Abstract
This article presents two case reports of Anaplasma platys detection in two women from Venezuela. Both patients were exposed to Rhipicephalus sanguineus, the presumed tick vector, and experienced chronic, nonspecific clinical signs including headaches and muscle pains. Intra-platelet inclusion bodies resembling A. platys were observed in buffy coat smears and A. platys DNA was amplified and sequenced from whole blood; however, treatment with doxycycline did not alleviate their symptoms. These cases provide further support for A. platys as a zoonotic tick-borne pathogen, most likely of low pathogenicity; nonetheless, the cause of illness in humans by A. platys is yet to be confirmed.
Introduction
Microorganisms invading platelets have been observed in people from Maracaibo, Venezuela since 1992, associated with clinical symptoms such as fever, chills, headache, muscular pain, arthralgia, weakness, insomnia, skin lesions, and other symptoms similar to those found in human Ehrlichosis and Anaplasmosis.1,2 Between 1993 and 2012, a total of 5,954 people had intra-platelet inclusions in buffy coat smear (BCS) evaluated at the Unidad de Investigaciones Clínicas-Facultad de Ciencias Veterinarias-La Universidad del Zulia (UIC- FCV-LUZ). Most had moderate to severe clinical symptoms, some were hospitalized, and all required treatment to recover health. Some patients responded well to tetracyclines, especially to doxycycline. When platelet-rich plasma from BCS-positive cases was prepared for ultrastructural examination by transmission electron microscopy, and organisms compared with ultrastructural studies described in the United States3 and in Venezuela,4 it was concluded that organisms infecting dogs and people appeared different. In canine organisms, a well-defined double membrane, characteristic of the Anaplasmataceae family, was evident and the intra-vacuolar space was clear, whereas in organisms from human cases, organism membranes were thickened and the intra-vacuolar space appeared electron-dense.5 To date, the etiology of these intra-platelet organisms has not been identified.
Case 1.
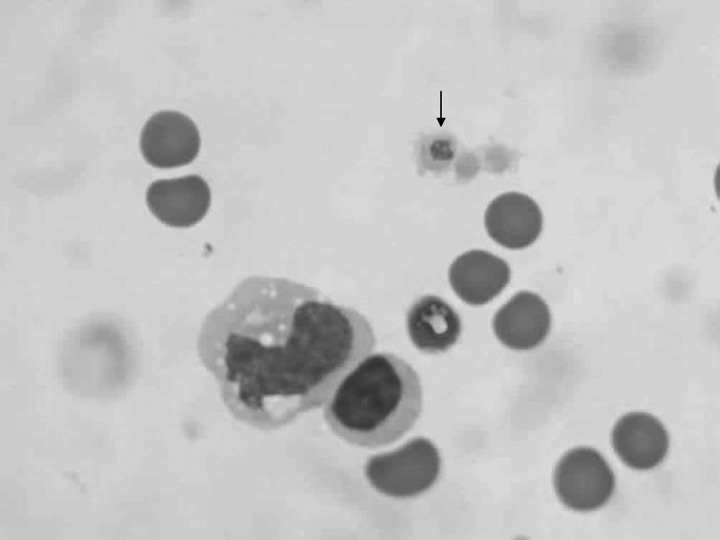
A 29-year-old woman from Maracaibo, Zulia State, Venezuela resided in a household consisting of three other people, three dogs, and two cats. In February 2012, the woman removed a tick from her chest and leg. During the second week of March she developed decreased appetite, generalized weakness, muscular pain, fatigue, and occasional headaches, all of which persisted during the next 2 months. On March 30, 2012, an EDTA-anti-coagulated blood specimen was sent to the UIC-FCV-LUZ. A BCS was prepared, stained with a Wright's Giemsa (Dip Quick) stain and examined by light microscopy at high magnification (100×). Intra-platelet inclusions, morphologically consistent with Anaplasma platys, were visualized. Complete blood count (CBC) values, obtained the same day, were within reference ranges, red blood cell (RBC) 4.48 × 1012/L (3.5–5.0 × 1012/L), Hct 44% (36–48%), Hgb 14.4g/dL (11–15g/dL), white blood cell (WBC) 6.7 × 109/L (4.3–10.8 × 109/L), with segmented neutrophils 4.69×109/L (3.0–5.0 × 109/L), lymphocytes 1.80 × 109/L (1.0–3.0 × 109/L), and eosinophils 0.223 × 109/L (0.02–0.35 × 109/L); platelet count was 311 × 109/L (150–400 × 109/L). A blood sample was obtained for DNA extraction, after which DNA was stored at −20°C for future polymerase chain reaction (PCR) testing to detect potential intracellular rickettsial pathogens. A second blood sample, obtained in April 2012, for BCS, also contained intra-platelet inclusions (Figure 1). Subsequently, a physician prescribed Acetaminophen, 500 mg every 8 hours until the symptoms disappeared, and Vitamin C, 500 mg daily for 7 days. Muscle pain and headaches resolved; however, occasionally during 2012 the woman experienced flu-like symptoms including nasal discharge, weakness, and headaches. The PCR testing providing molecular evidence supporting A. platys infection in the March 30 blood sample was not completed until August 2013. After this result became available, repeat blood smear examination again identified intra-platelet inclusions similar to A. platys, after which her physician prescribed doxycycline for treatment of the A. platys infection.
Figure 1.
Intra-platelet inclusions in a buffy coat smear (BCS) from case 1, infected with Anaplasma platys. 1,000×.
Concurrently, during February 2012 when the woman experienced tick attachments, a heavy infestation of Rhipicephalus sanguineus (R. sanguineus) ticks was found on the three adult dogs living in the patient's house. Petechial lesions were visible on the abdomen, thorax, and legs of two of the three dogs. The CBCs from the two dogs with clinical signs documented thrombocytopenia, 20.0 × 109/L and 43.0 × 109/L, respectively (reference range 175–500 × 109/L). No organisms were visualized on blood smears. Using a commercially available ELISA kit, SNAP4DX, (IDEXX Laboratories Inc., Westbrook, ME) that employs synthetic peptides of Ehrlichia spp., Anaplasma spp., Borrelia burgdorferi (Lyme Disease), and Dirofilaria immitis (Heartworm Disease) antigen, both thrombocytopenic dogs were only Anaplasma spp. seroreactive, most likely indicative of prior or concurrent infection with A. platys, an Anaplasma sp. known to be endemic to Venezuela. Both dogs were promptly treated with an acaricide to kill the ticks and doxycycline, 10 mg/kg, every 12 hours, was administered for 21 consecutive days with resultant clinical and hematological recovery, as indicated by normalization of platelet counts.
Case 2.
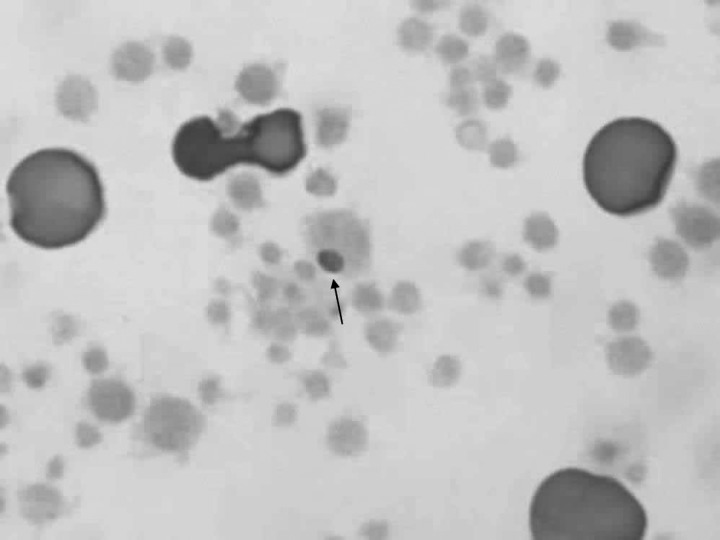
On June 5, 2012, a blood sample from a 50-year-old woman from Cabimas, Venezuela, with a history of leukopenia and neutropenia of unknown origin since 2001 and documented Epstein Barr virus, cytomegalovirus, and Dengue virus IgG positive serologies (as of October 2010) was sent to the UIC-FCV-LUZ for BCS examination. Intra-platelet inclusions were observed in the smear (Figure 2). Three weeks earlier, a CBC, had identified mild anemia (Hgb 10.7g/dL: Hct 33%), leukopenia (WBC 3.6 × 109/L), and neutropenia (segmented neutrophils 1.692 × 109/L) with lymphocytes (1.836 × 109/L), eosinophils (0.072 × 109/L), and platelets (311 × 109/L) within reference ranges. The DNA was extracted from a blood sample collected June 6, 2012, and stored at −20°C for future PCR analysis. Historically, the woman reported muscular pain, arthralgia, headaches, chills, and insomnia. In addition, she had recently obtained a dog that experienced recurrent R. sanguineus tick infestations; however, the woman was never knowingly bitten by a tick. Her doctor had requested the BCS examination to rule out the presence of morulae, associated with tick-borne diseases, such as anaplasmosis and ehrlichiosis. Rickettsia rickettsii, Rickettsia typhi, and Rickettsia conorii antibodies were negative. Treatment was not prescribed at the time intra-platelet organisms were visualized. During 2012–2013, case 2 continued to experience occasional headaches, muscular pain, and arthralgia. After A. platys infection was confirmed by PCR amplification and DNA sequencing in May 2013, a repeat blood sample was not provided; however, her physician prescribed doxycycline (4.4 mg/kg/day, divided into 2 doses) for 21 days. The previously documented leukopenia persisted unchanged.
Figure 2.
Intra-platelet inclusions in a buffy coat smear (BCS) from case 2, infected with Anaplasma platys. 1,000×.
Materials and Methods
BCSs.
Blood samples were obtained in tubes containing EDTA as an anticoagulant. The technique of quantitative BCA,6 modified at the UIC-FCV-LUZ laboratory, was used to concentrate platelets and leukocytes to more easily visualize hemotrophic agents in cells. Two microhematocrit capillary tubes were filled with blood and centrifuged for 5 minutes in a microcentrifuge (Clay Adams, Becton Dickinson, Franklin Lakes, NJ) to separate the blood cells from plasma, and then the WBC layer and a small portion of plasma was used to prepare the BCS. The BCS was stained with Dip Quick (Jorgensen Laboratories, Inc., Loveland, CO) and viewed on a microscope (Zeiss, Oberkochen, Germany) at high magnification (100×) to look for basophilic bodies consistent with A. platys inside platelets. Using this procedure, between 50 and 250 platelets per field were visualized.
DNA extraction.
Fresh blood samples were collected for Anaplasma and Ehrlichia sp. PCR. The DNA was obtained from 2.5 mL of whole blood from each using a BDtract Genomic DNA Isolation Kit (Maxim Biotech, Inc., San Francisco, CA), according to manufacturer's recommendations. Extracted DNA samples were stored at −20°C until use. The DNA integrity was verified in 1% agarose gels.
PCR.
DNA from both cases was tested by PCR assays that amplify Anaplasma and Ehrlichia spp. DNA of the following genes: Ehrlichia/Anaplasma 16S ribosomal RNA (rRNA), A. platys p44, E. canis p30 (msp4), and A. phagocytophilum p44. All PCRs were performed in an Eppendorf Mastercycler EPgradient (Eppendorf, Hauppauga, NY) with an aluminum block and included positive (plasmid or culture genomic DNA) and negative (RNAse-free, molecular grade water and uninfected canine genomic DNA) controls for each assay. The PCR product visualization was performed using the horizontal electrophoresis system Wide Mini-Sub Cell GT (Bio-Rad, Hercules, CA) and MultiImage Light Cabinet with camera and filters (Alpha Innotech Corporation, San Leandro, CA) and AlphaImager 3300 visualization computer software for Windows 2000/XP (ProteinSimple, Santa Clara, CA). Sequences were obtained by direct submission to GENEWIZ, Inc. (Research Triangle Park, NC). Alignments were compared with GenBank sequences using AlignX software (Vector NTI Advance Version 11.5, Invitrogen, Inc., Grand Island, NY).
Ehrlichia and Anaplasma 16S rRNA PCR.
Oligonucleotides and PCR conditions designed to amplify a 420 base pair (bp) fragment of the 16S rRNA gene have been described previously.7
Anaplasma platys p44 PCR.
The PCR screening for the A. platys p44 gene was performed using Apl_p44F3 (5′- GCT AAG TGG AGC GGT GGC GAT GAC AG) and Apl_p44R3 (5′- CGATCTCCGCCGC TTTCGTATTCTTC) as forward and reverse primers, respectively. Amplification, which yields a 520 bp amplicon, was performed in a 25 μL final volume reaction containing 12.5 μL of MyTaq HS Mix (2×) (Bioline cat: BIO-25046), 0.3 μL of 50 uM of each primer (Sigma-Aldrich, St. Louis, MO), 7 μL of filter-sterilized, molecular-grade water, and 5 μL of extracted DNA template. The PCR was performed using a single hot start cycle at 94°C for 3 minutes, followed by 55 cycles of denaturation at 94°C for 15 seconds (s), annealing at 70°C for 10 s, and extension at 72°C for 30 s, followed by a single cycle at 72°C for 1 minute.
Ehrlichia canis p30 (msp4) PCR.
The PCR screening for the E. canis p30 gene was performed using Ec_msp4_F2 (5′-GAATCATGGACT GGTGGTATCCTTG) and Ec_msp4_R (5′-GAAATGTTAG CTTCTGGGCTTATTGGA) as forward and reverse primers, respectively. Amplification, which yields a 470 bp amplicon, was performed in a 25 μL final volume reaction containing 12.5 μL of MyTaq HS Mix (2×) (Bioline cat: BIO-25046), 0.25 μL of 50 uM of each primer (Sigma-Aldrich), 7 μL of filter-sterilized, molecular-grade water and 5 μL of extracted DNA template. The PCR was performed using a single hot start cycle at 94°C for 3 minutes, followed by 55 cycles of denaturation at 94°C for 15 s, annealing at 62°C for 15 s, and extension at 72°C for 15 s, followed by a single cycle at 72°C for 1 minute.
Anaplasma phagocytophilum p44 PCR.
The PCR screening for the A. phagocytophilum p44 gene was performed using Aph_p44_F (5′-CTGGGAA TGATGTCAGGGCTCA) and Aph_p44_R (5′-CCCAATCCGAGGA TCAGGTGTG) as forward and reverse primers, respectively. Amplification, which yields a 230 bp amplicon, was performed in a 25 μL final volume reaction containing 12.5 μL of MyTaq HS Mix (2×) (Bioline cat: BIO-25046), 0.25 μL of 50 uM of each primer (Sigma-Aldrich), 7 μL of filter-sterilized, molecular-grade water, and 5 μL of extracted DNA template. The PCR was performed using a single hot start cycle at 94°C for 2 minutes, followed by 55 cycles of denaturation at 94°C for 15 s, annealing at 64°C for 15 s, and extension at 72°C for 15 s, followed by a single cycle at 72°C for 30 s.
Results
Previously extracted DNA from the two individuals' blood samples were sent to North Carolina State University, College of Veterinary Medicine, Intracellular Pathogens Research Laboratory (NCSC-CVM-IPRL). Anaplasma platys-like inclusion bodies had been previously visualized by a veterinary clinical pathologist working in Venezuela, who had experience with morulae identification in animal blood smears. Anaplasma platys DNA was PCR amplified from the two cases described previously. The PCR negative controls remained negative for all reactions. A 420 bp portion of the A. platys 16S rRNA gene was amplified from case 1 and sequence comparisons performed using the basic local alignment search tool (BLAST) against the GenBank database over a length of 350 nucleotides showed the highest sequence similarity (100% identical with 100% coverage) to A. platys 16S rRNA gene (AF286699). A 520 bp portion of the A. platys p44 gene was amplified from case 2 and sequence comparisons over a length of 450 bp had the highest sequence similarity (99% identical with 100% coverage) to A. platys p44 (GQ868750.1). The PCRs designed to amplify E. canis and A. phagocytophilum were negative.
Discussion
Molecular diagnostic evidence supporting A. platys infection was recently reported by Maggi and others8 in a woman from Grenada, who had experienced migraines and progressively severe seizures, including status epilepticus. As the woman was co-infected with A. platys, Bartonella henselae, and Candidatus Mycoplasma haematoparvum, the extent to which each organism contributed to the patient's symptoms could not be determined; however, after a 6-month course of doxycycline, A. platys, and Candidatus Mycoplasma haematoparvum DNA were no longer PCR amplified from the patient's blood, whereas B. henselae DNA persisted, despite the long antibiotic course. In the current study, we report two additional cases of A. platys infection that were initially identified by visualizing intra-platelet inclusion bodies. Subsequently, stored, extracted DNA was used for PCR amplification and DNA sequencing to confirm A. platys infections, however, each with a different gene target. One possible explanation for this is the large amount of human genomic DNA (200–1,200 ng/μL) in the extractions, which can interfere with amplification of small amounts of pathogen DNA. Anaplasma platys p44 was amplified from the undiluted sample in case 2, which contained the least amount of human genomic DNA (200 ng/μL); this may have contributed to the success of the A. platys p44 PCR reaction for case 2. The A. platys p44 partial gene could not be amplified from case 1, despite diluting the highly concentrated DNA. Potentially, pathogen DNA was diluted beyond the detection of that PCR assay. Following dilutions, 16S rDNA PCR was performed on all samples. In addition to amplifying A. platys from case 1, a faint amplicon of the appropriate size was generated from case 2; however, a 16S rDNA sequence could not be obtained from case 2. Therefore, it is likely gene targets, 16S rDNA, and A. platys p44, were amplified from case 2, although sequence confirmation was obtained for only one amplicon. Further supporting an A. platys infection, both women had a history of peri-domestic tick exposure, which in nearly all instances is a result of R. sanguineus, the likely vector for A. platys in Venezuela,9 and throughout the world.10–14
Anaplasma platys,(formerly Ehrlichia platys) was described in 1978 by Harvey and others15 in Florida, as basophilic inclusion bodies observed in platelets from a thrombocytopenic dog. In 1992, A. platys inclusions in dogs from Maracaibo, Venezuela were reported for the first time by Arraga.16 Subsequent studies, using techniques such as BCS analysis, serological assays (IFAT and ELISA), transmission electron microscopy, and PCR have shown A. platys is a prevalent tick-borne infection in dogs living in Venezuela.4,17–21 In dogs, A. platys induces a disease called infectious canine cyclic thrombocytopenia (ICCT)15,22; however, most infected dogs are not clinically ill.16,23 Based upon PCR testing in another study, 14 dogs from Grenada, located 600 miles north of Venezuela, were infected with A. platys.24 Other tick-transmitted alpha proteobacteria that are most often found in animals produce mild to severe disease in people, depending upon the individual's immune status. For example, Ehrlichia chaffeensis and E. ewingii, transmitted by Amblyomma americanum ticks in North America, are more likely to cause severe and potentially life-threatening disease in immune compromised patients.25 Similarly, E. muris-like infection was recently recognized for the first time as a human pathogen in immune compromised patients, primarily transplantation recipients.26 Infection with E. canis, a cause of canine monocytic ehrlichiosis, has also been implicated as a cause of mild disease in people from Venezuela.27 Ehrlichia canis DNA was not amplified from either patient in this report.
For decades A. platys was thought to only infect dogs; however, in 2005 A. platys-like inclusion bodies were detected in 7% of the platelets in a thrombocytopenic cat from Brazil.28 Subsequently, in 2010, molecular studies in Brazil documented A. platys infection in another thrombocytopenic cat that also had platelet inclusion bodies on a stained blood smear.29 In 2008, A. platys infection was confirmed in a goat from Cyprus by PCR followed by DNA sequence analysis.30 It was not until 2013, that the first DNA sequence-confirmed human case of A. platys infection was reported.8
Anaplasma platys infections have been reported in dogs throughout the world, including the Americas (United States, Venezuela, Grenada, Chile, Argentina, Costa Rica), Europe (France, Spain, Germany, Italy, Greece, Croatia), Africa and the Middle-East (Israel), Asia (Taiwan, Japan, China, Thailand, Turkey, Malaysia), Australia, and Oceania,24,31–38 therefore exposure to this rickettsial organism is widely distributed.
The 16S rDNA sequences for two different A. platys strains from Venezuela have been characterized9: A. platys Venezuela (GenBank accession no.: AF287153), obtained from a dog in Maracaibo21 and A. platys Lara (GenBank accession no.: AF399917), from Lara state.9 Both of these strains are 99% identical to the 16S rDNA sequence obtained from case 1. Strain variation in pathogenicity has been proposed because of reports of more severe disease manifestations, attributed to A. platys infections in dogs in Europe.23,31 However, as co-infections with multiple tick-borne pathogens are being increasingly recognized in dogs throughout the world, it is possible that the more severe disease manifestations in European dogs were caused by infection with another undocumented pathogen.13,39,40 In particular, the role of Bartonella spp. as co-infecting hemotrophic pathogens has only been recently described in dogs from Europe.
Anaplasma platys has not been confirmed as a cause of illness in humans; however, this study provides additional molecular evidence supporting A. platys infection in people, and physicians should recognize it as a potentially new zoonotic pathogen transmitted by ticks that typically infest dogs. Prospective studies are warranted to define the molecular prevalence of A. platys and to define whether other pathogenic organisms contribute to the platelet-inclusions described in human patients in Venezuela.
ACKNOWLEDGMENTS
We thank Tonya Lee for editorial assistance.
Disclaimer: The authors have no conflicts of interest related to this work.
Footnotes
Note: During the submission, review and publication of this manuscript, the NCSU-CVM-IPRL, in collaboration with the University of Texas Medical Branch Galveston, published another manuscript describing infection with Anaplasma platys, Ehrlichia chaffeensis and Ehrlichia ewingii in a dog, mother and daughter residing in North America. The reference citation for that manuscript is as follows: Breitschwerdt EB, Hegarty BC, BA, Qurollo BA, Saito TB, Maggi RG, Blanton LS, Bouyer BH. Intravascular persistence of Anaplasma platys, Ehrlichia chaffeensis, and Ehrlichia ewingii DNA in the blood of a dog and two family members. Parasites and Vectors 2014;7:298–305.
Financial support: Revenues of the VBDDL diagnostic service along with donations from industry and private sponsors are used for research projects otherwise unfunded that are pursued for their intellectual or societal benefits. Barbara Qurollo is a research postdoctoral fellow at North Carolina State University funded by IDEXX Laboratories Inc., Westbrook, ME.
Authors' addresses: Cruz M. Arraga-Alvarado, Omaira C. Parra, and Maribel A. Berrueta, Unidad de Investigaciones Clínicas, Facultad de Ciencias Veterinarias, Universidad del Zulia, Maracaibo, Venezuela, E-mails: cruzmariaarragadealvarado@gmail.com, opamaldo@gmail.com, and maribelberruetaf@gmail.com. Barbara A. Qurollo, Barbara C. Hegarty, and Edward B. Breitschwerdt, College of Veterinary Medicine, North Carolina State University, Raleigh, NC, E-mails: baquroll@ncsu.edu, barbara_hegarty@ncsu.edu, and ed_breitschwerdt@ncsu.edu.
Reprint requests: Edward B. Breitschwerdt, College of Veterinary Medicine, North Carolina State University, 1060 William Moore Dr., Raleigh, NC 27607.
References
- 1.Olano JP, Hogrefe W, Seaton B, Walker DH. Clinical manifestation, epidemiology, and laboratory diagnosis of human monocytotropic ehrlichiosis in a commercial laboratory setting. Clin Diagn Lab Immunol. 2003;10:891–896. doi: 10.1128/CDLI.10.5.891-896.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas RJ, Dumler JS, Carlyon JA. Current management of human granulocytic anaplasmosis, human monocytic ehrlichiosis and Ehrlichia ewingii ehrlichiosis. Expert Rev Anti Infect Ther. 2009;7:709–722. doi: 10.1586/eri.09.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Popov VL, Han VC, Chen S-M, Dumler JS, Feng H-M, Andreadisj TG, Tesh RB, Walker DH. Ultrastructural differentiation of the genogroups in the genus Ehrlichia. J Med Microbiol. 1998;47:235–251. doi: 10.1099/00222615-47-3-235. [DOI] [PubMed] [Google Scholar]
- 4.Arraga-Alvarado C, Parra O, Palmar M, Chango RE, Alvarado MC. Ehrlichia platys: preparación de antígenos y uso de la técnica de IFI en caninos y humanos. Rev Cientif FCV–LUZ VII. 1997:99–109. [Google Scholar]
- 5.Arraga-Alvarado C, Palmar M, Parra O, Salas P. Fine structural characterization of Rickettsia-like organism in human platelets from patients with symptoms of Ehrlichiosis. J Med Microbiol. 1999;48:991–997. doi: 10.1099/00222615-48-11-991. [DOI] [PubMed] [Google Scholar]
- 6.Wardlaw SC, Levine RA. Quantitative buffy coat analysis. A new laboratory tool functioning as a screening complete blood cell count. JAMA. 1983;249:617–620. doi: 10.1001/jama.249.5.617. [DOI] [PubMed] [Google Scholar]
- 7.Eddlestone SM, Dinz PP, Neer TM, Gaunt SD, Corstvet R, Cho D, Hosgood G, Hegarty B, Breitschwerdt EB. Doxycycline clearance of experimentally induced chronic Ehrlichia canis infection in dogs. J Vet Intern Med. 2007;21:1237–1242. doi: 10.1892/07-061.1. [DOI] [PubMed] [Google Scholar]
- 8.Maggi RG, Mascarelli PE, Havenga LN, Naidoo V, Breitschwerdt EB. Co-infection with Anaplasma platys, Bartonella henselae and Candidatus Mycoplasma haematoparvum in a veterinarian. Parasit Vectors. 2013;6:103–113. doi: 10.1186/1756-3305-6-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang H, Unver A, Pérez MJ, Orellana NG, Rikihisa Y. Prevalence and molecular analysis of Anaplasma platys in dogs in Lara, Venezuela. Braz J Microbiol. 2005;36:211–216. [Google Scholar]
- 10.Inokuma H, Raoult D, Brouqui P. Detection of Ehrlichia platys in brown dog ticks (Rhipicephalus sanguineus) in Okinawa Island, Japan. J Clin Microbiol. 2000;38:4219–4221. doi: 10.1128/jcm.38.11.4219-4221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inokuma H, Beppu T, Okuda M, Shimada Y, Sakata Y. Epidemiological survey of Anaplasma platys and Ehrlichia canis using ticks collected from dog in Japan. Vet Parasitol. 2003;115:343–348. doi: 10.1016/s0304-4017(03)00238-3. [DOI] [PubMed] [Google Scholar]
- 12.Sanogo YO, Davoust B, Inokuma H, Camicas JL, Parola P, Brouqui P. First evidence of Anaplasma platys in Rhipicephalus sanguineus (Acari: Ixodida) collected from dogs in Africa. Onderstepoort J Vet Res. 2003;70:205–212. [PubMed] [Google Scholar]
- 13.de Caprariis D, Dantas-Torres F, Capelli G, Mencke N, Stanneck D, Breitschwerdt EB, Otranto D. Evolution of clinical, hematological and biochemical findings in young dogs naturally infected by vector-borne pathogens. Vet Microbiol. 2011;149:206–212. doi: 10.1016/j.vetmic.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Oscherov EB, Milano AMF, Lobo B, Anda P, Escudero R. Detection of Anaplasma platys and other pathogens in ectoparasites from urban hosts in northeast Argentine. Rev Ibero-Latinoam Parasitol. 2011;70:42–48. [Google Scholar]
- 15.Harvey JW, Simpson CF, Gaskin JM. Cyclic thrombocytopenia induced by a Rickettsia-like agent in dogs. J Infect Dis. 1978;137:182–188. doi: 10.1093/infdis/137.2.182. [DOI] [PubMed] [Google Scholar]
- 16.Arraga-Alvarado C. Ehrlichiosis canina en Maracaibo, Estado Zulia-Venezuela: reporte de 55 casos. Rev Cientif FCV-LUZ. 1992;II:30–40. [Google Scholar]
- 17.Camacaro I, Pérez JR, Hernández R. Hepatozoonosis y Ehrlichosis en perro. Informe de un caso. Veterinaria Tropical. 1997;22:77–81. [Google Scholar]
- 18.Arraga-Alvarado C, Palmar M, Parra O, Salas P. Ehrlichia platys (Anaplasma platys) in Dogs from Maracaibo, Venezuela: an ultrastructural study of experimental and natural infection. Vet Pathol. 2003;40:149–156. doi: 10.1354/vp.40-2-149. [DOI] [PubMed] [Google Scholar]
- 19.Quijada J, García M, Bethencourt A, Medina O, Vivas I, Pérez A, García H. Rickettsias y parásitos Hemotrópicos en pacientes caninos de clínicas veterinarias de cuatro estados de Venezuela. REDVET. Rev. Electron Vet. 2012;13:7–13. [Google Scholar]
- 20.Almao M, García M, Mujica R. Ehrlichia canis en el Caserío” La Isla”, municipio Palavecino, estado Lara. Revista del Colegio de Médicos Veterinarios del Estado Lara. 2013;5:33–37. [Google Scholar]
- 21.Suksawat J, Pitulle C, Arraga-Alvarado C, Madrigal K, Hancock SI, Breitschwerdt EB. Co-infection with three Ehrlichia species in dogs from Thailand and Venezuela with emphasis on consideration of 16S ribosomal DNA secondary structure. J Clin Microbiol. 2001;39:90–93. doi: 10.1128/JCM.39.1.90-93.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.French TW, Harvey JW. Serologic diagnosis of infectious cyclic thrombocytopenia in dogs using an indirect fluorescent antibody test. Am J Vet Res. 1983;44:2407–2411. [PubMed] [Google Scholar]
- 23.Harrus S, Aroch I, Lavy E, Bark H. Clinical manifestations of infectious canine cyclic thrombocytopenia. Vet Rec. 1997;141:247–250. doi: 10.1136/vr.141.10.247. [DOI] [PubMed] [Google Scholar]
- 24.Yabsley MJ, McKibben J, Macpherson CN, Cattan PF, Cherry NA, Hegarty BC, Breitschwerdt EB, O'Connor T, Chandrashekar R, Paterson T, Perea ML, Ball G, Friesen S, Goedde J, Henderson B, Sylvester W. Prevalence of Ehrlichia canis, Anaplasma platys, Babesia canis vogeli, Hepatozoon canis, Bartonella vinsonii berkhoffii, and Rickettsia spp. in dogs from Grenada. Vet Parasitol. 2008;151:279–285. doi: 10.1016/j.vetpar.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Buller RS, Arens M, Hmiel SP, Paddock CD, Sumner JW, Rikhisa Y, Unver A, Gaudreault-Keener M, Manian FA, Liddell AM, Schmulewitz N, Storch GA. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999;341:148–155. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- 26.Pritt BS, Sloan LM, Johnson DK, Munderloh UG, Paskewitz SM, McElroy KM, McFadden JD, Binnicker MJ, Neitzel DF, Liu G, Nicholson WL, Nelson CM, Franson JJ, Martin SA, Cunningham SA, Steward CR, Bogumill K, Bjorgaard ME, Davis JP, McQuiston JH, Warshauer DM, Wilhelm MP, Patel R, Trivedi VA, Eremeeva ME. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N Engl J Med. 2011;365:422–429. doi: 10.1056/NEJMoa1010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez M, Bodor M, Zhang C, Xiong Q, Rikihisa Y. Human infection with Ehrlichia canis accompanied by clinical sings in Venezuela. Ann N Y Acad Sci. 2006;1078:110–117. doi: 10.1196/annals.1374.016. [DOI] [PubMed] [Google Scholar]
- 28.Santarém VA, Laposy CB, Farias MR. Anaplasma platys (Ehrlichia platys)-like inclusion bodies in platelets of a cat. Colloquium Agrariae. 2005;1:60–66. [Google Scholar]
- 29.Lima ML, Soares PT, Ramos CA, Araújo FR, Ramos RA, Souza II, Faustino MA, Alves LC. Molecular detection of Anaplasma platys in a naturally-infected cat in Brazil. Braz J Microbiol. 2010;41:381–385. doi: 10.1590/S1517-838220100002000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chochlakis D, Ioannou I, Sharif L, Kokkini S, Hristophi N, Dimitriou T, Tselentis Y, Psaroulaki A. Prevalence of Anaplasma sp. in goats and sheep in Cyprus. Vector Borne Zoonotic Dis. 2008;9:457–463. doi: 10.1089/vbz.2008.0019. [DOI] [PubMed] [Google Scholar]
- 31.Beaufils JP, Inokuma H, Martin-Granel J, Jumelle PH, Barbault-Jumelle M, Brouqui P. Anaplasma platys (Ehrlichia platys) infection in a dog in France: description of the case, and characterization of the agent. Revue Méd Vét. 2002;153:85–90. [Google Scholar]
- 32.de la Fuente J, Torina A, Naranjo V, Nicosia S, Alongi A, La Mantia F, Kocan KM. Molecular characterization of Anaplasma platys strains from dogs in Sicily, Italy. BMC Vet Res. 2006;2:24. doi: 10.1186/1746-6148-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abarca K, López J, Perret C, Guerrero J, Godoy P, Veloz A, Valiente-Echeverría F, León U, Gutjahr C, Azócar T. Anaplasma platys in dogs, Chile. Emerg Infect Dis. 2007;13:1392–1395. doi: 10.3201/eid1309.070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uluta B, Bayramli G, Karagenc T. First case of Anaplasma (Ehrlichia) platys infection in a dog in Turkey. Turk J Vet Anim Sci. 2007;31:279–282. [Google Scholar]
- 35.Dyachenko V, Pantchev N, Balzer HJ, Meyersen A, Straubinger RK. First case of Anaplasma platys infection in a dog from Croatia. Parasit Vectors. 2012;5:49. doi: 10.1186/1756-3305-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mokhtar AS, Lim SF, Tay ST. Molecular detection of Anaplasma platys and Babesia gibsoni in dogs in Malaysia. Trop Biomed. 2013;30:345–348. [PubMed] [Google Scholar]
- 37.Eiras DF, Craviotto MB, Vezzani D, Eyal O, Baneth G. First description of natural Ehrlichia canis and Anaplasma platys infections in dogs from Argentina. Comp Immunol Microbiol Infect Dis. 2013;36:169–173. doi: 10.1016/j.cimid.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Ábrego L, Dolz G, Romero JJ, Vargas B, Meneses A. Revista de Ciencias Veterinarias; 2013. Molecular detection of Anaplasma platys in Costa Rican dogs.http://hdl.handle.net/11056/8208 Available at. Accessed September 27, 2013. [Google Scholar]
- 39.Gaunt S, Beall M, Stillman B, Lorentzen L, Diniz P, Chandrashekar R, Breitschwerdt E. Experimental infection and co-infection of dogs with Anaplasma platys and Ehrlichia canis: hematologic, serologic and molecular findings. Parasit Vectors. 2010;3:33–44. doi: 10.1186/1756-3305-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nascimento Ramos RA, Giannelli A, Lia RP, Brianti E, Tarallo VD, Breitshwerdt EB, Dantas-Torres F, Stanneck D, Otranto D. Incidence of Cercopithifilaria bainae in dogs and probability of co-infection with other tick-borne pathogens. PLoS ONE. 2014;9:1–3. doi: 10.1371/journal.pone.0088198. [DOI] [PMC free article] [PubMed] [Google Scholar]