Abstract
Chloroplasts isolated from tall fescue, Festuca arundinacea Schreb., showed high rates of electron transport, comparable to rates observed for spinach chloroplasts.
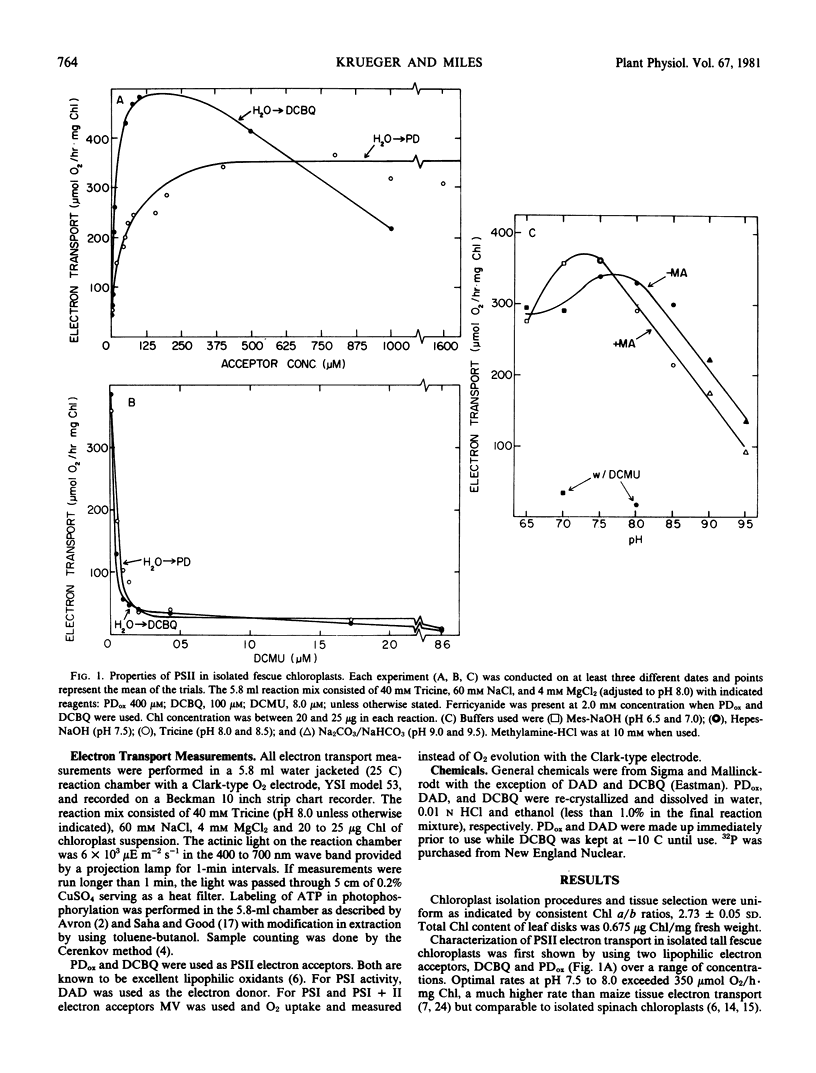
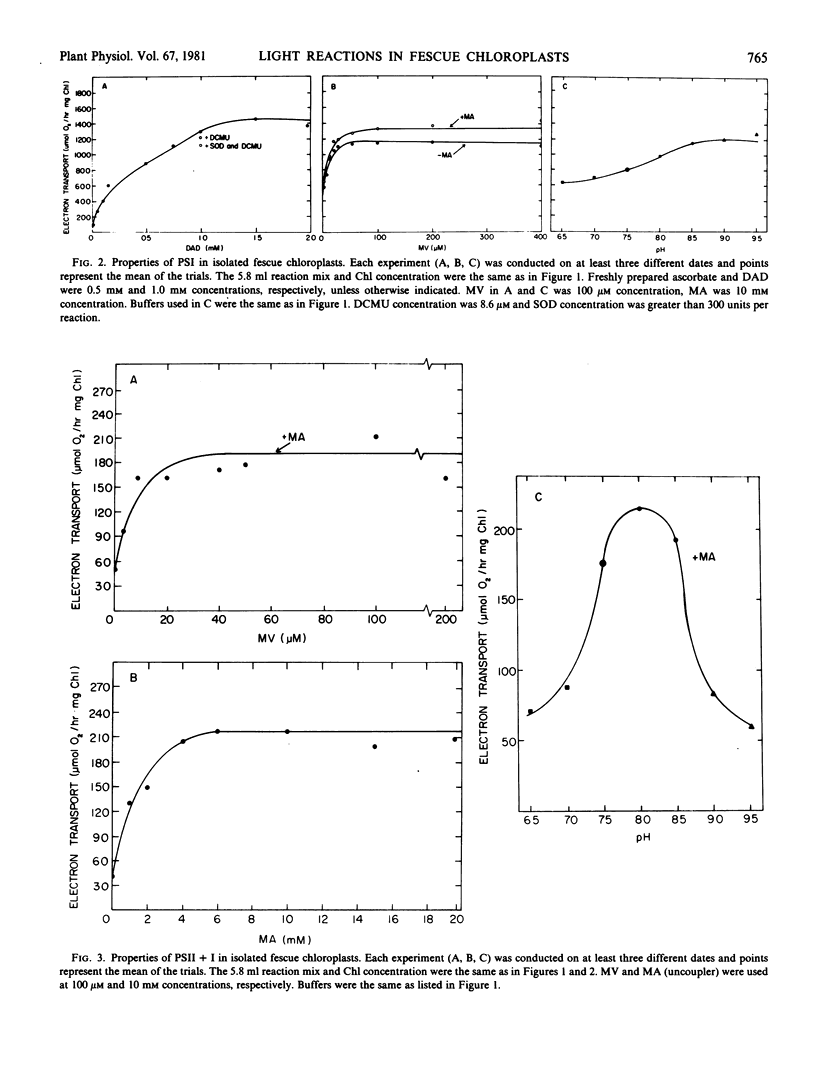
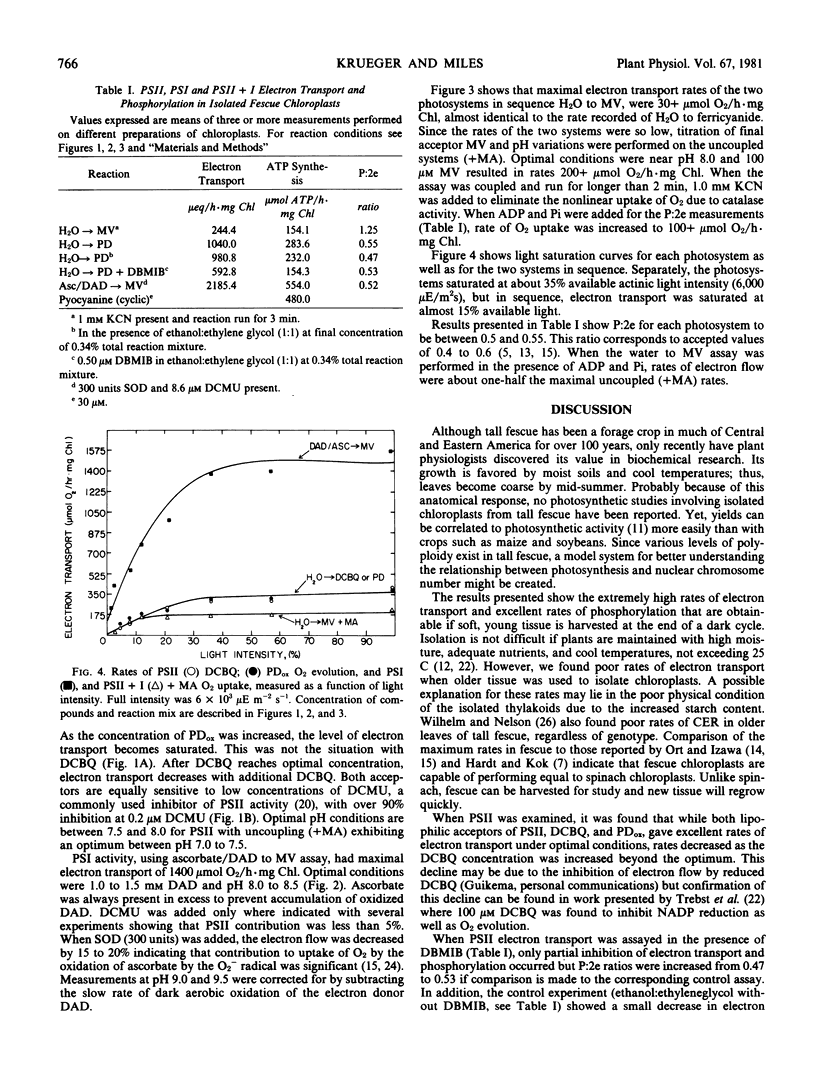
Chloroplasts were well coupled and rates of electron transport from water to methyl viologen (photosystem II and I) were increased two to five times when ADP and inorganic phosphate or methylamine (uncoupler) were added to the reaction mixture. Ratios of P:2e for photosystem II plus I were found to be near 1.2. Electron transport rates from water to p-phenylenediamine or 2,6-dichlorobenzoquinone (photosystem II) were over 300 micromoles O2 per hour per milligram chlorophyll, while P:2e ratios were found to be over 0.5. The highest rates of electron transport were found in electron flow from diaminodurene to methyl viologen (photosystem I) and P:2e ratios remained near 0.5.
Light intensity saturation curves for photosystem II and I, as well as the photosystems independently, resembled curves for spinach, with saturation of electron transport in photosystem I and photosystem II separately occurring at 35% of the available light intensity (6000 microeinsteins per square meter per second). Photosystem II and I in sequence were saturated at about half this light intensity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould J. M., Cather R., Winget G. D. Advantages of the use of Cerenkov vounting for determination of P 32 in photophosphorylation research. Anal Biochem. 1972 Dec;50(2):540–548. doi: 10.1016/0003-2697(72)90064-4. [DOI] [PubMed] [Google Scholar]
- Gould J. M., Izawa S. Photosystem-II electron transport and phosphorylation with dibromothymoquinone as the electron acceptor. Eur J Biochem. 1973 Aug 1;37(1):185–192. doi: 10.1111/j.1432-1033.1973.tb02974.x. [DOI] [PubMed] [Google Scholar]
- Guikema J. A., Yocum C. F. Steady-state kinetic analyses of photosystem II activity catalyzed by lipophilic electron acceptors. Biochim Biophys Acta. 1979 Aug 14;547(2):241–251. doi: 10.1016/0005-2728(79)90007-0. [DOI] [PubMed] [Google Scholar]
- Hardt H., Kok B. Comparison of photosynthetic activities of spinach chloroplasts with those of corn mesophyll and corn bundle sheath tissue. Plant Physiol. 1978 Jul;62(1):59–63. doi: 10.1104/pp.62.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa S., Gould J. M., Ort D. R., Felker P., Good N. E. Electron transport and photophosphorylation in chloroplasts as a function of the electron acceptor. 3. A dibromothymoquinone-insensitive phosphorylation reaction associated with photosystem II. Biochim Biophys Acta. 1973 Apr 27;305(1):119–128. doi: 10.1016/0005-2728(73)90237-5. [DOI] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. The measurement of cyclic photophosphorylation in isolated chloroplasts by determination of hydrogen ion consumption. An evaluation of the method using titration at constant pH. Biochim Biophys Acta. 1973 Sep 26;314(3):354–359. doi: 10.1016/0005-2728(73)90119-9. [DOI] [PubMed] [Google Scholar]
- Ort D. R., Izawa S. Studies on the Energy-coupling Sites of Photophosphorylation: II. Treatment of Chloroplasts with NH(2)OH Plus Ethylenediaminetetraacetate to Inhibit Water Oxidation while Maintaining Energy-coupling Efficiencies. Plant Physiol. 1973 Dec;52(6):595–600. doi: 10.1104/pp.52.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort D. R., Izawa S. Studies on the Energy-coupling Sites of Photophosphorylation: V. Phosphorylation Efficiencies (P/e(2)) Associated with Aerobic Photooxidation of Artificial Electron Donors. Plant Physiol. 1974 Mar;53(3):370–376. doi: 10.1104/pp.53.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort D. R. Quantitative relationship between photosystem I electron transport and ATP foramtion. Arch Biochem Biophys. 1975 Feb;166(2):629–638. doi: 10.1016/0003-9861(75)90429-4. [DOI] [PubMed] [Google Scholar]
- Randall D. D., Nelson C. J., Asay K. H. Ribulose bisphosphate carboxylase: altered genetic expression in tall fescue. Plant Physiol. 1977 Jan;59(1):38–41. doi: 10.1104/pp.59.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S., Good N. E. Products of the photophosphorylation reaction. J Biol Chem. 1970 Oct 10;245(19):5017–5021. [PubMed] [Google Scholar]
- Saha S., Ouitrakul R., Izawa S., Good N. E. Electron transport and photophosphorylation in chloroplasts as a function of the electron acceptor. J Biol Chem. 1971 May 25;246(10):3204–3209. [PubMed] [Google Scholar]
- Trebst A., Reimer S. Properties of photoreductions by photosystem II in isolated chloroplasts. 3. The effect of uncouplers on phenylenediamine shuttles accross the membrane in the presence of dibromothymoquinone. Biochim Biophys Acta. 1973 Dec 14;325(3):546–557. doi: 10.1016/0005-2728(73)90214-4. [DOI] [PubMed] [Google Scholar]
- Yocum C. F., Guikema J. A. Photophosphorylation Associated with Photosystem II: I. Photosystem II Cyclic Photophosphorylation Catalyzed by p-Phenylenediamine. Plant Physiol. 1977 Jan;59(1):33–37. doi: 10.1104/pp.59.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]


