Abstract
Dengue is the most important mosquito-borne viral disease to humans. Bats are potential reservoirs for flaviviruses, including dengue virus (DENV). In this work, Artibeus jamaicensis bats were inoculated with two serotypes of DENV using different routes. For experimental inoculations (EI) 1 and 2, bats were inoculated subcutaneously or intraperitoneally with DENV-4; for EI-3 bats were inoculated intraperitoneally with DENV-1. Mock inoculated bats were kept as controls. In EI-4, bats were bitten by Aedes aegypti mosquitoes infected with DENV-1 or 4. Reverse transcription-polymerase chain reaction assays in plasma and spleen tissue collected from Day 1 to Days 9–17 after inoculation failed to reveal the presence of viral RNA in any of the samples. No evidence of circulating NS1 or specific anti-DENV IgG was detected in the plasma of the inoculated bats. These results indicate that A. jamaicensis bats are incapable of sustaining dengue virus replication and are unlikely to act as reservoirs for this virus.
Introduction
Dengue virus (DENV) is classified within the family Flaviviridae, in the genus Flavivirus; a genus that groups several other important human pathogens associated with encephalitis and hemorrhagic fevers. Four antigenically different but genetically related serotypes of DENV (DENV-1 to DENV-4) are known. The DENV mature virion is composed of a small icosahedral capsid, of about 45 nm in diameter, made of a single protein (C), surrounded by a lipid envelope containing the E and M proteins. The virus presents a single-stranded RNA genome, of positive polarity, of ∼10 kb. In addition to the three structural proteins, the genome encodes for seven non-structural proteins named NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5, which all participate in virus replication.1–3 In addition, NS1 is secreted to the extracellular milieu and circulates in patients' sera early during infection along with infectious virus.4 Thus, NS1 detection is the basis of several commercial diagnostic assays.5
Dengue currently is considered the most important mosquito-borne viral disease for humans. The disease is endemic in more than 100 countries where an estimated 100 million cases are reported annually, associated with more than 25,000 fatalities, mainly in children < 5 years of age.6 Mexico is a country endemic for dengue, where all 4 DENV serotypes are known to circulate. Infection with any of the 4 DENV serotypes usually results in subclinical or mild dengue; however, a small fraction of the patients can evolve to severe dengue, the life threatening form of the disease. Mild dengue is characterized by high fever, headache, muscle and joint pain, and rash, whereas patients with severe dengue (also known as dengue hemorrhagic fever) present hemorrhages, plasma leakage, and respiratory distress.2,3 Despite the great disease burden associated with dengue, currently there are neither vaccines nor specific treatment of this disease.7 In addition, no widely available animal model for dengue virus infection is available.8
The DENV is maintained in nature in two ecologically and evolutionary distinct transmission cycles: the human and the sylvatic cycle.9 The mosquito Aedes aegypti is the main vector for the human cycle, which involves only humans as reservoirs or amplification hosts. The sylvatic cycle involves non-human primates as reservoirs and amplification hosts and is maintained by mosquitoes in the genus Aedes. The DENV sylvatic cycles have been well documented only in West Africa and in Southeast Asia.10 However, there is scattered evidence suggesting that a sylvatic cycle for DENV among several species of small mammals, may also exist in the Americas. A serological survey conducted by de Thoisy and others,11 comprising 27 wild forest mammal species collected in the French Guiana, found sporadic evidence of DENV infection in several of the species sampled. Serologic evidence for DENV infection in several species of chiroptera collected in Ecuador, Costa Rica, and Mexico has also been reported.12–14 In addition, reverse transcription-polymerase chain reaction (RT-PCR) testing of spleen or heart tissue samples from bats captured in dengue-endemic areas in Mexico, detected the presence of DENV-2 in frugivorous (Artibeus jamaicensis, Artibeus lituratus, Glossophaga soricina) and insectivorous (Myotis nigricans) bats.13,15 In an elegant study carried out in the French Guiana, RNA corresponding to the 4 DENV serotypes was detected in tissue samples collected from several species of wild mammals. Interestingly, evidence of DENV infection was found in mammals, including several species of Chiroptera, captured in remote sylvatic areas, where no evidence of dengue cycle exists.11 All these results suggest that wild animals, including several species of bats, may carry and presumably sustain DENV infection in nature.
Bats belong to the order Chiroptera, one of the most abundant, species diverse and widely disseminated mammal orders.16 Over 350 bats species are found in the Americas and the Caribbean.17,18 In recent years, bats are increasingly being recognized as reservoirs for a variety of important human viruses19,20 including flaviviruses,11 and have become an important study subject from a public health point of view. Because mounting evidence indicates that bats may be a reservoir for DENV, the main objective of this study was to directly test the susceptibility of the very wide spread species of bat A. jamaicensis to DENV infection. Experimental inoculations with DENV-1 and DENV-4, using several inoculation routes suggest that A. jamaicensis bats are incapable of sustaining DENV replication.
Materials and Methods
Bats capture.
Artibeus jamaicensis bats were captured at Morelos State (located in Central Mexico). Bats were trapped in mist nets opened for 4 hours after sunset. Bat species were identified according to the guide of Medellin and others.21 Lactating and pregnant females were released immediately without any further manipulation. Captured bats were placed in cloth bags for transportation to the enclosure at the animal facility of the National Public Health Institute (INSP, Cuernavaca, Mexico). No bat died or suffered injury as a result of capture or handling procedures. The collection of specimens was performed according to the guidelines of the American Society for the Use of Mammalogists of Wild Mammals in Research and under a collecting permit issued by the General Direction of Wildlife of Mexico (permission number SGPA/DGVS/00471/11).
Bats enclosure and handling.
Enclosures were constructed using PVC pipes of 1.5 inch in diameter and PVC connectors to build a structure of 1 cubic foot, covered with shade netting tied to the base frame tubes. The enclosure had two apertures on the top side for introduce nourishment. After placement in the cages, bats were left in quarantine for 1 week and fed fresh fruits daily ad libitum.
Virus propagation and titration.
The DENV serotype 1, strain Hawaii (a kind gift of Dr. Juan Salas, Instituto Politecnico Nacional, Mexico), and DENV serotype 4, strain H241 were both propagated in C6/36 HT as previously described.22 Briefly, monolayers grown in 75 mm2 flasks were inoculated with DENV at multiplicity of infection of 1 for 1 h. After inoculation, cells were maintained in minimum essential medium (MEM) (Gibco, Grand Island, NY) supplemented with 7% fetal calf serum (FCS) and maintained at 34°C for 1 week. Cells were freeze-thawed twice, the media clarified by low speed centrifugation and stored in aliquots (0.6 mL) at −70°C until used. The DENV titers were determined by plaque assay inoculating 10-fold dilutions on confluent monolayers of BHK-21 cells grown in 24-well plates, following protocol described by Ludert and others.23
Bat experimental inoculations.
A total of 52 A. jamaicensis bats were used in four experimental inoculations as follows. For experimental inoculation (EI) 1, 10 bats were inoculated subcutaneously in the chest with 200 μL of DENV-4 inoculum. For EI-2, 8 bats were inoculated intraperitoneally, also with 200 μL of DENV-4. For EI-3, 10 bats were inoculated intraperitoneally with 200 μL of DENV-1. The inoculum titers were 2.4 × 104 plaque-forming units (PFU)/mL and 8.4 × 104 PFU/mL, for DENV-1 and DENV-4, respectively. The viability of the viral inoculum was tested in parallel by inoculation of suckling mice in the brain; inoculated mice were watched for the appearance of neurological signs 5–7 days after inoculation and their brains collected to test for the presence of viral RNA by RT-PCR. For each EI, five bats were mock inoculated under identical conditions with supernatant of uninfected C6/36 cells, and kept as controls. Mock inoculated bats were maintained in separate enclosures. Finally, for EI-4, mosquitoes from group “a” and “b”, taken at Day 9 after the infection with DENV24 were deprived of sucrose for 4 hours and placed in a small container with a mosquito net. Bats (N = 5) were settled inside the container but outside of the mosquito net and five mosquitoes allowed to feed on each bat until the mosquito engorged (∼20 min).25 Following exposure to the mosquito bites, bats were returned to the enclosures until sampled or euthanized. Calendars and experimental program of each EI are shown in Table 1.
Table 1.
Experimental inoculations schedule
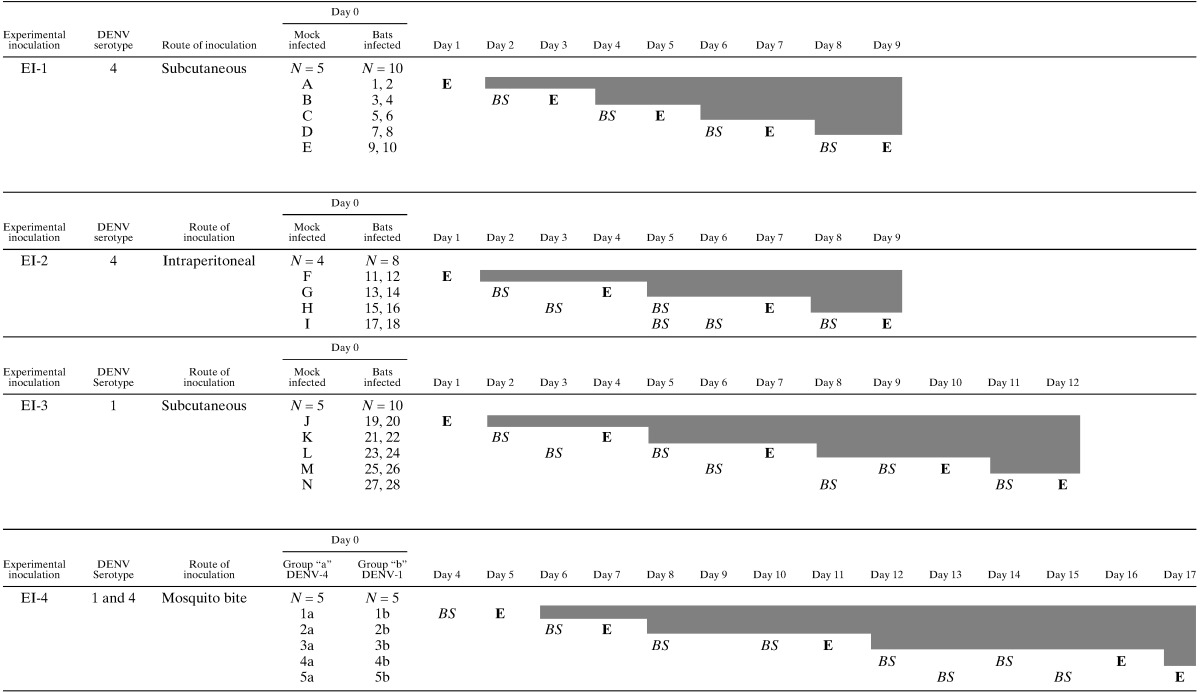
Procedure: E. Euthanized: BS Blood sampled.
Experiment 4: Group a: Bats bitten for mosquitoes infected with DENV-4.
Group b: Bats bitten for mosquitoes infected with DENV-1.
Collection of spleen tissue and plasma.
Before their enrollment in any of the experimental inoculations, all bats were bled by puncture of the cephalic vein using a needle of 21G Becton Dickinson (BD), 3 or 4 days after capture and just the day before the experimental inoculation. Blood (∼80 μL) was collected with heparinized capillary tubes and stored at −70°C until RNA extraction to test for the presence of genomic DENV RNA by RT-PCR.
Following the experimental program (Table 1), bats were anesthetized with Pentobarbital (0.2 mL/mL total volume) and euthanized for exsanguination by intracardiac puncture using a sterile syringe with 100 μL of heparin per 1 mL of blood. Blood was centrifuged at 1,300 × g for 5 minutes to obtain the plasma. After obtaining blood samples, all animals were necropsied and the spleen collected. Organs and plasma were frozen at −70°C and kept until RNA extraction to test for the presence of genomic DENV RNA by RT-PCR.
An aliquot of ∼0.5 mL of whole blood from each of the euthanized animals from EI-3 was immediately put in mosquito feeders, and used to feed a pool of 10 mosquitoes. Mosquitoes were maintained under controlled conditions, and 7 days after the blood meal, each pool was macerated in phosphate buffered saline, the mixture clarified by low speed centrifugation and the clarified supernatant tested for the presence of soluble NS1 by a commercial enzyme-linked immunosorbent assay (ELISA).26
Mosquito strains.
Aedes aegypti mosquitoes (Rockefeller strain), were maintained under controlled conditions at 26 to 28°C and 70–80% relative humidity with 12-h/12-h light-dark cycle at the insectary of the National Public Health Institute (INSP), Cuernavaca, Mexico. Mosquito eggs were hatched and larvae were reared in water containers at a density of 200–300 larvae per liter and daily fed with high quality food as described by Moreno-Garcia and others.27 Pupae were transferred to screened cages and emergent adults were maintained with an ad libitum diet of 10% sucrose solution.
Oral mosquito infections.
Adults mosquitoes (4–6 days old) were deprived of sucrose for 4 hours, and afterward fed with an infections blood meal for 40 minutes through a piece of Parafilm M (Chicago, IL) clamped to mosquito feeders. Infection meals consisted of 500 μL of fresh heparinized rabbit blood mixed with an equal volume of viral inoculum. During the meal, the feeding mixture was maintained at 37°C by circulating warm water though the feeders. Group “a” mosquitoes were fed with DENV-4 (4.2 × 104 PFU/mL) and group “b” mosquitoes with DENV-1 (1.2 × 104 PFU/mL). Doses in this range have proven to be infectious for A. aegypti females28. Three days after the blood meal and up to day 12, five female mosquitoes from each group were dissected daily and the thorax and abdomen each pooled and macerated in 100 μL of TRIzol Reagent (Invitrogen, Carlsbad, CA) following the manufacturer's protocol. Extracted RNA was immediately tested for the presence of DENV RNA by RT-PCR. In agreement with previous results Salazar and others,24 the maximum infections rates of the mosquitoes were observed at Day 5 in the abdomen and at Day 8 in the thorax.
Detections of dengue virus genome (RT-PCR).
Total RNA was extracted from all plasma or organs with TRIzol Reagen (Invitrogen) following the manufacturer's protocol. The RNA was reverse transcribed into cDNA for 50 min at 42°C using hexamer random primers (New England, Biolabs, Ipswich, MA) and Invitrogen SuperScript II Reverse Transcriptase (Invitrogen) according to the manufacturer's recommendations. Two microliters of cDNA were used in a PCR carried out using the Taq DNA polymerase (Kapa Biosystems, Wilmington, MA) and primers D1 (forward) and D2 (reverse) as reported in Lanciotti and others.29 Water instead of nucleic acids and nucleic acids extracted from mice brain inoculated with DENV-1 or 4 were used in the RT-PCR reactions as negative and positive controls, respectively. As an additional positive control, the cDNA generated with the random primers was amplified in parallel by PCR using primers specific for the housekeeping gene GAPDH (forward 5′-GCAGGGGGGAGCCAAAAGGG-3′; reverse 5′-TGCCAGCCCCAGCGTCAAAG 3′). Both amplifications were carried out as follows: initial incubation at 94°C for 10 min, then 30 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension a 72°C for 30 s, followed by a final incubation at 72°C for 10 min. The PCR products were analyzed by 1.5% agarose gel electrophoresis and visualized by staining with ethidium bromide.
Enzyme immune assay (ELISA).
All plasmas collected from the DENV inoculated bats (N = 38) were tested in duplicate for the presence of DENV NS1 protein, using the commercial assay Platelia Dengue NS1 Ag (Bio-Rad, Hercules, CA). This diagnostic test is a one-step sandwich format microplate enzyme immunoassay designed for the qualitative or semi-quantitative detection of DENV NS1 antigen in human sera or plasma.5 The assay was carried and the cut-off values were established following the manufacturer's protocol.
Plasma samples from the DENV (N = 22) and mock (N = 5) inoculated bats were evaluated for the presence of specific immunoglobulin G (IgG) using the reagents and materials provided in the Panbio Diagnostics Dengue IgG capture ELISA. This commercial assay is intended as an aid in the diagnosis of DENV infection and is based on the capture of human IgG by anti-human IgG antibodies coated onto the plate. Because bat IgG was not captured in the plate (data not shown), a competition ELISA was set-up. Briefly, bat plasma (1:25 final dilution) was reacted with the DENV 1-4 antigen provided with the kit for 1 h at 37°C before the Mab tracer (anti-E Mab labeled with horse radish peroxidase) provided with the assay was added to the mixture and further incubated for 1 h at 20–25°C. In parallel, the assay plate was reacted with the human serum positive for DENV IgG provided with the assay as a positive (reactive) control for 1 h at 37°C. The antigen mixture was added to the plate, previously washed 6 times, and the plate covered and incubated for 1 h at 37°C. Finally, the plate was washed again (6×) and 100 μL of the tetramethylbenzidine (TMB) chromogen reagent added to each well. After 10 min. incubation at 20–25°C, the colorimetric reaction was stopped by adding 100 μL of acid stop solution. Absorbance of each well was read immediately at a wavelength of 450 nm. As competition controls, one human serum, tested negative for anti-dengue IgG by the Panbio Dengue capture ELISA and by plaque reduction neutralization titer (PRNT) (PRNT50 < 20) and one human serum positive for DENV IgG by the Panbio Dengue ELISA and by PRNT (PRNT50 = 320) were used. As an additional control, a mouse monoclonal anti-E antibody (4G2) (PRNT50 =1,250) was also used. The calibrator, reactive and negative controls were also run in parallel in a standard assay as quality controls. Any reading below the mean optical density (O.D.) plus three standard deviations, obtained with the negative human serum was taken as indicative of the presence of anti-DENV IgG in the bat's plasma.
Results
No clinical signs were observed in any of the bats experimentally inoculated with DENV virus by any of the inoculation routes used. In addition, no evident changes in the behavior or in the feeding pattern of the bats were observed. Upon necropsies, no skin damages at the inoculation site, hemorrhages, or apparent pathological signs were observed.
The RT-PCR analysis of RNA extracted from plasma and spleen of any of the bats experimentally inoculated by any of the routes or directly exposed to mosquito bites, failed to show evidence of viremia or the presence of DENV nucleic acid in the tested organs (Table 2). The spleen sample from one bat from EI-1 killed on Day 7 post-inoculation showed a faint amplicon of ∼500 base pairs after the PCR assay. Similar amplicons were also observed for the spleens of bats killed on Days 4, 7, and 9 from EI-2 and Days 10 and 12 from EI-3. However, these results could not be reproduced after using either the original cDNA or new tissue samples. Moreover, direct sequencing of the amplicons confirmed their spurious nature (data not shown). No positive RT-PCR results were obtained from bats of EI-4. In contrast, the amplification of the GAPDH mRNA carried out in parallel as a control of nucleic acid extraction for each sample clearly showed the 600 base pairs of the expected amplicon. These results indicate that no viral replication took place in the DENV inoculated animals. In view of these results and the limited amount of plasma available, assessing viremia by plaque assay was not attempted.
Table 2.
Results of experimental inoculation in Artibeus jamaicensis bats
| Days post inoculum | EI-1 | E1-2 | ||||
|---|---|---|---|---|---|---|
| Bats positive to the following tests | Bats positive to the following tests | |||||
| Platelia NS1 | RT-PCR Lanciotti | RT-PCT GAPDH | Platelia NS1 | RT-PCR Lanciotti | RT-PCT GAPDH | |
| Day 1 | – | – | 1, 2 | – | – | 11, 12 |
| Day 2 | – | – | No samples | – | – | No samples |
| Day 3 | – | – | 3, 4 | – | – | No samples |
| Day 4 | – | – | No samples | – | 13 (suspected) | 13, 14 |
| Day 5 | – | – | 5, 6 | – | – | No samples |
| Day 6 | – | – | No samples | – | – | No samples |
| Day 7 | 8 | 8 (suspected) | 7, 8 | 15 | 16 (suspected) | 15, 16 |
| Day 8 | – | – | No samples | – | – | No samples |
| Day 9 | – | – | 9, 10 | 18 (suspected) | 17, 18 | |
| Days post inoculum | E1-3 | E1-4 | ||||
| Bats positive to the following tests | Bats positive to the following tests | |||||
| Platelia NS1 | RT-PCR Lanciotti | RT-PCT GAPDH | Platelia NS1 | RT-PCR Lanciotti | RT-PCT GAPDH | |
| Day 1 | 19, 20 | – | 19, 20 | No samples collected | ||
| Day 2 | – | – | No samples | |||
| Day 3 | – | – | No samples | |||
| Day 4 | 21, 22 | – | 21, 22 | |||
| Day 5 | – | – | No samples | – | – | 1a, 1b |
| Day 6 | – | – | No samples | – | – | No samples |
| Day 7 | – | – | 23, 24 | – | 2a (suspected) | 2a, 2b |
| Day 8 | – | – | No samples | – | – | No samples |
| Day 9 | – | – | No samples | – | – | No samples |
| Day 10 | – | 26 (suspected) | 25, 26 | – | – | No samples |
| Day 11 | – | – | No samples | – | – | 3a, 3b |
| Day 12 | – | 28 (suspected) | 27, 28 | – | – | No samples |
| Day 13 | – | – | No samples | |||
| Day 14 | – | – | No samples | |||
| Day 15 | – | – | No samples | |||
| Day 16 | 4b | – | 4a, 4b | |||
| Day 17 | 5a | 5a (suspected) | 5a, 5b | |||
Results obtained with Mock inoculated bats are not present for simplicity. The reverse-transcription-polymerase chain reaction (RT-PCR) and NS1 assays carried out the samples collected from these animals all resulted as negatives.
The presence of circulating NS1 was evaluated in the plasma collected from the DENV inoculated animals by ELISA (Table 2). All plasma were tested for the presence of NS1 regardless of the RT-PCR results, because they expect kinetics in bats of NS1 antigemia with respect to viremia could not be anticipated. Plasma collected from mock infected bats (N = 14) were also analyzed as controls. Readings slightly above the positive cut-off value of the assay were observed for one plasma collected from EI-1 and one from EI-2 on Day 7 post infection (p.i.) and also for plasmas collected each on p.i. Days 16 and 17 of EI-4, but these results were not reproducible. In addition, strong O.D. readings were observed for plasma collected from the two animals killed on Days 1 (bats 19 and 20) and 4 (bats 21 and 22) p.i. of EI-3. However, levels of circulating NS1 were undetectable in plasma collected at later times (p.i Days 7, 10, and 12) from the same EI-3. These results suggest that not NS1 sustained antigenemia appeared after the experimental inoculation of the bats.
One of the 10 pools of mosquitoes fed with the fresh blood collected from EI-3 inoculated bats, tested positive for the presence of NS1. This pool corresponded to a bat euthanized on Day 1 after inoculation (bat 19). Mosquitoes fed with blood from mock infected bats or with blood collected from inoculated bats afterward were negative for the presence of NS1 (data not shown).
None of the bat plasma samples tested caused a reduction in the O.D. readings in the competition ELISA set to detect the presence of DENV specific IgG (mean O.D. reading 1.168 ± 0.066 versus 1.061 ± 0.049 for the negative human serum). In contrast, over 98% reduction in the O.D. reading was observed with the human serum (0.022 ± 0.010) and anti-E Mab (0.009 ± 0.004) included as positive controls. These results suggest that no serological immune response was induced in the DENV inoculated bats and no anti-DENV IgG was present in the plasma of the mock inoculated animals.
Discussion
Bats are a natural host for a large number of viruses, some of which are important human pathogens. Recent metagenomic analyses have identified viruses belonging to the Retroviridae, Herpesviridae, Poxviridae, Flaviviridae, Reoviridae, Picobirnaviridae families, among others, as part of the bat viromes.30–34 Moreover, the existence of several yet unknown mammalian viruses in bats is indicated by these studies. Dengue is the most important viral disease transmitted by mosquitoes to humans. The implementation of control measures for this disease is of public health interest and a clear understanding of the life cycle of the DENV in nature, including identification of possible natural hosts is a necessity. The aim of our study was to test the susceptibility of the species of bat A. jamaicensis to DENV infection. We decided to use this species as an experimental model because it is an abundant species of fruit-eating habits and very tolerant to habitat disturbance,18 which allows A. jamaicensis to cohabit with humans in rural and semi-rural areas where DENV is endemic. Moreover, evidence for DENV infection in wild Artibeus sp. bats has been reported.11,13,15
For EI-1, DENV-4, and the subcutaneous route were used in an attempt to generate an initial localized infection of the skin resident cells of the immune system.2 Skin resident dendritic cells and macrophages are supposed to be first cells encountered by the DENV after a natural infection by a mosquito bite.35,36 The subcutaneous route has been used successfully for the humanized mouse models of DENV infection36 and also has been used in the experimental inoculation of bats with West Nile virus (WNV) and Japanese encephalitis virus (JEV).37,38 In view of the results obtained after EI-1 and in an attempt to augment tissue virus adsorption, for EI-2 the same DENV-4 strain was used as inoculum but inoculated using the intraperitoneal route. This route has been used in experimental protocols to inoculate mice with DENV39 and bats with DENV and other flaviviruses.40,41 However, no evidence of viremia, antigenemia or serological immune response was found after EI-1 or EI-2. Analysis of spleen tissue, an organ found to be infected in humans42 and bats15 also failed to show evidence of DENV replication.
In a study conducted by Barr and others43 cell lines of different species, including the TB Lu cell line I, which is a pulmonary epithelium cell line derived from the Tadaria brasilensis bat, were infected in vitro with the 4 DENV serotypes. Evidence of infection in the bat cell line was found by qRT-PCR 72 h p.i., only with the DENV-1 inoculum. Furthermore, DENV-1 sequences have been isolated from wild bats.11 Therefore, a DENV serotype 1 strain was used as inoculum for EI-3. In addition, because no data about the possible kinetics a DENV infection in bats are available, the follow-up time after inoculum was extended from 9 to 12 days in EI-3. Although no evidence of viremia or viral replication in the spleen was found in the inoculated bats, the plasma collected on Days 1 and 4 after inoculation showed positive values for NS1 (antigemia). Dengue NS1 protein levels are known to rapidly rise in sera during acute phase and to decrease about 7 days after fever onset.44,45 However, antigenemia levels usually parallel viremia levels in patients. Thus, in the absence of any evidence for viremia, these results are difficult to interpret. In addition, because the DENV-1 inoculum titer was three times higher than the DENV-4 titer and the Platelia Dengue NS1 Ag assay is much more sensitive detecting DENV-1 NS1 than DENV-4 NS1,46 the possibility that the NS1 detected in the plasma of bat from EI-3 correspond to the inoculum is highly plausible. Of note, mosquitoes fed from the blood of the bats that tested positive for NS1 also tested positive for this antigen.
The importance of the mosquito saliva to enhance dengue infection of the vertebrate host is well established.25,47–49 Therefore for EI-4, infections of the bats by mosquito bite was attempted. Both DENV serotypes 1 and 4 were used and the bats followed up for 17 days. Aedes aegypti mosquitoes of the Rockefeller strain, known to be highly susceptible to DENV infection, were used. The kinetics of mosquito infection, followed by RT-PCR, was in agreement with previous reports24 and no differences in kinetics were observed between DENV-1 and 4. The number of infected mosquitoes per bat (N = 5) was as reported by Cox and others25 to infect humanized mice and mosquitoes were retired only after observed to have had a blood meal. Despite all these precautions and controls, no evidence of viremia or organ viral replication was observed in any of the EI- 4 bats.
The results from all the EIs taken together strongly suggest that A. jamaicensis bats are incapable of sustaining DENV replication. This conclusion is supported by the fact that highly sensitive and specific methods such as RT-PCR for virus genome detection and ELISA detection of circulating NS1 were used to follow-up infection in the bats. In addition, mosquitoes highly susceptible to DENV infections were used to detect or to induce infection in EI-3 and EI-4, respectively. Deficiencies in the quality or the amount of the inoculum to explain these results are unlikely. The viability of the inoculum was corroborated by parallel titrations in neonate mice and the amount of viruses used in each inoculum (103 to 104 PFU/bat) is similar or comparable to the inoculum used in the mouse animal models.8 The parallel detection of mRNA from housekeeping genes (GAPDH) from spleen tissue indicates appropriate nucleic acid extraction and amplification protocols. In addition, no apparent changes in bats feeding habits or behavior were observed during the experiments, suggesting that neither the manipulation procedure nor the captivity affected significantly the animals. Our results are in agreement with previous results obtained by Perea-Martinez and others41 after the intraperitoneal experimental inoculation of 27 Artibeus intermedius bats with DENV serotype 2. Our results are also in agreement with previous reports of experimental inoculation of 2 species of North American bats with WNV or Australian black flying foxes with JEV, two flavivirus closely related to DENV, and one species of Asian bats with yokose virus, a flavivirus distantly related to DENV.37,38,40 In contrast, low to relatively high levels of susceptibility were observed in three species of insectivorous bats experimentally inoculated with several strains of JEV or St. Louis encephalitis virus (SLEV).50
One limitation of this work is that field-collected bats were tested only for active DENV infection and not for anti-DENV antibodies that may have prevented infection, before the EIs. However, if these animals presented past DENV infections, a robust memory IgG response was to be expected after the EIs and this was not observed. The same rationale will hold true if putative cross-reactive flaviviral antibodies caused by past infection with other arthropod-borne flavivirus were to be present.
The results obtained in this and other experimental inoculations of flaviviruses in bats and the several reports of DENV infection in wild bats are not easy to reconcile. However, the current evidence of DENV infection in bats is based on serology or the amplification of DENV-related sequences. Cross-reactivity among the more than 70 members of the Flaviviridae family is well established51 thus adding confusion to the serological data, and the significance of isolated DENV sequences is hard to assess. On the other hand, despite their abundance, many things about the biology and ecology of bats remain unknown, and the possibility that conditions in captivity fail to fully reproduce the conditions in which bats encounter viruses in nature cannot be excluded. Further experiments, including bonafide DENV isolates and robust flavivirus metagenomic analysis from bats, are still required to fully evaluate the role played by bats in DENV ecology.
Footnotes
Financial support: This study was partially supported by CONACYT grant 132811 and DGAPA-PAPIIT grant In202711 to ARM, grant In209314 to VSC and a award from the Fundación Miguel Alemán A,C. to JEL.
Authors' addresses: Salomé Cabrera-Romo, Ana C. Alcalá, and Rosa María del Ángel, CINVESTAV - Infectomics and Molecular Pathogenesis, Mexico City, Mexico, E-mails: gorilagorila@yahoo.com.mx, ana.aca1711@gmail.com, and rmangel@cinvestav.mx. Benito Recio-Tótoro and Humberto Lanz, Instituto Nacional de Salud Pùblica, Center for Research on Infectious Diseases, Cuernavaca, Mexico, E-mails: enitort@gmail.com and humberto@insp.mx. Victor Sánchez-Cordero and Ángel Rodríguez-Moreno, Institute of Biology - UNAM, Mexico City, Mexico, E-mails: victor@ibunam2.ibiologia.unam.mx and tanicandil@hotmail.com.
References
- 1.Clyde K, Kyle JL, Harris E. Recent advances in deciphering viral and host determinants of dengue virus replication and pathogenesis. J Virol. 2006;80:11418–11431. doi: 10.1128/JVI.01257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martina BE, Koraka P, Osterhaus AD. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev. 2009;22:564–581. doi: 10.1128/CMR.00035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simmons CP, Farrar JJ, van Vinh Cahu N, Wills B. Dengue. N Engl J Med. 2012;366:1423–1432. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 4.Muller DA, Young PR. The flavivirus NS1 protein: molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antiviral Res. 2013;98:192–208. doi: 10.1016/j.antiviral.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Peeling RW, Artsob H, Pelegrino JL, Buchy P, Cardosa MJ, Devi S, Enria DA, Farrar J, Gubler DJ, Guzman MG, Halstead SB, Hunsperger E, Kliks S, Margolis HS, Nathanson CM, Nguyen VC, Rizzo N, Vázquez S, Yoksan S. Evaluation of diagnostic tests: dengue. Nat Rev Microbiol. 2010;8:S30–S38. doi: 10.1038/nrmicro2459. [DOI] [PubMed] [Google Scholar]
- 6.Guzmán MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martı'nez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;12((Suppl)):S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitehorn J, Simmons CP. The pathogenesis of dengue. Vaccine. 2011;29:7221–7228. doi: 10.1016/j.vaccine.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Zompi S, Harris E. Animal models of dengue virus infection. Viruses. 2012;4:62–82. doi: 10.3390/v4010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weaver SC, Vasilakis N. Molecular evolution of dengue viruses: contributions of phylogenetics to understanding the history and epidemiology of the preeminent arboviral disease. Infect Genet Evol. 2009;9:523–540. doi: 10.1016/j.meegid.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasilakis N, Cardosa J, Hanley KA, Holmes EC, Weaver SC. Fever from the forest: prospects for the continued emergence of sylvatic dengue virus and its impact on public health. Nat Rev Microbiol. 2011;9:532–541. doi: 10.1038/nrmicro2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Thoisy B, Lacoste V, Germain A, Munoz-Jordan J, Colon C, Mauffrey JF, Delaval M, Catzeflis F, Kazanji M, Matheus S, Dussart P, Morvan J, Aguilar-Setien A, Deparis X, Lavergne A. Dengue infection in neotropical forest mammals. Vector Borne Zoonotic Dis. 2009;9:157–170. doi: 10.1089/vbz.2007.0280. [DOI] [PubMed] [Google Scholar]
- 12.Platt KB, Mangiafico JA, Rocha OJ, Zaldivar ME, Mora J, Trueba G, Rowley WA. Detection of dengue virus neutralizing antibodies in bats from Costa Rica and Ecuador. J Med Entomol. 2000;37:965–967. doi: 10.1603/0022-2585-37.6.965. [DOI] [PubMed] [Google Scholar]
- 13.Aguilar-Setien A, Romero-Almaraz ML, Sanchez-Hernandez C, Figueroa R, Juarez-Palma LP, Garcia-Flores MM, Vazquez-Salinas C, Salas-Rojas M, Hidalgo-Martinez AC, Pierle SA, Garcia-Estrada C, Ramos C. Dengue virus in Mexican bats. Epidemiol Infect. 2008;136:1678–1683. doi: 10.1017/S0950268808000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machain-Williams C, Lopez-Uribe M, Talavera-Aguilar L, Carrillo-Navarrete J, Vera-Escalante L, Puerto-Manzano F, Ulloa A, Farfan-Ale JA, Garcia-Rejon J, Blitvich BJ, Lorono-Pino MA. Serologic evidence of flavivirus infection in bats in the Yucatan Peninsula of Mexico. J Wildl Dis. 2013;49:684–689. doi: 10.7589/2012-12-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sotomayor-Bonilla J, Chaves A, Rico-Chavez O, Rostal MK, Ojeda-Flores R, Salas-Rojas M, Aguilar-Setien A, Ibanez-Bernal S, Barbachano-Guerrero A, Gutierrez-Espeleta G, Aguilar-Faisal JL, Aguirre AA, Daszak P, Suzan G. Dengue virus in bats from southeastern Mexico. Am J Trop Med Hyg. 2014;91:129–131. doi: 10.4269/ajtmh.13-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Z. Bat and virus. Protein Cell. 2010;1:109–114. doi: 10.1007/s13238-010-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altringham JD. Bats: From Evolution to Conservation. Oxford, UK: Oxford University Press; 2011. [Google Scholar]
- 18.Medellín RA, Equihua M, Amin MA. Bat diversity and abundance as indicators of disturbance in neotropical rainforests. Conserv Biol. 2000;14:1666–1675. doi: 10.1111/j.1523-1739.2000.99068.x. [DOI] [PubMed] [Google Scholar]
- 19.Dobson AP. Virology. What links bats to emerging infectious diseases? Science. 2005;310:628–629. doi: 10.1126/science.1120872. [DOI] [PubMed] [Google Scholar]
- 20.Wong S, Lau S, Woo P, Yuen KY. Bats as a continuing source of emerging infections in humans. Rev Med Virol. 2007;17:67–91. doi: 10.1002/rmv.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medellin RA, Arita HT, Sánchez HO. Identificación de los Murciélagos de México: Claves de campo. Second edition. México: Instituto de Ecología, UNAM; 2008. [Google Scholar]
- 22.Mosso C, Galvan-Mendoza IJ, Ludert JE, del Angel RM. Endocytic pathway followed by dengue virus to infect the mosquito cell line C6/36 HT. Virology. 2008;378:193–199. doi: 10.1016/j.virol.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Ludert JE, Mosso C, Ceballos-Olvera I, del Angel RM. Use of a commercial enzyme immunoassay to monitor dengue virus replication in cultured cells. Virol J. 2008;5:51. doi: 10.1186/1743-422X-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salazar MI, Richardson JH, Sanchez-Vargas I, Olson KE, Beaty BJ. Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 2007;7:9. doi: 10.1186/1471-2180-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox J, Mota J, Sukupolvi-Petty S, Diamond MS, Rico-Hesse R. Mosquito bite delivery of dengue virus enhances immunogenicity and pathogenesis in humanized mice. J Virol. 2012;86:7637–7649. doi: 10.1128/JVI.00534-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voge NV, Sanchez-Vargas I, Blair CD, Eisen L, Beaty BJ. Detection of dengue virus NS1 antigen in infected Aedes aegypti using a commercially available kit. Am J Trop Med Hyg. 2013;88:260–266. doi: 10.4269/ajtmh.2012.12-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreno-Garcia M, Lanz-Mendoza H, Cordoba-Aguilar A. Genetic variance and genotype-by-environment interaction of immune response in Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2010;47:111–120. doi: 10.1603/me08267. [DOI] [PubMed] [Google Scholar]
- 28.Lambrechts L, Fansiri T, Pongsiri A, Thaisomboonsuk B, Klungthong C, Richardson JH, Ponlawat A, Jarman RG, Jarman RG, Scott TW. Dengue-1 virus clade replacement in Thailand associated with enhanced mosquito transmission. J Virol. 2012;86:1853–1861. doi: 10.1128/JVI.06458-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Epstein JH, Quan PL, Briese T, Street C, Jabado O, Conlan S, Ali Khan S, Verdugo D, Hossain MJ, Hutchison SK, Egholm M, Luby SP, Daszak P, Lipkin WI. Identification of GBV-D, a novel GB-like flavivirus from old world frugivorous bats (Pteropus giganteus) in Bangladesh. PLoS Pathog. 2010;6:e1000972. doi: 10.1371/journal.ppat.1000972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donaldson EF, Haskew AN, Gates JE, Huynh J, Moore CJ, Frieman MB. Metagenomic analysis of the viromes of three North American bat species: viral diversity among different bat species that share a common habitat. J Virol. 2010;84:13004–13018. doi: 10.1128/JVI.01255-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Z, Ren X, Yang L, Hu Y, Yang J, He G, Zhang J, Dong J, Sun L, Du J, Liu L, Xue Y, Wang J, Yang F, Zhang S, Jin Q. Virome analysis for identification of novel mammalian viruses in bat species from Chinese provinces. J Virol. 2012;86:10999–11012. doi: 10.1128/JVI.01394-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He B, Yang F, Yang W, Zhang Y, Feng Y, Zhou J, Xie J, Bao X, Guo H, Li Y, Xia L, Li N, Matthijnssens J, Zhang H, Tu C. Characterization of a novel G3P[3] rotavirus isolated from a lesser horseshoe bat: a distant relative of feline/canine rotaviruses. J Virol. 2013;87:12357–12366. doi: 10.1128/JVI.02013-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dacheux L, Cervantes-Gonzalez M, Guigon G, Thiberge JM, Vandenbogaert M, Maufrais C, Caro V, Bourhy H. A preliminary study of viral metagenomics of French bat species in contact with humans: identification of new mammalian viruses. PLoS ONE. 2014;9:e87194. doi: 10.1371/journal.pone.0087194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11:532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- 36.Kyle JL, Beatty PR, Harris E. Dengue virus infects macrophages and dendritic cells in a mouse model of infection. J Infect Dis. 2007;195:1808–1817. doi: 10.1086/518007. [DOI] [PubMed] [Google Scholar]
- 37.Davis A, Bunning M, Gordy P, Panella N, Blitvich B, Bowen R. Experimental and natural infection of North American bats with West Nile virus. Am J Trop Med Hyg. 2005;73:467–469. [PubMed] [Google Scholar]
- 38.van den Hurk AF, Smith CS, Field HE, Smith IL, Northill JA, Taylor CT, Jansen CC, Smith GA, Mackenzie JS. Transmission of Japanese encephalitis virus from the black flying fox, Pteropus alecto, to Culex annulirostris mosquitoes, despite the absence of detectable viremia. Am J Trop Med Hyg. 2009;81:457–462. [PubMed] [Google Scholar]
- 39.Paes MV, Pinhao AT, Barreto DF, Costa SM, Oliveira MP, Nogueira AC, Takiya CM, Farias-Filho JC, Schatzmayr HG, Alves AM, Barth OM. Liver injury and viremia in mice infected with dengue-2 virus. Virology. 2005;338:236–246. doi: 10.1016/j.virol.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe S, Omatsu T, Miranda ME, Masangkay JS, Ueda N, Endo M, Kato K, Tohya Y, Yoshikawa Y, Akashi H. Epizootiology and experimental infection of Yokose virus in bats. Comp Immunol Microbiol Infect Dis. 2010;33:25–36. doi: 10.1016/j.cimid.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perea-Martinez L, Moreno-Sandoval HN, Moreno-Altamirano MM, Salas-Rojas M, Garcia-Flores MM, Arechiga-Ceballos N, Tordo N, Marianneau P, Aguilar-Setien A. Experimental infection of Artibeus intermedius bats with serotype-2 dengue virus. Comp Immunol Microbiol Infect Dis. 2013;36:193–198. doi: 10.1016/j.cimid.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Jessie K, Fong MY, Devi S, Lam SK, Wong KT. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J Infect Dis. 2004;189:1411–1418. doi: 10.1086/383043. [DOI] [PubMed] [Google Scholar]
- 43.Barr KL, Anderson BD, Heil GL, Friary JA, Gray GC, Focks DA. Dengue serotypes 1–4 exhibit unique host specificity in vitro. Virus Adaptation and Treatment. 2012;4:85–91. [Google Scholar]
- 44.Young PR, Hilditch PA, Bletchly C, Halloran W. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J Clin Microbiol. 2000;38:1053–1057. doi: 10.1128/jcm.38.3.1053-1057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alcon S, Talarmin A, Debruyne M, Falconar A, Deubel V, Flamand M. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J Clin Microbiol. 2002;40:376–381. doi: 10.1128/JCM.40.2.376-381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramirez AH, Moros Z, Comach G, Zambrano J, Bravo L, Pinto B, Vielma S, Cardier J, Liprandi F. Evaluation of dengue NS1 antigen detection tests with acute sera from patients infected with dengue virus in Venezuela. Diagn Microbiol Infect Dis. 2009;65:247–253. doi: 10.1016/j.diagmicrobio.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 47.Conway MJ, Watson AM, Colpitts TM, Dragovic SM, Li Z, Wang P, Feitosa F, Shepherd DT, Ryman KD, Klimstra WB. Mosquito saliva serine protease enhances dissemination of dengue virus into the mammalian host. J Virol. 2014;88:164–175. doi: 10.1128/JVI.02235-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Surasombatpattana P, Ekchariyawat P, Hamel R, Patramool S, Thongrungkiat S, Denizot M, Delaunay P, Thomas F, Luplertlop N, Yssel H. Aedes aegypti saliva contains a prominent 34-kDa protein that strongly enhances dengue virus replication in human keratinocytes. J Invest Dermatol. 2013;134:281–284. doi: 10.1038/jid.2013.251. [DOI] [PubMed] [Google Scholar]
- 49.Ader DB, Celluzzi C, Bisbing J, Gilmore L, Gunther V, Peachman KK, Rao M, Barvir D, Sun W, Palmer DR. Modulation of dengue virus infection of dendritic cells by Aedes aegypti saliva. Viral Immunol. 2004;17:252–265. doi: 10.1089/0882824041310496. [DOI] [PubMed] [Google Scholar]
- 50.Sulkin SE, Allen R, Sims R. Studies of arthropod-borne virus infections in Chiroptera. I. Susceptibility of insectivorous species to experimental infection with Japanese B and St. Louis encephalitis viruses. Am J Trop Med Hyg. 1963;12:800–814. [PubMed] [Google Scholar]
- 51.Vaughan K, Greenbaum J, Blythe M, Peters B, Sette A. Meta-analysis of all immune epitope data in the Flavivirus genus: inventory of current immune epitope data status in the context of virus immunity and immunopathology. Viral Immunol. 2010;23:259–284. doi: 10.1089/vim.2010.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]


