Abstract
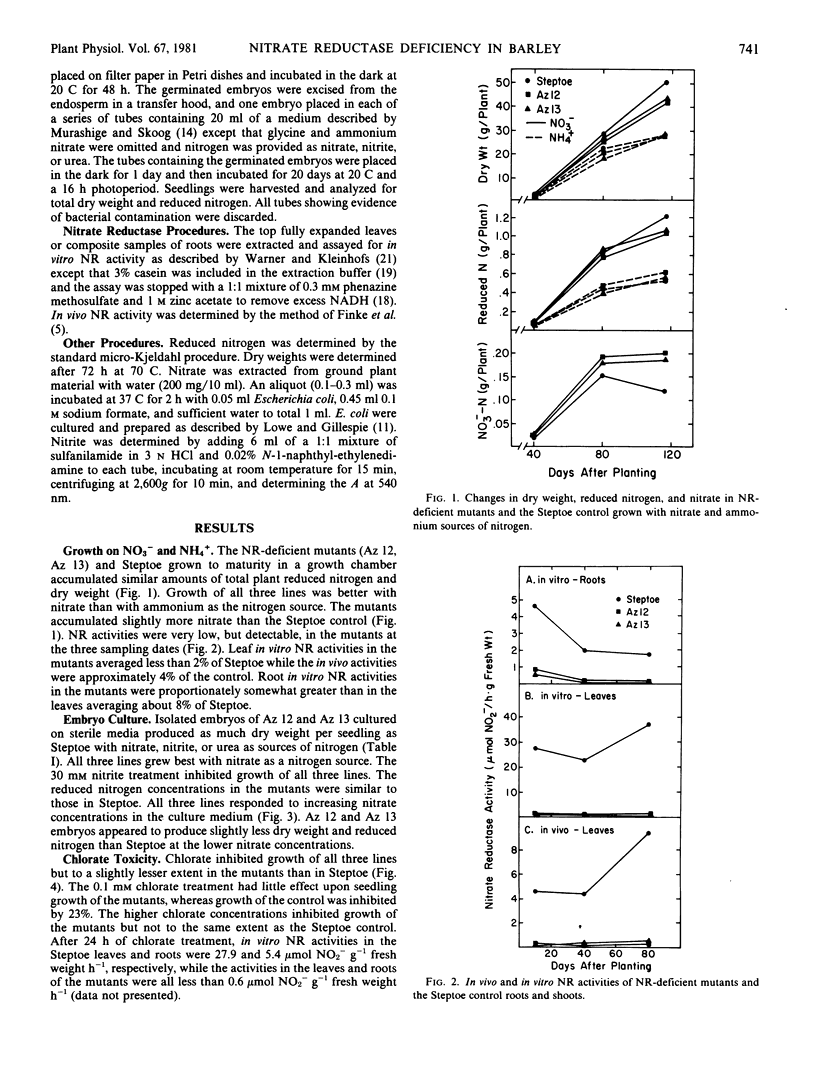
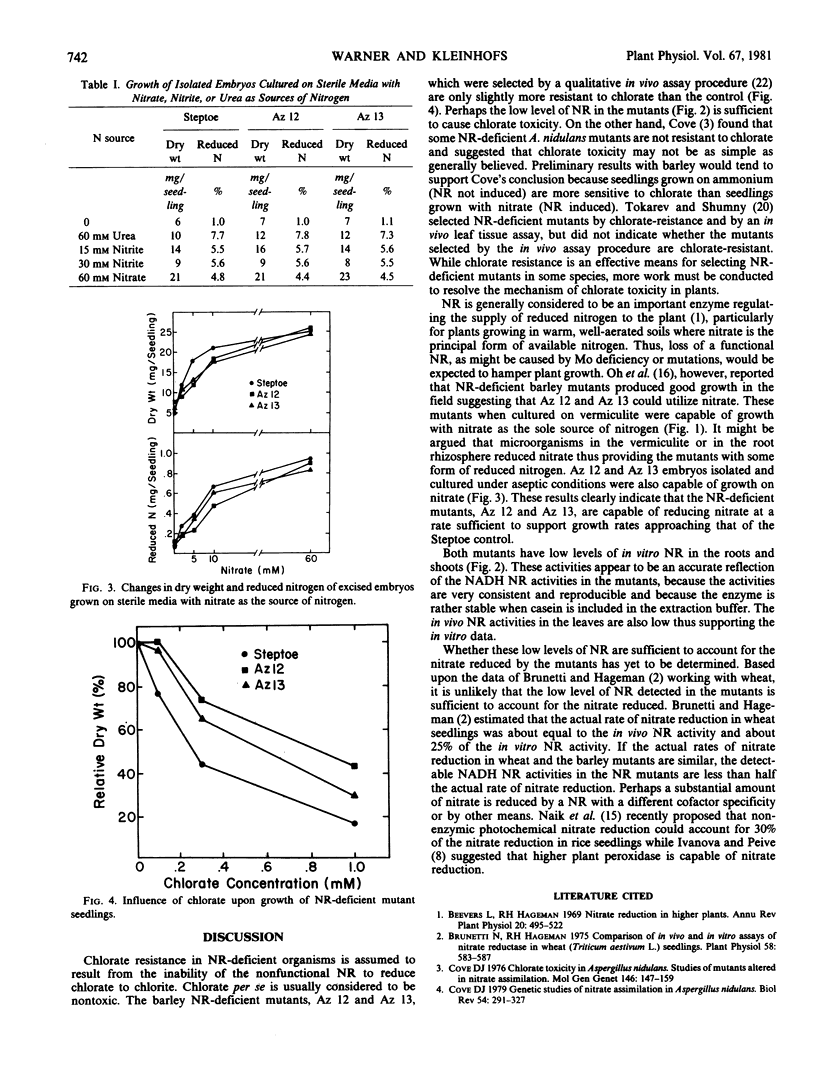
Two nitrate reductase-deficient barley mutants were studied for growth on nitrate and ammonium sources of nitrogen and for resistance to chlorate. Although nitrate reductase-deficient mutants in some species are chlorate-resistant (unable to reduce chlorate to chlorite), the barley mutants used in these studies when grown on nitrate and treated with chlorate were only slightly more resistant to chlorate than the control. When grown to maturity on vermiculite supplemented with either nitrate or ammonium nutrient solutions, the mutants produced as much dry weight and reduced nitrogen per plant as the control. The in vivo and in vitro nitrate reductase activities in the roots and shoots of the mutants grown on nitrate were consistently less than 10% of the control. To avoid the possibility that the mutants received reduced nitrogen from microbial sources, excised embryos were cultured under sterile conditions. Again the mutants were capable of growth and reduced nitrogen accumulation with nitrate as the sole source of nitrogen. In spite of the low apparent nitrate reductase activity, the nitrate reductase-deficient mutants are capable of substantial nitrate reduction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunetti N., Hageman R. H. Comparison of in Vivo and in Vitro Assays of Nitrate Reductase in Wheat (Triticum aestivum L.) Seedlings. Plant Physiol. 1976 Oct;58(4):583–587. doi: 10.1104/pp.58.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cove D. J. Chlorate toxicity in Aspergillus nidulans. Studies of mutants altered in nitrate assimilation. Mol Gen Genet. 1976 Jul 23;146(2):147–159. doi: 10.1007/BF00268083. [DOI] [PubMed] [Google Scholar]
- Cove D. J. Genetic studies of nitrate assimilation in Aspergillus nidulans. Biol Rev Camb Philos Soc. 1979 Aug;54(3):291–327. doi: 10.1111/j.1469-185x.1979.tb01014.x. [DOI] [PubMed] [Google Scholar]
- Garrett R. H., Amy N. K. Nitrate assimilation in fungi. Adv Microb Physiol. 1978;18:1–65. doi: 10.1016/s0065-2911(08)60414-2. [DOI] [PubMed] [Google Scholar]
- Ivanova N. N., Peive Y. V. Nitrate reduction by higher plant peroxidase. FEBS Lett. 1973 Apr 15;31(2):229–232. doi: 10.1016/0014-5793(73)80110-3. [DOI] [PubMed] [Google Scholar]
- Lowe R. H., Gillespie M. C. An Escherichia coli strain for use in nitrate analysis. J Agric Food Chem. 1975 Jul-Aug;23(4):783–785. doi: 10.1021/jf60200a018. [DOI] [PubMed] [Google Scholar]
- Scholl R. L., Harper J. E., Hageman R. H. Improvements of the nitrite color development in assays of nitrate reductase by phenazine methosulfate and zinc acetate. Plant Physiol. 1974 Jun;53(6):825–828. doi: 10.1104/pp.53.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader L. E., Cataldo D. A., Peterson D. M. Use of protein in extraction and stabilization of nitrate reductase. Plant Physiol. 1974 May;53(5):688–690. doi: 10.1104/pp.53.5.688. [DOI] [PMC free article] [PubMed] [Google Scholar]


