodic pattern, with almost no Mf at 21:00 and 05:00 hours but high counts of > 25,000 Mf/mL at 13:00 hours (Figure 3). Subjects 1 and 5 had diurnally subperiodic patterns, and two individuals (subjects 6 and 7) had aperiodic patterns, with > 20,000 Mf/mL at all three time points. These results show that L. loa exhibits atypical periodicity in some subjects in the study area, and many subjects had high Mf counts in night blood.
Figure 3.
Periodicity of L. loa Mf in seven patients with L. loa Mf in night blood smears. One subject (subject 4) had no Mf at the time of reexamination. The others had variable periodicity patterns that were diurnal (subjects 2 and 3), diurnally subperiodic (subject 5), and aperiodic (subjects 6 and 7); 60-μL finger prick blood samples were collected at (a) 21:00, (b) 05:00, and (c) 13:00 hours, and Mf densities were determined by microscopy. The species identification was confirmed to be L. loa in all cases by qPCR.
Discussion
The purpose of this study was to map the distribution of LF in the Ituri and Haut Uele regions of the DRC before initiation of MDA for LF elimination. With only 1 W. bancrofti infection identified (by PCR only) from > 2,700 people tested, it is safe to say that MDA is not required for these regions. This finding underlines the importance of careful mapping before initiating MDA for LF in central Africa, where the infection is often highly focal and historical information on prevalence is spotty.
In contrast to W. bancrofti, infections with L. loa and M. perstans were highly prevalent in the study areas. The presence of loiasis was not surprising, because recent rapid assessment of prevalence of Loa loa (RAPLOA) surveys had documented high rates of loiasis in these regions.13,14 However, high rates of L. loa Mf in night blood samples in this study were a major surprise. This result contrasts with the common assumption that people in central Africa with large sheathed Mf in night blood have bancroftian filariasis, whereas those with large sheathed Mf in day blood have loiasis.2 Periodicity studies showed that L. loa Mf counts did not exhibit the expected diurnal pattern in some subjects in the study areas. Additional research is needed to determine the relative frequency of these atypical periodicity patterns (diurnal subperiodic and aperiodic) and assess whether particular genotypes are associated with aperiodic L. loa.
The presence of high numbers of L. loa Mf in night blood samples has important implications for mapping LF in areas of central Africa with loiasis. It is not possible to rule out the presence of W. bancrofti Mf by microscopy when L. loa Mf counts are high in night blood samples. Although we assumed that this problem could be avoided by antigen testing, our results suggest that the ICT test may not be specific for W. bancrofti infection in loiasis coendemic areas of Africa. Prior studies have shown that, although the epitope recognized by the monoclonal antibody AD12.1 is present in antigen extracts from many nematode species, filarial antigen tests, such as the Binax Now Filariasis Test, that use this antibody (or the closely related Og4C3 monoclonal antibody) have until now been considered to be specific for W. bancrofti infection. Prior studies of the Binax Now Filariasis Test have not detected filarial antigen in serum samples from patients infected with B. malayi, O. volvulus, M. perstans, or L. loa (apart from a few samples from people who had also been exposed to W. bancrofti).15–17 The ICT card test has been extensively used in areas with onchocerciasis, and no evidence for cross-reactivity has been observed.18,19 However, relatively few serum or plasma samples from patients with loiasis were tested in these prior studies, and some of these samples were from Americans who had acquired the infection as missionaries or Peace Corps volunteers in Africa. It seems likely that some people with loiasis in the study area have a circulating L. loa antigen that is immunologically cross-reactive with the 200-kDa W. bancrofti adult worm antigen that is detected by the Binax Now Filariasis ICT Test.16 Because most (77%) blood samples from subjects with L. loa Mf had negative ICT test results, ICT positivity in loiasis is likely to be associated with high L. loa adult worm loads. Prior studies have shown that circulating filarial antigen levels are correlated with adult female worm counts in dogs infected with Dirofilaria immitis and jirds infected with B. pahangi.20,21 An association with adult worm loads would explain the increased frequency of ICT positivity in subjects with very high night blood Mf counts and the lack of positivity in prior studies with loiasis serum from expatriates and Africans from other Loa-endemic countries. The relatively high frequency of negative ICT tests in people with Loa Mf counts > 2,000/mL (41%) might be explained by immune clearance of the putative circulating L. loa antigen in some subjects.
Because day blood Mf counts were not performed in this study, we do not know whether the ICT test might be useful in areas without LF for detecting persons with very high L. loa Mf counts (> 30,000/mL) who have an increased risk of developing neurological severe adverse events after ivermectin.22 Additional studies are needed (with day blood testing in areas with high rates of loiasis) to answer this question.
The specificity problem that we observed with the ICT card test in central Africa may be even worse with the Alere Filariasis Test Strip, which also uses the AD12.1 monoclonal antibody, because the Test Strip is more sensitive than the ICT card test.15 It has to be stressed that positive ICT results caused by cross-reactivity of a Loa antigen are true-positive results that can be confirmed by retesting. These results are different from false-positive ICT tests caused by improper reading of the diagnostic device that are not confirmed when tests are read correctly after 10 minutes. This finding leads to the question of how best to map and monitor LF in areas with high rates of loiasis. Traditional night blood Mf testing might be sufficient for this purpose in areas where L. loa Mf have strictly diurnal periodicity. Antibody testing with the recombinant filarial antigen Wb-123 could be helpful, but there is no commercially available version of the test at this time.23 Similar to the situation with the ICT test, the specificity of Wb-123 antibodies for W. bancrofti infection has not yet been thoroughly evaluated with serum or plasma samples from Loa-endemic areas in central Africa. For now, we think that the best option for mapping LF in Loa-endemic regions would be to test night blood samples for W. bancrofti DNA by qPCR.24 Dried blood samples can be pooled to reduce costs, and night blood surveys should be powered to provide high confidence for detecting W. bancrofti infection rates that exceed 1% in implementation units, because that is the trip point for initiating MDA. Several reference laboratories in Africa have experience with qPCR testing for LF.
In conclusion, this study did not detect a significant presence of LF in Mambasa or Watsa Territories, and MDA is not required for that infection in these areas. However, the study identified factors that will complicate mapping and monitoring activities for LF elimination programs in areas of central Africa that are highly endemic for loiasis. They include high rates of L. loa Mf in night blood samples that interfere with detection of W. bancrofti Mf by microscopy and the unexpected finding that some subjects with high-intensity L. loa infections have positive filarial antigen tests. LF elimination programs in areas of central Africa with high rates of loiasis may need to use alternative diagnostic tools, such as antibody tests or qPCR, for mapping, monitoring, and evaluation.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the residents of the study villages who agreed to participate in these surveys. Ms. Yuefang Huang helped with the analysis of blood samples by qPCR.
Disclaimer: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of the Special Programme for Research and Training in Tropical Diseases or the Bill and Melinda Gates Foundation. The filarial antigen test used in this study uses reagents licensed from Barnes-Jewish Hospital, an affiliation of G.J.W. All royalties from sales of these tests are donated to the Barnes-Jewish Hospital Foundation, a registered not-for-profit organization (http://www.barnesjewish.org/groups/default).
Footnotes
Financial support: This research was funded by Bill and Melinda Gates Foundation Grant GH5342 (the Death to Onchocerciasis and Lymphatic Filariasis [DOLF] Project). D.K.B. received support during part of this study from the Special Programme for Research and Training in Tropical Diseases, World Health Organization.
Authors' addresses: Didier K. Bakajika, Programme National de Lutte contre L'Onchocercose, Kinshasa, Democratic Republic of Congo, and Centre de Recherche en Maladies Tropicales de l'Ituri, Ituri, Congo, Democratic Republic of Congo, E-mail: dbakajika@yahoo.fr. Maurice M. Nigo, Jean Pierre Lotsima, and Germain A. Masikini, Centre de Recherche en Maladies Tropicales de l'Ituri, Ituri, Congo, Democratic Republic of Congo, E-mails: mmnigo58@yahoo.fr, jeanpierrelotsima@yahoo.fr, and abhafule@gmail.com. Kerstin Fischer, Melanie M. Lloyd, Gary J. Weil, and Peter U. Fischer, Infectious Diseases Division, Department of Internal Medicine, Washington University School of Medicine, St. Louis, MO, E-mails: Kefische@dom.wustl.edu, Mlloyd@dom.wustl.edu, Gweil@dom.wustl.edu, and Pufische@dom.wustl.edu.
References
- 1.WHO . The Global Programme to Eliminate Lymphatic Filariasis: Progress Report 20002009 and Strategic Plan 2010–2020. Geneva: World Health Organization; 2011. pp. 1–78. [Google Scholar]
- 2.Simonsen PE, Fischer PU, Hoerauf A, Weil GJ. The filariases. In: Farrar J, Hotez PJ, Junghanss T, Kang G, Lalloo D, White NJ, editors. Manson's Tropical Diseases. Philadelphia, PA: Elsevier Saunders; 2014. pp. 737–765. [Google Scholar]
- 3.Price DL, Mann GV, Roels OA, Merrill JM. Parasitism in Congo pygmies. Am J Trop Med Hyg. 1963;12:383–387. doi: 10.4269/ajtmh.1963.12.383. [DOI] [PubMed] [Google Scholar]
- 4.Tekle AH, Zoure H, Wanji S, Leak S, Noma M, Remme JH, Amazigo U. Integrated rapid mapping of onchocerciasis and loiasis in the Democratic Republic of Congo: impact on control strategies. Acta Trop. 2011;120((Suppl 1)):S81–S90. doi: 10.1016/j.actatropica.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Gass K, Beau de Rochars MV, Boakye D, Bradley M, Fischer PU, Gyapong J, Itoh M, Ituaso-Conway N, Joseph H, Kyelem D, Laney SJ, Legrand AM, Liyanage TS, Melrose W, Mohammed K, Pilotte N, Ottesen EA, Plichart C, Ramaiah K, Rao RU, Talbot J, Weil GJ, Williams SA, Won KY, Lammie P. A multicenter evaluation of diagnostic tools to define endpoints for programs to eliminate bancroftian filariasis. PLoS Negl Trop Dis. 2012;6:e1479. doi: 10.1371/journal.pntd.0001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao RU, Atkinson LJ, Ramzy RM, Helmy H, Farid HA, Bockarie MJ, Susapu M, Laney SJ, Williams SA, Weil GJ. A real-time PCR-based assay for detection of Wuchereria bancrofti DNA in blood and mosquitoes. Am J Trop Med Hyg. 2006;74:826–832. [PMC free article] [PubMed] [Google Scholar]
- 7.Fink DL, Kamgno J, Nutman TB. Rapid molecular assays for specific detection and quantitation of Loa microfilaremia. PLoS Negl Trop Dis. 2011;5:e1299. doi: 10.1371/journal.pntd.0001299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Core Team R. R: A Language and Environment for Statistical Computing. Vienna. Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 9.Venables WN, Ripley BD. Modern Applied Statistics with S. New York, NY: Springer; 2002. [Google Scholar]
- 10.Becker RA, Wilks AR, Brownrigg R, Minka TP. maps: Draw Geographical Maps. 2013. cran.r-project.org/web/packages/maps/maps.pdf Available at.
- 11.Becker RA, Allen RW, Brownrigg R. mapdata: Extra Map Databased. 2013. cran.r-project.org/web/packages/mapdata/mapdata/pdf Available at.
- 12.Simonsen PE, Magesa SM. Observations on false positive reactions in the rapid NOW filariasis card test. Trop Med Int Health. 2004;9:1200–1202. doi: 10.1111/j.1365-3156.2004.01326.x. [DOI] [PubMed] [Google Scholar]
- 13.Zoure HG, Wanji S, Noma M, Amazigo UV, Diggle PJ, Tekle AH, Remme JH. The geographic distribution of Loa in Africa: results of large-scale implementation of the rapid assessment procedure for loiasis (RAPLOA) PLoS Negl Trop Dis. 2011;5:e1210. doi: 10.1371/journal.pntd.0001210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wanji S, Akotshi DO, Mutro MN, Tepage F, Ukety TO, Diggle PJ, Remme JH. Validation of the rapid assessment procedure for loiasis (RAPLOA) in the Democratic Republic of Congo. Parasit Vectors. 2012;5:25. doi: 10.1186/1756-3305-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weil GJ, Curtis KC, Fakoli L, Fischer K, Gankpala L, Lammie PJ, Majewski AC, Pelletreau S, Won KY, Bolay FK, Fischer PU. Laboratory and field evaluation of a new rapid test for detecting Wuchereria bancrofti antigen in human blood. Am J Trop Med Hyg. 2013;89:11–15. doi: 10.4269/ajtmh.13-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weil GJ, Jain DC, Santhanam S, Malhotra A, Kumar H, Sethumadhavan KV, Liftis F, Ghosh TK. A monoclonal antibody-based enzyme immunoassay for detecting parasite antigenemia in bancroftian filariasis. J Infect Dis. 1987;156:350–355. doi: 10.1093/infdis/156.2.350. [DOI] [PubMed] [Google Scholar]
- 17.Weil GJ, Lammie PJ, Weiss N. The ICT Filariasis Test: a rapid-format antigen test for diagnosis of bancroftian filariasis. Parasitol Today. 1997;13:401–404. doi: 10.1016/s0169-4758(97)01130-7. [DOI] [PubMed] [Google Scholar]
- 18.Richards FO, Eigege A, Miri ES, Kal A, Umaru J, Pam D, Rakers LJ, Sambo Y, Danboyi J, Ibrahim B, Adelamo SE, Ogah G, Goshit D, Oyenekan OK, Mathieu E, Withers PC, Saka YA, Jiya J, Hopkins DR. Epidemiological and entomological evaluations after six years or more of mass drug administration for lymphatic filariasis elimination in Nigeria. PLoS Negl Trop Dis. 2011;5:e1346. doi: 10.1371/journal.pntd.0001346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiferaw W, Kebede T, Graves PM, Golasa L, Gebre T, Mosher AW, Tadesse A, Sime H, Lambiyo T, Panicker KN, Richards FO, Hailu A. Lymphatic filariasis in western Ethiopia with special emphasis on prevalence of Wuchereria bancrofti antigenaemia in and around onchocerciasis endemic areas. Trans R Soc Trop Med Hyg. 2012;106:117–127. doi: 10.1016/j.trstmh.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Weil GJ, Chandrashekar R, Liftis F, McVay CS, Bosshardt SC, Klei TR. Circulating parasite antigen in Brugia pahangi-infected jirds. J Parasitol. 1990;76:78–84. [PubMed] [Google Scholar]
- 21.Weil GJ, Malane MS, Powers KG, Blair LS. Monoclonal antibodies to parasite antigens found in the serum of Dirofilaria immitis-infected dogs. J Immunol. 1985;134:1185–1191. [PubMed] [Google Scholar]
- 22.Gardon J, Gardon-Wendel N, Demanga N, Kamgno J, Chippaux JP, Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa infection. Lancet. 1997;350:18–22. doi: 10.1016/S0140-6736(96)11094-1. [DOI] [PubMed] [Google Scholar]
- 23.Steel C, Golden A, Kubofcik J, LaRue N, de Los Santos T, Domingo GJ, Nutman TB. Rapid Wuchereria bancrofti-specific antigen Wb123-based IgG4 immunoassays as tools for surveillance following mass drug administration programs on lymphatic filariasis. Clin Vaccine Immunol. 2013;20:1155–1161. doi: 10.1128/CVI.00252-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Supali T, Ismid IS, Wibowo H, Djuardi Y, Majawati E, Ginanjar P, Fischer P. Estimation of the prevalence of lymphatic filariasis by a pool screen PCR assay using blood spots collected on filter paper. Trans R Soc Trop Med Hyg. 2006;100:753–759. doi: 10.1016/j.trstmh.2005.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.