Abstract
We report here the sequencing and analysis of the genome of the purple non-sulfur photosynthetic bacterium Rubrivivax gelatinosus CBS. This microbe is a model for studies of its carboxydotrophic life style under anaerobic condition, based on its ability to utilize carbon monoxide (CO) as the sole carbon substrate and water as the electron acceptor, yielding CO2 and H2 as the end products. The CO-oxidation reaction is known to be catalyzed by two enzyme complexes, the CO dehydrogenase and hydrogenase. As expected, analysis of the genome of Rx. gelatinosus CBS reveals the presence of genes encoding both enzyme complexes. The CO-oxidation reaction is CO-inducible, which is consistent with the presence of two putative CO-sensing transcription factors in its genome. Genome analysis also reveals the presence of two additional hydrogenases, an uptake hydrogenase that liberates the electrons in H2 in support of cell growth, and a regulatory hydrogenase that senses H2 and relays the signal to a two-component system that ultimately controls synthesis of the uptake hydrogenase. The genome also contains two sets of hydrogenase maturation genes which are known to assemble the catalytic metallocluster of the hydrogenase NiFe active site. Collectively, the genome sequence and analysis information reveals the blueprint of an intricate network of signal transduction pathways and its underlying regulation that enables Rx. gelatinosus CBS to thrive on CO or H2 in support of cell growth.
Introduction
Rubrivivax gelatinosus CBS was originally isolated from soil in Denver, Colorado. It is a purple non-sulfur (PNS) photosynthetic bacterium belonging to the family of Rhodospirillaceae [1]. Similar to most PNS in this family, Rx. gelatinosus is versatile with various modes of growth and energy metabolism. It carries out anoxygenic photosynthesis using electrons derived from organic acids and energy from sunlight, aerobic respiration using organic acids, and N2 fixation to ammonium in support of cell growth [2], [3]. Moreover, upon exposure to carbon monoxide (CO) in the culture gas phase, two Rx. gelatinosus strains, i.e., CBS and S1 can both carry out a water-gas shift reaction according to the equation CO + H2O → CO2 + H2 [4]-[7]. The CO2 and H2 products are then assimilated into new cell mass either in the light or in darkness, both under anaerobic condition. The latter growth mode in darkness can use CO as the sole carbon and energy source, hence coupling CO oxidation to energy conservation by a proton/sodium gradient generated by electron transfer via the CO-linked hydrogenase [8], [9].
The CO metabolic pathway in the PNS Rhodospirillum rubrum is well characterized and has served as the model system to unravel the genes and enzymes involved in CO metabolism. Upon exposure to CO, two coo (CO oxidation) operons are induced, with the cooFSCTJ operon encoding CO dehydrogenase (CODH) and related Ni-insertion proteins [10], [11], and the cooMKLXUH operon encoding a NiFe-hydrogenase [12], [13]. Transcription of both operons is under the control of the heme-containing CO-sensing transcription factor cooA [14]. The CooF protein contains iron-sulfur (FeS) clusters which likely mediates electron transfer between CODH and the CO-linked CooMKLXUH hydrogenase [15], [16]. Prior research in Rx. gelatinosus CBS has identified a cooMKLXUH operon encoding a CO-inducible hydrogenase [17]. This hydrogenase is likely a hexameric protein with high degree of amino acid identity to its homologs in Rs. rubrum, Carboxydothermus hydrogenoformans [18], Methanosarcina barkeri [19], and Thermoanaerobacter tengcongensis [20]. This type of hexameric NiFe-hydrogenases shares a common feature in that the hydrophilic subunits CooLXUH display sequence similarity to the energy-conserving NADH: quinone oxidoreductase (complex I). This class of hydrogenases is classified as the Group 4 energy-converting hydrogenase (Ech) [21], consistent with its role in proton-pumping reaction to yield energy from CO oxidation and H2 production [9], [22]. The CO-dependent H2 production hence has important ramifications in microbial energy generation during C1 carbon metabolism.
Although the genomes of the Rx. gelatinosus strains ATCC17011 and IL144 have been sequenced, neither contains homologs of the cooFSCTJ or the cooMKLXUH genes [23]. When tested, the strain ATCC17011 failed to metabolize CO (NREL, unpublished work). As such the versatility of energy metabolism in Rx. gelatinosus CBS especially regarding CO metabolism and CO-linked H2 production prompted us to sequence its genome [24], which is the first sequenced genome for a Rx gelatinosus strain capable of metabolizing CO.
Detailed genome annotation revealed a cooFSC and cooMKLXUH gene cluster likely encoding the protein machinery responsible for CO oxidation and H2 production. We uncovered a set of hydrogenase maturation genes (hypABFCDE), which is clustered near the coo operon and presumably assembles the active site of the Ech hydrogenase [25], [26]. Moreover, we uncovered a H2-uptake hydrogenase and a H2-sensing hydrogenase along with a second set of hyp maturation genes, with a genome arrangement similar to that in Ralstonia eutropha [27]. The Rx. gelatinosus CBS genome hence provides the blueprint to an intricate signal transduction network governing H2 and CO sensing, regulation, and metabolism.
Materials and Methods
Genome Sequencing
SMRTbell template libraries were prepared as previously described [28]. Two different sized SMRTbell template libraries were employed. Genomic DNA samples were either sheared to an average size of ∼800 base pairs via adaptive focused acoustics (Covaris; Woburn, MA, USA) or to a target size of approximately 8–10 kilobase pairs using Covaris g-TUBEs (Woburn, MA, USA). Fragmented DNA was then end repaired and ligated to hairpin adapters. Incompletely formed SMRTbell templates were digested with a combination of Exonuclease III (New England Biolabs; Ipswich, MA, USA) and Exonuclease VII (Affymetrix; Cleveland, OH, USA). SMRT Sequencing was carried out on the Pacific Biosciences RS (Menlo Park, CA, USA) using C2 chemistry with standard protocols for either small or large insert SMRTbell template libraries.
Genome Assembly
The de novo genome assembly was performed using hybrid assembly protocols, which is based on error correction of the long-insert library of SMRT sequencing reads with the short-insert library, circular consensus sequencing (CCS) SMRT sequencing reads [29], [30]. The algorithm is available in SMRT Analysis version 1.3.3. The initial assembly resulted in three contigs with sizes of 4,724,250, 362,142 and 241,393 bases. The 241 kb contig was determined to be a distinct genomic element without any sequence similarity to the other two contigs, and was concluded to be circularly closed due to overlapping ends from the assembler (Figure S1). The remaining two contigs were found to have a region of high sequence similarity near the end of the 4.7 Mb and the very beginning of the 362 kb contig. It overlaps with a coverage anomaly at the end of the 4.7 Mb contig, indicating the presence of a 47 kb high-copy phage/plasmid element that has sequence identity with this region in the chromosome over a stretch of ∼43 kb (Figure S2). The 47 kb high-copy element was represented by a new contig, and the 4.7 Mb and 362 kb contigs were connected to yield the full-length, 5.1 Mb bacterial chromosome. The chromosome had overlapping, self-similar ends, indicating that it is circularly closed. Such end-overlaps are typical for de novo assemblies on circularly closed genomic elements, and were trimmed manually. Approximately 93% of the long-insert library reads mapped back to the de novo assembly which is typical for SMRT sequencing.
We also applied a more recent hierarchical genome assembly algorithm (HGAp) on the long-insert library data only, and obtained identical results for the final genome assembly. HGAp is available at www.pacbiodevnet.com/hgap. Dot plots were generated using Gepard [31]. For the quality control of remapping reads back to the assembly, reads were mapped using the BLASR mapper (http://www.pacbiodevnet.com/SMRT-Analysis/Algorithms/BLASR) and the Pacific Biosciences' SMRT Analysis pipeline (http://www.pacbiodevnet.com/SMRT-Analysis/Software/SMRT-Pipe) using the standard mapping protocol.
Results and Discussion
Overview of Subsystem Category
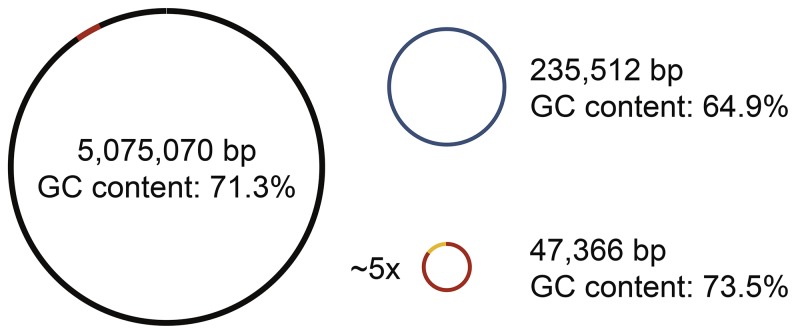
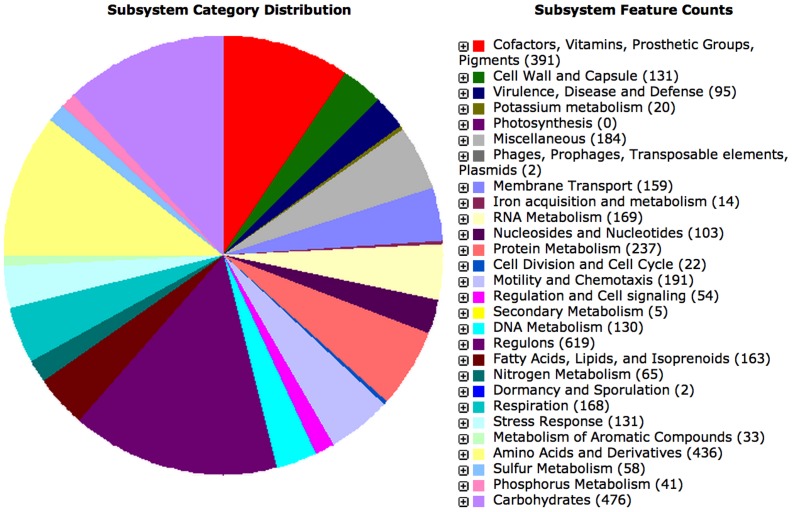
Hybrid, de novo genome assembly from long-insert library SMRT sequencing reads and short-insert library CCS SMRT sequencing reads resulted in three contigs representing one circularly closed bacterial chromosome with a size of 5,075,070 bases and a GC content of 71.3%, a 235,5122 basepair plasmid with a GC content of 64.9%, and a 47,366 basepair plasmid with a 73.5% GC content (Figure 1). In order to annotate the Rx. gelatinosus CBS genome, we submitted all contigs to RAST (Rapid Annotation using Subsystem Technology), a fully-automated service for annotating bacterial and archaeal genomes [32]. With RAST, we discovered 4,910 genes in Rx. gelatinosus CBS. The subsystem distribution in the genome shown in Figure 2 reveals the majority of genes encode regulons and genes involved in metabolism of carbohydrates, amino acids and derivatives. Specifically, we found 41 predicted hydrogenase genes and 158 predicted dehydrogenase genes in the genome, including 6 new hydrogenases genes and 5 new dehydrogenases genes that have not been reported in [24].
Figure 1. De novo genome assembly results for Rubrivivax gelatinosus CBS, resulting in one bacterial chromosome and two satellite DNA elements.
The common section of the small satellite and the chromosome are highlighted in red. Not drawn to scale.
Figure 2. Organism Overview for Rubrivivax gelatinosus CBS.
There are 643 subsystems, 4852 coding sequences, and 58 RNAs.
CO Metabolism via CO Dehydrogenase
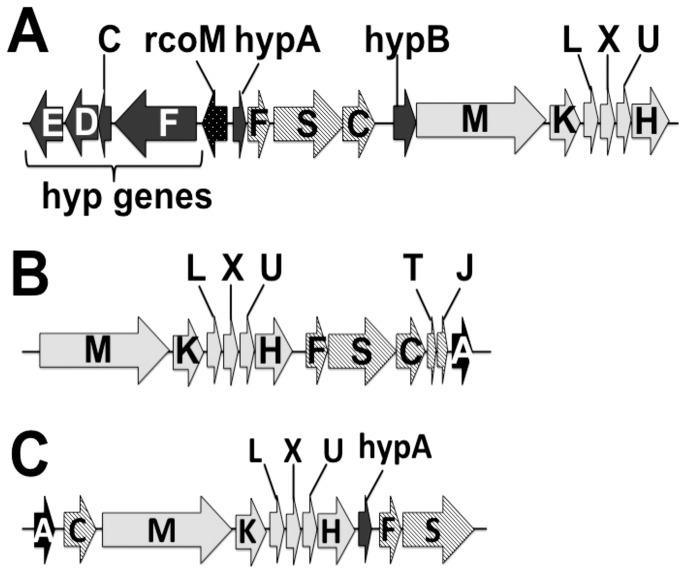
Genome annotation in Rx. gelatinosus CBS identified cooFCS genes (Figure 3), which display high levels of homology with their counterparts in Rs. rubrum, C. hydrogenoformans, and D. vulgaris str. Hildenborough (Table 1). These genes are presumably responsible in Rx. gelatinosus CBS for CO oxidation catalyzed by CODH (encoded by cooS), followed by electron transfer to CooF, an FeS protein. Critical residues for the Ni-Fe-S active site in CooS are highly conserved [33], and in CBS these conserved residues are H266, C293, G457, C458, C489, C540, T581, and K583. CooS is predicted to have one FeS cluster in the N-terminal half of the protein coordinated by conserved cysteine residues at C48, C51, C56, and C70. The CooF protein in Rs. rubrum contains conserved cysteine motifs to coordinate up to four FeS clusters [15], [16], while the CBS CooF protein sequence predicts only three FeS clusters. However, EPR data of Rs. rubrum CooF revealed that there are likely only two FeS clusters present per CooF monomer [16]. CooF likely mediates electron transfer from the CooS active site to the Coo hydrogenase (discussed below).
Figure 3. Organization of genes encoding anaerobic CO metabolism in multiple bacteria.
(A) Gene structure of CO metabolism genes in Rubrivivax gelatinosus CBS. The hyp genes putatively responsible for coo hydrogenase maturation are clustered among the coo CODH and hydrogenase genes. The gene encoding RcoM, the putative transcription factor for the coo CODH and hydrogenase genes is located among the hyp genes within this cluster of CO metabolism genes. (B) Gene structure of CO metabolism genes in Rhodospirillum rubrum. The cooA gene, encoding the CooA CO-responsive transcription factor, directly follows the CODH genes. (C) Gene structure of CO metabolism genes in Carboxydothermus hydrogenoformans. C. hydrogenoformans has five CODH complexes, but the genes for only one CODH are co-located with the genes for an energy-conserving hydrogenase [36]. The cooA and hypC genes precede the cooMKLXUH hydrogenase genes, which are followed by hypA and the cooFS CODH genes.
Table 1. Similarity and identity of select CO dehydrogenase proteins and CO-responsive transcription factors between Rubrivivax gelatinosus CBS with other bacterial species, generated by EMBOSS 6.3.1: matcher search [62].
| Protein and organism | Similarity% | Identity% |
| CooF | ||
| Rs. rubrum | 71 | 60 |
| C. hydrogenoformans | 63 | 49 |
| D. vulgaris | 65 | 48 |
| CooS | ||
| Rs. rubrum | 76 | 61 |
| C. hydrogenoformans | 77 | 59 |
| D. vulgaris | 60 | 44 |
| CooC | ||
| Rs. rubrum | 70 | 52 |
| C. hydrogenoformans | 65 | 46 |
| D. vulgaris | 58 | 46 |
| RcoM | ||
| B. xenovorans RcoM-1 | 52 | 32 |
| B. xenovorans RcoM-2 | 58 | 35 |
| Rs. rubrum | 43 | 28 |
| CooA | ||
| Rx. gelatinosus IL144 | 100 | 98 |
| Rx. benzoatilyticus JA2 | 95 | 90 |
| Rs. rubrum | 57 | 36 |
| C. hydrogenoformans | 61 | 37 |
| CowN | ||
| Rx. gelatinosus IL144 | 100 | 99 |
| Rx. benzoatilyticus JA2 | 92 | 91 |
| Rs. rubrum | 50 | 31 |
| CoxS | ||
| Rx. gelatinosus IL144 | 100 | 100 |
| O. carboxidovorans | 74 | 58 |
| CoxL | ||
| Rx. gelatinosus IL144 | 99 | 99 |
| O. carboxidovorans | 54 | 38 |
| CoxM | ||
| Rx. gelatinosus IL144 | 100 | 100 |
| O. carboxidovorans | 58 | 43 |
| CoxD | ||
| Rx. gelatinosus IL144 | 100 | 99 |
| O. carboxidovorans | 64 | 48 |
Strain Hildenborough was chosen for the comparison of Desulfovibrio gigas. Since Rx. benzoatilyticus does not contain the cox genes for aerobic CO oxidation, only predicted protein sequences from Rx. gelatinosus IL144 and Oligotropha carboxidovorans were used for comparison with Rx. gelatinosus CBS.
Similar to other complex metalloproteins, the CODH enzyme requires maturation factors, or chaperones, for the correct insertion of metals to form the holoprotein. In Rs. rubrum, it was found that CooCTJ are involved in the insertion of Ni into the CooS protein [10]. CooC is a membrane-associated protein that is believed to couple ATP hydrolysis with Ni insertion into the CODH active site [34]. There is less Ni insertion into the active site of CooS in either cooT or cooJ mutants, but there was virtually no CODH activity in a cooC mutant [34], [35]. This implies that CooC is the most important of these proteins for Ni insertion. A CooC homolog is evident in Rx. gelatinosus CBS, but the genes encoding CooT and J are not present (Figure 3), similar to C. hydrogenoformans. Yet even without clear homologs of cooT and cooJ, both Rx. gelatinosus CBS and C. hydrogenoformans produces an active CODH, as evidenced by growth on CO as a sole carbon source [1], [36]. This suggests that either CooC alone may suffice for Ni insertion into the CooS active site in the latter microbes, or Ni insertion is assisted by some yet to be identified proteins.
In addition to the coo genes for anaerobic CO oxidation, Rx. gelatinosus CBS was found to also have the coxSLMD genes for aerobic CO oxidation. Aerobic CO oxidation has been best studied in Oligotropha carboxidovorans, and in this organism coxSLM encode the carbon monoxide dehydrogenase enzyme. CoxS is the small subunit and contains 2 FeS clusters. CoxM, the medium-sized subunit, contains an FAD binding site, and the enzymatic CoxL is the large, catalytic subunit and contains the Mo-Cu active site. The CoxD protein is involved in biosynthesis of the active site metallocluster [37]. While Rx. benzoatilyticus does not contain any of the coxSLMD genes, the Rx. gelatinosus IL144 genome does. The IL144 strain is similar to Rx. gelatinosus CBS in genome size (around 5 Mb) and GC content (around 71%), as well as numbers of predicted genes (close to 5000) [23]. As shown in Table 1, the Rx. gelatinosus IL144 CoxSLMD predicted proteins share 99–100% sequence identity with those predicted in Rx. gelatinosus CBS. While the genes encoding coxSLMD have been identified in both the Rx. gelatinosus CBS and Rx. gelatinosus IL144 genomes, no expression data exists for these genes in either organism and the functionality of an aerobic mode of CO metabolism has not been confirmed in either strain.
Transcription Regulation of CO Metabolism
Anaerobic carbon monoxide metabolism in both Rs. rubrum and Rx. gelatinosus CBS is regulated by CO. In Rs. rubrum, CO induces increased expression of the genes encoding CODH and hydrogenase [12], [38], [39]. CooA is the CO-responsive transcription factor that regulates expression of coo genes in Rs. rubrum [12], [14], [40], [41]. The CooA protein contains an N-terminal b-type heme for gas sensing and a C-terminal helix-turn-helix motif to bind DNA [42]. CO metabolism in Rx. gelatinosus CBS is also regulated by the presence of CO with CO-dependent growth and the appearance of Coo hydrogenase proteins CooH and CooL strictly depending on the presence of CO [1], [17]. Genome annotation revealed a cooA gene in Rx. gelatinosus CBS, yet it is not clustered with the cooFSC genes as in Rs rubrum. CooA in Rx. gelatinosus CBS is only 36% identical and 57% similar to the well-studied CooA in Rs. rubrum (Table 1). As expected based on protein alignment, the CooA in Rx. gelatinosus CBS is most similar to its counterpart in Rx. gelatinosus IL144 and Rx. benzoatilyticus (Table 1). However, neither of the latter two microbes metabolizes CO anaerobically and genes encoding the CODH and Coo hydrogenase necessary for anaerobic CO metabolism are not present [23].
Interestingly, the Rx. gelatinosus CBS cooA gene is adjacent to the cowN gene in the genome. In Rs. rubrum, the CowN protein is responsible for the protection of nitrogenase from inactivation by CO [43]. While it is known that Rx. gelatinosus CBS can fix nitrogen while consuming CO as the sole carbon source [1], the mechanism of the protection of nitrogenase is unknown. The Rx. gelatinosus CBS, Rx. gelatinosus IL144, and Rx. benzoatilyticus genomes do all contain the cowN gene, with protein sequence identities ranging from 91-99% with the predicted Rx. gelatinosus CBS CowN protein (Table 1). Since neither Rx. gelatinosus IL144 nor Rx. benzoatilyticus contain genes for the anaerobic metabolism of CO, the role of CooA and CowN proteins is unclear if these genes are indeed expressed. It is worth noting that the genome arrangement in Rx. gelatinosus CBS is the reverse of what is observed in Rs. rubrum, where cooA is clustered with coo genes and the gene encoding the RcoM transcription factor (described below) is adjacent to the cowN gene [43], suggesting that cooA may regulate cowN expression in Rx. gelatinosus CBS.
Rx. gelatinosus CBS harbors a single copy of the rcoM gene, which is clustered with the coo genes responsible for anaerobic CO oxidation (Figure 3). RcoM (Regulator of CO Metabolism) was identified in 2008 and is expected to regulate genes responsible for CO metabolism in Geobacter spp. and Pelobacter carbinolicus DSM 2380, since these microbes do not harbor a CooA homolog [44]. Like CooA, RcoM is a single-component transcription factor; with an N-terminal PAS sensor domain presumably used to sense CO and a C-terminal domain containing a LytTR DNA-binding domain [44], [45]. The Rx. gelatinosus CBS RcoM protein is predicted to contain these conserved motifs [46] and is similar to RcoM in other organisms (Table 1). Therefore, based on the location of the rcoM gene in the genome along with evidence that rcoM is absent in strains of Rubrivivax that do not oxidize CO, we predict that RcoM regulates anaerobic CO metabolism genes in Rx. gelatinosus CBS.
Hydrogen Metabolism and Multiple Hydrogenases
CO-linked Hydrogenase
The Rx. gelatinosus CBS genome contains a cooMKLXUH operon (Figure 3), with genes displaying high levels of homology with their counterparts in Rs. rubrum and C. hydrogenoformans [17]. These genes encode a membrane anchored hexameric NiFe hydrogenase [47] that is responsible in Rx. gelatinosus CBS for CO-linked H2 production [7]. This operon was initially identified via transposon mutagenesis; a cooH mutant strain completely lost CO-linked H2 production [17]. The CooH subunit harbors the NiFe active site; CooL contains an FeS cluster predicted to serve as an electron relay to/from the active site in the CooH subunit; CooX also harbors FeS clusters for electron relay based on a study on its counterpart in Methanosarcina barkeri [17], [48]. CooU is predicted to be a soluble protein of 180 amino acids (20 kDa). Its amino acid sequence shows 39% identity and 56% similarity to CooU (annotated as NADH dehydrogenase subunit) in Rs. rubrum, and 34% identity and 57% similarity to CooU in C. hydrogenoformans. However, the specific role of CooU in the CooMKLXUH hydrogenase has yet to be determined. Gene expression from this operon is regulated by CO, as discussed above. Not only is hydrogenase activity in Rx. gelatinosus CBS induced by CO, so is gene transcription (data not shown) and accumulation of subunits CooL and CooH [17].
The CooM and CooK hydrogenase subunits are membrane-associated proteins. CooM is predicted to be a large membrane protein of 1255 amino acids (130 kDa) with 29–33 transmembrane segments, as predicted by DAS (http://www.sbc.su.se/~miklos/DAS/). The amino acid sequence shows 52% identity and 66% similarity to Rs. rubrum CooM (annotated as NADH dehydrogenase) and 46% identity and 62% similarity to C. hydrogenoformans CooM (annotated as carbon monoxide-induced hydrogenase, membrane anchor subunit). CooK is predicted to be a membrane protein of 319 amino acids (34 kDa), with 8 transmembrane segments predicted by DAS. Its amino acid sequence exhibits 57% identity and 74% similarity to CooK in Rs. rubrum (annotated as membrane bound hydrogenase subunit, MbhM), and 50% identity and 66% similarity to CooK in C. hydrogenoformans. Besides serving as a membrane anchor for the hydrogenase, CooM and CooK may be involved in energy generation from the CO oxidation-H2 production pathway catalyzed by Ech hydrogenase including that in Rx. gelatinosus CBS [9], [47].
The cooMKLXUH operon is absent from the genome of Rx. gelatinosus IL144 [23]. Comparison of the two Rx. gelatinosus genomes shows that this operon may have been present in a common ancestor and subsequently lost in the IL144 strain. This is evidenced by the observation that genes flanking the upstream and downstream of the operon are present in both microbes, including a 48-bp region identical to the 3′ of hypE in Rx. gelatinosus CBS (Figure S3).
H2-Uptake and Sensor Hydrogenases
Membrane-bound hydrogenases (MBH) are a class of NiFe hydrogenases that couple H2 oxidation to the respiratory chain for energy generation, hence allowing organisms to utilize H2 as an electron and energy source [21]. The extensively studied MBH of R. eutropha H16 serves as the model enzyme for comparison [49], [50]. The MBH operon of R. eutropha H16 contains 22 genes, including genes encoding the MBH structural subunits (hoxKG) and a regulatory or sensor hydrogenase (hoxBC), as well as associated signaling factors (hoxJ and hoxA). HoxBC (homologs of HupUV in other organisms) functions with a two-component system composed of a histidine kinase (HoxJ/HupT) and a DNA-binding response regulator (HoxA/HupR) which regulate MBH operon expression in the presence of H2 [51]–[53]. Analysis of the Rx. gelatinosus CBS genomic sequence reveals a 17,891 basepair MBH operon with 20 genes. Comparison at the nucleotide level reveals that the MBH operon in Rx. gelatinosus CBS displays 98% identity with the MBH operon in Rx. gelatinosus IL144 spanning 99% query coverage, yet shows only 80% nucleotide identity with the MBH operon in R. eutropha spanning 20% query coverage. Therefore, we adopted the Rx. gelatinosus IL144 “hup” gene nomenclature for the genes in the Rx. gelatinosus CBS MBH operon. Nevertheless, high amino acid conservation is observed between Rx. gelatinosus CBS, Rx. gelatinosus IL144, and R. eutropha H16 as to the respective MBH small subunit (HupA/HoxK), large subunit (HupB/HoxG), and the sensor hydrogenase small subunit (HupU/HoxB) and large subunit (HupV/HoxC) (Table 2), with their expression likely under the influence of H2 [51]–[53].
Table 2. Similarity, identity, and coverage of select hydrogenase proteins comparing the Rubrivivax gelatinosus CBS sensor and uptake hydrogenases to other bacterial species including Ralstonia eutropha H16.
| Similarity% | Identity% | Coverage% | ||
| Sensor Hydrogenase | ||||
| HupU | Rx. gelatinosus IL144 | 99 | 98 | 100 |
| R. eutropha H16 (HoxB) | 84 | 74 | 98 | |
| HupV | Rx. gelatinosus IL144 | 99 | 98 | 100 |
| R. eutropha H16 (HoxC) | 75 | 66 | 100 | |
| Uptake Hydrogenase | ||||
| HupA | Rx. gelatinosus IL144 | 100 | 100 | 100 |
| R. eutropha H16 (HoxK) | 96 | 89 | 87 | |
| HupB | Rx. gelatinosus IL144 | 99 | 99 | 100 |
| R. eutropha H16 (HoxG) | 92 | 86 | 100 | |
HupUV is a sensor hydrogenase, with HupU being the small subunit and HupV being the large, catalytic subunit. HupAB comprises a membrane-bound uptake hydrogenase with a small HupA subunit and a large, catalytic HupB subunit. Analysis was done using a NCBI P-BLAST search.
Examining the sequence of the Rx. gelatinosus CBS MBH small subunit HupA reveals that it is likely an O2-tolerant hydrogenase. It contains two conserved supernumerary cysteines (Cys98 and Cys197) that align with the characterized Cys19 and Cys120 in the O2-tolerant NiFe-hydrogenases of R. eutropha H16 MBH [54] and E. coli Hyd-1 [55], whereas the O2-sensitive hydrogenase contain glycine residues instead. Rx. gelatinosus CBS contains HupI (HoxR homolog in R. eutropha H16), a rubredoxin-type FeS protein deemed essential in assembling the O2-tolerant MBH in R. eutropha H16 when cultured in aerobic environment [54], [56]. Future studies may reveal whether the supernumerary cysteines contribute to the O2-tolerance of the MBH in Rx. gelatinosus CBS [7].
NiFe Hydrogenase Maturation Factors
Genome sequencing also reveals the presence of two copies of the pluripotent hyp hydrogenase maturation genes. One copy (hereafter hyp1FCDEAB) clusters near the coo operon (Figure 3) and another (hereafter hyp2ABFCDE) is located in the MBH operon. The respective proteins from hyp1 and hyp2 share identities ranging from 35% to 54% (Table 3). However, Rx. gelatinosus CBS Hyp2 proteins display much higher identity (ranging from 60 to 77%) with the respective counterparts in the R. eutropha H16 MBH operon (Table 3). It is therefore likely that the hyp1 cluster is responsible for assembling the CO-linked, H2-evolving hydrogenase while the hyp2 cluster assembles the MBH in Rx. gelatinosus CBS. Genetic knockout of hyp1 and/or hyp2 will directly test this hypothesis. No hyp genes were found near the coo operon in the Rs. rubrum sequenced genome (Figure 3). Two sets of hyp maturation genes were found in the Rs. rubrum genome, with one partial set (hypABC) clustered near its MBH (HupAB) and a second set (hypFCDEB) clustered near a ferredoxin hydrogenase. The latter is likely a fermentative hydrogenase linking H2 production to formate oxidation [57] since a nearby operon contains genes encoding formate dehydrogenase, a formate transporter, and a chaperon protein for the biosynthesis of molybdenum cofactor. A second hypA was found in a distant location in the genome. Rs. rubrum therefore might recruit these hyp genes for maturation of its CO-linked hydrogenase.
Table 3. Identity percentage of Rubrivivax gelatinosus CBS Hyp1, Hyp2 proteins and the Hyp proteins from Ralstonia eutropha H16, generated by NCBI P-BLAST search.
| Rx. gelatinosus Hyp1 vs. Hyp2% | Rx. gelatinosus Hyp1 vs. R. eutropha Hyp % | Rx. gelatinosus Hyp2 vs. R. eutropha Hyp % | |
| HypA | 35 | 31 | 66 |
| HypB | 54 | 52 | 67 |
| HypC | 39 | 38 | 60 |
| HypD | 51 | 47 | 77 |
| HypE | 54 | 54 | 74 |
| HypF | 37 | 38 | 60 |
Physiological Relevance of CO and H2 Metabolism
Genetic elements and gene arrangements controlling the CO-dependent H2 evolution system are very similar between Rx. gelatinosus CBS and Rs. rubrum. It is therefore logical to compare the physiological relevance of the two strains regarding CO and H2 metabolism. CODH was first purified from Rs. rubrum without adding CO for its induction [38], although adding CO does increase its CODH activity significantly and CO is required for the CO-induced hydrogenase activity [58]. To the contrary, in Rx. gelatinosus CBS the presence of CO during growth is required to afford CODH activity linking CO oxidation to the reduction of methyl viologen [7]. The CO-inducible hydrogenase activity in Rs. rubrum is reported to be insensitive to CO, retaining 40% activity in 100% CO gas phase (880 µM) [39]. However, the chromatophore membranes used for the above hydrogenase assay also contained CODH activity; its high rate of CO oxidation could protect hydrogenase from CO inhibition. Yet once partially purified from extracts, the CO-inducible hydrogenase in Rx. gelatinosus CBS is extremely sensitive to CO, with 50% inhibition observed at 3.9 µM dissolved CO [7]. The latter is consistent with the 5.8 to 40 µM Ki values of CO reported for most hydrogenases [59], [60]. This dramatic difference in tolerance to CO is likely attributed to the removal of the bulk of CODH from the partially purified Rx. gelatinosus CBS hydrogenase preparation used for the assay. While Rs. rubrum, Rx. gelatinosus strain CBS and strain S1 can grow in CO in darkness, with CO serving as a carbon substrate and energy source [4], [9], [61], an equal comparison of growth rates is complicated by the fact that all strains were grown under different conditions and with differing media components. No matter the growth rate, it is now well recognized that CO-inducible hydrogenase couples H2 evolution to proton translocation based on additional evidence including the effects of the electron transfer uncoupler carbonyl-cyanide m-chlorophenylhydrazone (CCCP) and the ATP synthesis inhibitor N, N′-dicyclohexylcarbodiimide (DCCD), with effects of the latter observed in both Rs. rubrum and Rx. gelatinosus CBS [9], [22].
Conclusion
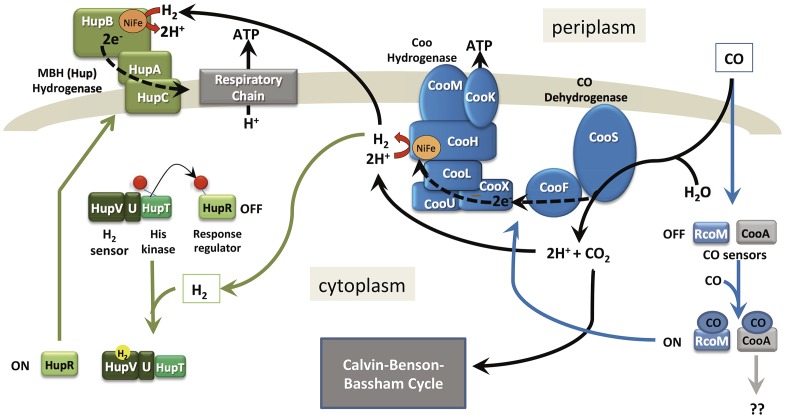
The genomic information compiled in this report for Rx. gelatinosus CBS reveals features responsible for an autotrophic life style based on CO utilization. The coo, hup and hyp gene repertoire along with genes encoding the Calvin-Benson-Bassham (CBB) pathways (data not shown) enable growth using CO as the sole carbon substrate (Figure 4). This microbe contains a CO sensor, likely encoded by rcoM, which upon sensing CO initiates the transcription of the coo operon to enable CO oxidation and H2 production. This microbe also contains the H2-sensing hydrogenase HupUV, which is expected to work in concert with its cognate regulatory two-component system HupRT to regulate the expression of an H2-uptake hydrogenase HupAB. H2 oxidation ultimately provides electrons; that along with ATP generated from photosynthesis, participate in the CO2 fixation reaction via the CBB pathway leading to cell growth. Rx. gelatinosus CBS is therefore a model photosynthetic bacterium to study a network of intricate signal transduction pathways and the underlying regulations controlling the assimilation of one-carbon compounds such as CO and CO2.
Figure 4. An overview of the CO and H2 signal transduction pathways and metabolism in Rubrivivax gelatinosus CBS.
Supporting Information
The 242 kb contig from the Rubrivivax gelatinosus CBS de novo assembly is a distinct, circular genomic element. (a) Sequencing coverage from remapping SMRT sequencing reads onto the 242 kb contig from the de novo assembly. (b) Dot plot of the contig against itself, showing overlapping sequence at both ends (circled). (c) Dot plots between the 242 kb contig and the other two de novo assembly contigs, highlighting the absence of any sequence similarity between the 242 kb contig and the other contigs.
(TIF)
Curation of the bacterial chromosome assembly of Rubrivivax gelatinosus CBS. (a) Sequencing coverage from remapping SMRT sequencing reads onto the 4.7 Mb contig from the de novo assembly. The broad undulation in coverage is of biological origin due to the presence of more DNA in the sample near the origin of replication (ori) when cells are harvested in log growth phase. The large spike in coverage is highlighted (arrow). (b) Sequencing coverage over the 363 kb contig. (c) SMRTView zoom-in of the end of the 4.7 Mb contig showing the sequence read structure. Uniquely mapped reads are shown in gray, ambiguously mapping reads are highlighted in red. The dotplots show the end of the 4.7 Mb contig against itself (top), and the end of the 4.7 Mb contig against the beginning of the 362 kb contig (bottom).
(TIF)
coo operon comparison in the genome of Rubrivivax gelatinosus CBS strain and IL144. Genes flanking the upstream and downstream of the operon are present in both microbes, including a 48 bp region identical to the 3′ of hypE in Rx. gelatinosus CBS.
(TIF)
Methylome determination of Rubrivivax gelatinosus CBS. (a) Example section of the bacterial chromosome with kinetic signals indicating adenine methylation. (b) Scatter plot of kinetic scores over all 3 genomic elements. The threshold used for methyltransferase specificity determination is indicated by the dashed line. (c) Determined methyltransferase specificities. (d) Summary of detected methylated positions across the genome.
(TIF)
Kinetic score distributions for the identified methyltransferase specificities in Rubrivivax gelatinosus CBS.
(TIF)
Methylome. The Methylome supplementary information describes the methodology used to determine the methylome in Rx. gelatinosus CBS. We uncover both type I and type II methyltransferases, both of which are N6-methyladenine. Further analysis involving cloning or knock outs of the methyltransferase genes would be necessary to determine the gene responsible for this specificity.
(DOCX)
Acknowledgments
We acknowledge Tyson A. Clark and Khai Luong at Pacific Biosciences for assistance with the sequencing and data analysis.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files or in the public database which can be accessed via http://www.ncbi.nlm.nih.gov/bioproject/246247.
Funding Statement
This work was supported by the U.S. Department of Energy Fuel Cell Technologies Office (to SN, CE, JY, and PCM), Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy award number DE-FG02-91ER20021 (to JC), and the University of Wyoming start-up funds (to KW). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Maness P-C, Weaver PF (1994) Production of poly-3-hydroxyalkanoates from CO and H2 by a novel photosynthetic bacterium. Applied Biochemistry and Biotechnology 45–46:395–406 10.1007/BF02941814 [DOI] [Google Scholar]
- 2. Cogdell RJ, Isaacs NW, Howard TD, McLuskey K, Fraser NJ, et al. (1999) How Photosynthetic Bacteria Harvest Solar Energy. Journal of Bacteriology 181:3869–3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tang K-H, Tang YJ, Blankenship RE (2011) Carbon Metabolic Pathways in Phototrophic Bacteria and Their Broader Evolutionary Implications. Front Microbio 2 10.3389/fmicb.2011.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uffen RL (1983) Metabolism of carbon monoxide by Rhodopseudomonas gelatinosa: cell growth and properties of the oxidation system. Journal of Bacteriology 155:956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Champine JE, Uffen RL (1987) Membrane topography of anaerobic carbon monoxide oxidation in Rhodocyclus gelatinosus. Journal of Bacteriology 169:4784–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maness P-C, Weaver PF (2002) Hydrogen production from a carbon-monoxide oxidation pathway in Rubrivivax gelatinosus. International Journal of Hydrogen Energy 27:1407–1411 10.1016/S0360-3199(02)00107-6 [DOI] [Google Scholar]
- 7. Maness PC, Smolinski S, Dillon AC, Heben MJ, Weaver PF (2002) Characterization of the Oxygen Tolerance of a Hydrogenase Linked to a Carbon Monoxide Oxidation Pathway in Rubrivivax gelatinosus. Appl Environ Microbiol 68:2633–2636 10.1128/AEM.68.6.2633-2636.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Uffen RL (1976) Anaerobic growth of a Rhodopseudomonas species in the dark with carbon monoxide as sole carbon and energy substrate. Proc Natl Acad Sci USA 73:3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maness P-C, Huang J, Smolinski S, Tek V, Vanzin G (2005) Energy Generation from the CO Oxidation-Hydrogen Production Pathway in Rubrivivax gelatinosus. Appl Environ Microbiol 71:2870–2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kerby RL, Hong SS, Ensign SA, Coppoc LJ, Ludden PW, et al. (1992) Genetic and physiological characterization of the Rhodospirillum rubrum carbon monoxide dehydrogenase system. Journal of Bacteriology 174:5284–5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kerby RL, Ludden PW, Roberts GP (1997) In vivo nickel insertion into the carbon monoxide dehydrogenase of Rhodospirillum rubrum: molecular and physiological characterization of cooCTJ. Journal of Bacteriology 179:2259–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fox JD, He Y, Shelver D, Roberts GP, Ludden PW (1996) Characterization of the region encoding the CO-induced hydrogenase of Rhodospirillum rubrum. Journal of Bacteriology 178:6200–6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drennan CL, Heo J, Sintchak MD, Schreiter E, Ludden PW (2001) Life on carbon monoxide: X-ray structure of Rhodospirillum rubrum Ni-Fe-S carbon monoxide dehydrogenase. Proceedings of the National Academy of Sciences 98:11973–11978 10.1073/pnas.141230698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shelver D, Kerby RL, He Y, Roberts GP (1995) Carbon monoxide-induced activation of gene expression in Rhodospirillum rubrum requires the product of cooA, a member of the cyclic AMP receptor protein family of transcriptional regulators. Journal of Bacteriology 177:2157–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ensign SA, Ludden PW (1991) Characterization of the CO oxidation/H2 evolution system of Rhodospirillum rubrum. Role of a 22-kDa iron-sulfur protein in mediating electron transfer between carbon monoxide dehydrogenase and hydrogenase. Journal of Biological Chemistry 266:18395–18403. [PubMed] [Google Scholar]
- 16. Singer SW, Hirst MB, Ludden PW (2006) CO-dependent H2 evolution by Rhodospirillum rubrum: Role of CODH: CooF complex. Biochimica et Biophysica Acta (BBA) - Bioenergetics VL - 1757:1582–1591 10.1016/j.bbabio.2006.10.003 [DOI] [PubMed] [Google Scholar]
- 17. Vanzin G, Yu J, Smolinski S, Tek V, Pennington G, et al. (2010) Characterization of Genes Responsible for the CO-Linked Hydrogen Production Pathway in Rubrivivax gelatinosus. Appl Environ Microbiol 76:3715–3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Soboh B, Linder D, Hedderich R (2002) Purification and catalytic properties of a CO-oxidizing: H2-evolving enzyme complex from Carboxydothermus hydrogenoformans. European Journal of Biochemistry 269:5712–5721 10.1046/j.1432-1033.2002.03282.x [DOI] [PubMed] [Google Scholar]
- 19. Kunkel A, Vorholt JA, Thauer RK, Hedderich R (1998) An Escherichia coli hydrogenase-3-type hydrogenase in methanogenic archaea. European Journal of Biochemistry 252:467–476 10.1046/j.1432-1327.1998.2520467.x [DOI] [PubMed] [Google Scholar]
- 20. Soboh B, Linder D, Hedderich R (2004) A multisubunit membrane-bound [NiFe] hydrogenase and an NADH-dependent Fe-only hydrogenase in the fermenting bacterium Thermoanaerobacter tengcongensis. Microbiology 150:2451–2463 10.1099/mic.0.27159-0 [DOI] [PubMed] [Google Scholar]
- 21. Vignais PM, Billoud B, Meyer J (2001) Classification and phylogeny of hydrogenases. FEMS Microbiology Reviews 25:455–501 10.1111/j.1574-6976.2001.tb00587.x [DOI] [PubMed] [Google Scholar]
- 22. Fox JD, Kerby RL, Roberts GP, Ludden PW (1996) Characterization of the CO-induced, CO-tolerant hydrogenase from Rhodospirillum rubrum and the gene encoding the large subunit of the enzyme. Journal of Bacteriology 178:1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nagashima S, Kamimura A, Shimizu T, Nakamura-Isaki S, Aono E, et al. (2012) Complete Genome Sequence of Phototrophic Betaproteobacterium Rubrivivax gelatinosus IL144. Journal of Bacteriology 194:3541–3542 10.1128/JB.00511-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu P, Juan L, Wawrousek K, Yu J, Maness P-C, et al. (2012) Draft genome sequence of Rubrivivax gelatinosus CBS. Journal of Bacteriology 194:3262 10.1128/JB.00515-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lutz S, Jacobi A, Schlensog V, Böhm R, Sawers G, et al. (1991) Molecular characterization of an operon (hyp) necessary for the activity of the three hydrogenase isoenzymes in Escherichia coli. Mol Microbiol 5:123–135 10.1111/j.1365-2958.1991.tb01833.x [DOI] [PubMed] [Google Scholar]
- 26. Hube M, Blokesch M, Böck A (2002) Network of Hydrogenase Maturation in Escherichia coli: Role of Accessory Proteins HypA and HybF. Journal of Bacteriology 184:3879–3885 10.1128/JB.184.14.3879-3885.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pohlmann A, Fricke WF, Reinecke F, Kusian B, Liesegang H, et al. (2006) Genome sequence of the bioplastic-producing |[ldquo]|Knallgas|[rdquo]| bacterium Ralstonia eutropha H16. Nat Biotechnol 24:1257–1262 10.1038/nbt1244 [DOI] [PubMed] [Google Scholar]
- 28. Travers KJ, Chin CS, Rank DR, Eid JS, Turner SW (2010) A flexible and efficient template format for circular consensus sequencing and SNP detection. Nucleic Acids Research 38:e159–e159 10.1093/nar/gkq543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bashir A, Klammer AA, Robins WP, Chin C-S, Webster D, et al. (2012) A hybrid approach for the automated finishing of bacterial genomes. Nat Biotechnol 30:701–707 10.1038/nbt.2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koren S, Schatz MC, Walenz BP, Martin J, Howard JT, et al. (2012) Hybrid error correction and de novo assembly of single-molecule sequencing reads. Nat Biotechnol 30:693–700 10.1038/nbt.2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krumsiek J, Arnold R, Rattei T (2007) Gepard: a rapid and sensitive tool for creating dotplots on genome scale. Bioinformatics 23:1026–1028. [DOI] [PubMed] [Google Scholar]
- 32. Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, et al. (2008) The RAST Server: Rapid Annotations using Subsystems Technology. BMC Genomics 9:75 10.1186/1471-2164-9-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Doukov TI, Iverson TM, Seravalli J, Ragsdale SW, Drennan CL (2002) A Ni-Fe-Cu Center in a Bifunctional Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase. Science 298:567–572 10.1126/science.1075843 [DOI] [PubMed] [Google Scholar]
- 34. Jeon WB, Cheng J, Ludden PW (2001) Purification and Characterization of Membrane-associated CooC Protein and Its Functional Role in the Insertion of Nickel into Carbon Monoxide Dehydrogenase from Rhodospirillum rubrum. Journal of Biological Chemistry 276:38602–38609. [DOI] [PubMed] [Google Scholar]
- 35. Watt RK, Ludden PW (1999) Ni2+ Transport and Accumulation inRhodospirillum rubrum. Journal of Bacteriology 181:4554–4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu M, Ren Q, Durkin AS, Daugherty SC, Brinkac LM, et al. (2005) Life in Hot Carbon Monoxide: The Complete Genome Sequence of Carboxydothermus hydrogenoformans Z-2901. PLoS Genet 1:e65 10.1371/journal.pgen.0010065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pelzmann A, Ferner M, Gnida M, Meyer-Klaucke W, Maisel T, et al. (2009) The CoxD Protein of Oligotropha carboxidovorans Is a Predicted AAA+ ATPase Chaperone Involved in the Biogenesis of the CO Dehydrogenase [CuSMoO2] Cluster. Journal of Biological Chemistry 284:9578–9586 10.1074/jbc.M805354200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bonam D, Murrell SA, Ludden PW (1984) Carbon monoxide dehydrogenase from Rhodospirillum rubrum. Journal of Bacteriology 159:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bonam D, Lehman L, Roberts GP, Ludden PW (1989) Regulation of carbon monoxide dehydrogenase and hydrogenase in Rhodospirillum rubrum: effects of CO and oxygen on synthesis and activity. Journal of Bacteriology 171:3102–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. He Y, Shelver D, Kerby RL, Roberts GP (1996) Characterization of a CO-responsive Transcriptional Activator from Rhodospirillum rubrum. Journal of Biological Chemistry 271:120–123 10.1074/jbc.271.1.120 [DOI] [PubMed] [Google Scholar]
- 41. Aono S, Nakajima H, Saito K, Okada M (1996) A Novel Heme Protein That Acts as a Carbon Monoxide-Dependent Transcriptional Activator inRhodospirillum rubrum. Biochemical and Biophysical Research Communications 228:752–756 10.1006/bbrc.1996.1727 [DOI] [PubMed] [Google Scholar]
- 42. Lanzilotta WN, Schuller DJ, Thorsteinsson MV, Kerby RL, Roberts GP, et al. (2000) Structure of the CO sensing transcription activator CooA. Nat Struct Biol 7:876–880 10.1038/82820 [DOI] [PubMed] [Google Scholar]
- 43. Kerby RL, Roberts GP (2011) Sustaining N2-Dependent Growth in the Presence of CO. Journal of Bacteriology 193:774–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kerby RL, Youn H, Roberts GP (2008) RcoM: A New Single-Component Transcriptional Regulator of CO Metabolism in Bacteria. Journal of Bacteriology 190:3336–3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nikolskaya AN, Galperin MY (2002) A novel type of conserved DNA-binding domain in the transcriptional regulators of the AlgR/AgrA/LytR family. Nucleic Acids Research 30:2453–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pellequer J-L, Wager-Smith KA, Kay SA, Getzoff ED (1998) Photoactive yellow protein: A structural prototype for the three-dimensional fold of the PAS domain superfamily. Proc Natl Acad Sci USA 95:5884–5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hedderich R, Forzi L (2005) Energy-Converting [NiFe] Hydrogenases: More than Just H2 Activation. J Mol Microbiol Biotechnol 10:92–104 10.1159/000091557 [DOI] [PubMed] [Google Scholar]
- 48. Forzi L, Koch J, Guss AM, Radosevich CG, Metcalf WW, et al. (2005) Assignment of the [4Fe-4S] clusters of Ech hydrogenase from Methanosarcina barkeri to individual subunits via the characterization of site-directed mutants. FEBS Journal 272:4741–4753 10.1111/j.1742-4658.2005.04889.x [DOI] [PubMed] [Google Scholar]
- 49. Burgdorf T, Lenz O, Buhrke T, van der Linden E, Jones AK, et al. (2005) [NiFe]-Hydrogenases of Ralstonia eutropha H16: Modular Enzymes for Oxygen-Tolerant Biological Hydrogen Oxidation. J Mol Microbiol Biotechnol 10:181–196 10.1159/000091564 [DOI] [PubMed] [Google Scholar]
- 50. Ludwig M, Cracknell JA, Vincent KA, Armstrong FA, Lenz O (2008) Oxygen-tolerant H2 Oxidation by Membrane-bound [NiFe] Hydrogenases of Ralstonia Species: Coping with Low Level H2 in Air. Journal of Biological Chemistry 284:465–477 10.1074/jbc.M803676200 [DOI] [PubMed] [Google Scholar]
- 51. Lenz O, Friedrich B (1998) A novel multicomponent regulatory system mediates H2 sensing in Alcaligenes eutrophus. Proceedings of the National Academy of Sciences 95:12474–12479 10.1073/pnas.95.21.12474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vignais PM, Elsen S, Colbeau A (2005) Transcriptional regulation of the uptake [NiFe]hydrogenase genes in Rhodobacter capsulatus. Biochemical Society Transactions: 28–32. [DOI] [PubMed]
- 53. Elsen S, Duché O, Colbeau A (2003) Interaction between the H2 Sensor HupUV and the Histidine Kinase HupT Controls HupSL Hydrogenase Synthesis in Rhodobacter capsulatus. Journal of Bacteriology 185:7111–7119 10.1128/JB.185.24.7111-7119.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fritsch J, Scheerer P, Frielingsdorf S, Kroschinsky S, Friedrich B, et al. (2011) The crystal structure of an oxygen-tolerant hydrogenase uncovers a novel iron-sulphur centre. Nature 479:249–252 10.1038/nature10505 [DOI] [PubMed] [Google Scholar]
- 55. Volbeda A, Amara P, Darnault C, Mouesca J-M, Parkin A, et al. (2012) X-ray crystallographic and computational studies of the O2-tolerant [NiFe]-hydrogenase 1 from Escherichia coli. Proceedings of the National Academy of Sciences 109:5305–5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fritsch J, Siebert E, Priebe J, Zebger I, Lendzian F, et al. (2014) Rubredoxin-related Maturation Factor Guarantees Metal Cofactor Integrity during Aerobic Biosynthesis of Membrane-bound [NiFe] Hydrogenase. Journal of Biological Chemistry 289:7982–7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maness P-C, Weaver PF (2001) Evidence for three distince hydrogenase activities in Rhodospirillum rubrum Appl Microbiol Biotechnol: 751–756. Available: http://f. Accessed 15 May 2013. [DOI] [PubMed]
- 58. Bonam D, Ludden PW (1987) Purification and characterization of carbon monoxide dehydrogenase, a nickel, zinc, iron-sulfur protein, from Rhodospirillum rubrum. J Biol Chem 262:2980–2987. [PubMed] [Google Scholar]
- 59. Adams MW, Mortenson LE, Chen JS (1980) Hydrogenase. Biochimica et Biophysica Acta (BBA) - Bioenergetics VL - 594:105–176. [DOI] [PubMed] [Google Scholar]
- 60. Peters JW (1998) X-ray Crystal Structure of the Fe-Only Hydrogenase (CpI) from Clostridium pasteurianum to 1.8 Angstrom Resolution. Science 282:1853–1858 10.1126/science.282.5395.1853 [DOI] [PubMed] [Google Scholar]
- 61. Kerby RL, Ludden PW, Roberts GP (1995) Carbon monoxide-dependent growth of Rhodospirillum rubrum. Journal of Bacteriology 177:2241–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rice P, Longden I, Bleasby A (2000) EMBOSS: The European Molecular Biology Open Software Suite. Trends in Genetics 16:276–277 10.1016/S0168-9525(00)02024-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The 242 kb contig from the Rubrivivax gelatinosus CBS de novo assembly is a distinct, circular genomic element. (a) Sequencing coverage from remapping SMRT sequencing reads onto the 242 kb contig from the de novo assembly. (b) Dot plot of the contig against itself, showing overlapping sequence at both ends (circled). (c) Dot plots between the 242 kb contig and the other two de novo assembly contigs, highlighting the absence of any sequence similarity between the 242 kb contig and the other contigs.
(TIF)
Curation of the bacterial chromosome assembly of Rubrivivax gelatinosus CBS. (a) Sequencing coverage from remapping SMRT sequencing reads onto the 4.7 Mb contig from the de novo assembly. The broad undulation in coverage is of biological origin due to the presence of more DNA in the sample near the origin of replication (ori) when cells are harvested in log growth phase. The large spike in coverage is highlighted (arrow). (b) Sequencing coverage over the 363 kb contig. (c) SMRTView zoom-in of the end of the 4.7 Mb contig showing the sequence read structure. Uniquely mapped reads are shown in gray, ambiguously mapping reads are highlighted in red. The dotplots show the end of the 4.7 Mb contig against itself (top), and the end of the 4.7 Mb contig against the beginning of the 362 kb contig (bottom).
(TIF)
coo operon comparison in the genome of Rubrivivax gelatinosus CBS strain and IL144. Genes flanking the upstream and downstream of the operon are present in both microbes, including a 48 bp region identical to the 3′ of hypE in Rx. gelatinosus CBS.
(TIF)
Methylome determination of Rubrivivax gelatinosus CBS. (a) Example section of the bacterial chromosome with kinetic signals indicating adenine methylation. (b) Scatter plot of kinetic scores over all 3 genomic elements. The threshold used for methyltransferase specificity determination is indicated by the dashed line. (c) Determined methyltransferase specificities. (d) Summary of detected methylated positions across the genome.
(TIF)
Kinetic score distributions for the identified methyltransferase specificities in Rubrivivax gelatinosus CBS.
(TIF)
Methylome. The Methylome supplementary information describes the methodology used to determine the methylome in Rx. gelatinosus CBS. We uncover both type I and type II methyltransferases, both of which are N6-methyladenine. Further analysis involving cloning or knock outs of the methyltransferase genes would be necessary to determine the gene responsible for this specificity.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files or in the public database which can be accessed via http://www.ncbi.nlm.nih.gov/bioproject/246247.