Abstract
Setting
The impact of diabetes on tuberculosis in United States and foreign-born populations in San Francisco has not been studied.
Objective
To determine the characteristics, prevalence and temporal trends of diabetes in US and foreign-born persons attending the San Francisco Tuberculosis Clinic.
Design
We analyzed data from individuals seeking medical attention at the San Francisco Tuberculosis Clinic. We included patients with diagnosis of tuberculosis, latent infection, or not infected with Mycobacterium tuberculosis. We assessed the temporal trend and the characteristics of individuals with and without diabetes.
Result
Between 2005 and 2012, there were 4371 (19.0%) individuals without evidence of tuberculosis infection, 17,856 (77.6%) with latent tuberculosis, and 791 (3.4%) with tuberculosis. 66% were born in the United States, China, Mexico, and the Philippines. The prevalence of diabetes was the highest among individuals with tuberculosis and increased during the study period. Patients with tuberculosis and diabetes were more likely to be male, older than 45 years and born in the Philippines. There was a disproportionate association of TB and DM relative to LTBI and DM among Filipinos in individuals older than 45 years old.
Conclusions
Our data suggest that Filipinos older than 45 years old are more likely to have tuberculosis probably due to a higher prevalence of diabetes. In San Francisco, tuberculosis-screening programs in individuals with diabetes and latent tuberculosis may be beneficial in patients older than 45 years old especially from the Philippines.
Introduction
The prevalence of diabetes mellitus (DM) among patients with tuberculosis (TB) increased in United States from 8% in 2010 [1] to 14% in 2012 [2]. Globally, it is estimated that 10% of TB cases are associated with DM [3]. A meta-analysis of cohort studies demonstrated that DM was associated with TB with a relative risk (RR) of 3.1 (95% confidence interval (CI) 2.3 to 4.3) compared with non-diabetic individuals, although in observational studies the odds ratios were highly variable [4]. Patients with DM and TB were more likely to have an unfavorable treatment outcome (treatment failure or death) (RR 1.7, 95%CI 1.4 to 2.1) and more likely to relapse (RR 3.9, 95%CI 2.4 to 6.2) than patients without DM [5]. Although there is no evidence that DM increases susceptibility to infection by Mycobacterium tuberculosis [6], DM is associated with alterations in inflammatory responses such as activation of the inflammation cascade that appear to induce and maintain the subacute inflammatory state associated with obesity [7].
The estimated prevalence of DM in persons 20 to 79 years old increased globally from 5.1% in 2003 to 8.3% in 2012 [8], [9]. It is predicted that the world prevalence of DM will increase from 285 million in 2010 to 439 million by 2030, with 36 million diabetics in the United States (US) alone [10]. In 2012, the estimated prevalence of DM in US in persons born in Mexico, the Philippines, China, and US was 15.6%, 9.6%, 8.8%, and 9.3%, respectively [9].
In 2010, 85% of the TB cases in San Francisco occurred in foreign-born persons, mainly from the Philippines, China and Mexico with an estimated incidence of 90, 32 and 23 cases per 100,000 persons, respectively, compared with an incidence of 2.8 per 100,000 persons among the US-born population [11].
We undertook this analysis to determine the prevalence and temporal trends of DM in three main groups attending the San Francisco Tuberculosis Clinic (SFTB clinic): persons with latent tuberculosis infection (LTBI), persons with TB and persons with no evidence of LTBI or TB. We also examined the clinical, epidemiological and bacterial characteristics of patients with TB with and without DM in the different US- and foreign-born populations. Among the bacterial characteristics, we also included the analysis of the lineage of M. tuberculosis [12], as there is evidence of variable inflammatory response by different lineages [13]. Some of the results of this study have been previously reported in abstract form at the American Thoracic Society 2012 International Conference [14].
Materials and Methods
We performed a retrospective study of the data collected by the San Francisco Tuberculosis Control Section. We included information from all individuals seeking medical attention who had a final diagnosis of LTBI, TB or no evidence of LTBI or TB. We analyzed demographic and clinical information from all individuals as well as microbiological characteristics in patients with TB.
Ethics statement
The study was approved by the University of California, San Francisco, Human Research Protection Program. Consent form was not obtained as the study was based on de-identified information collected as part of standard of care.
Lineages of M.tb
[12] There are 7 lineages based on genomic regions of difference and single nucleotide polymorphisms [12], [15]. In this study, we used methods to determine the six lineages prevalent in San Francisco (the seventh lineage was described in Ethiopia and has not been detected in SF (data not shown)) [12], [15] The six lineages are: M. tuberculosis Indo-Oceanic lineage (or lineage 1), East Asian (or lineage 2), East African Indian (or lineage 3), Euro-American lineage (lineage 4), West African 1 (or lineage 5), and West African 2 (or lineage 6).
TB categories
All individuals seeking medical attention in the San Francisco TB control section were classified in one of the following TB categories [16]:
no evidence of M. tuberculosis infection (TB categories 0-no exposure to TB or 1-not infected based on the ATS classification [16]): no evidence of TB and negative tuberculin skin test or an interferon gamma release assay.
LTBI (category 2 on the ATS classification [16]): positive tuberculin skin test or interferon gamma release assay and no evidence of TB.
TB (category 3 on the ATS classification [16]) reported as a case of TB.
Treatment outcome of TB
We included: completed treatment, treatment failure [17], default, transferred and death [17], [18].
Diagnosis of DM
This was based on the information reported by the patient and medical records. During the study period, there was no change in screening policies for DM among individuals seeking care at the San Francisco TB control section.
Statistical Analysis
The temporal trend of DM prevalence in the 3 TB categories was assessed using the Cochran-Armitage test. We compared the prevalence of DM within the three TB categories among different populations stratified by age by comparing odds ratios using Z-score. Clinical, epidemiological and bacterial characteristics were compared between TB patients with and without DM among the different US- and foreign-born populations using Chi-squared test and logistic regression. We included interaction terms to the regression model to determine interactions between diabetes and place of birth on TB versus LTBI. We used Wilcoxon rank-sum to test for differences between medians of treatment length and time to conversion of the sputum. Statistical analyses were performed with SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Study population
Between April 2005 and March 2012, 25,293 individuals seeking medical attention were evaluated for TB and LTBI. If treated for either TB or LTBI, management was usually conducted at the SFTB clinic. Of the total evaluated, 23,018 had a category determined: 4371 (19.0%) did not have evidence of M. tuberculosis infection, 17,856 (77.6%) had LTBI; and 791 (3.4%) had TB. Of those with a TB category determined, 6732 (29.2%) were born in the US, 3582 (15.6%) in China, 2530 (11.0%) in Mexico, 2544 (11.1%) in the Philippines, and 7630 (33.1%) in other countries (Table 1).
Table 1. Prevalence of DM in the different TB categories and in the different FB and US-born population, April 2005-March 2012.
| Populations | DM in individuals with TB (%) [95%CI] | DM in individuals with LTBI (%) [95%CI] | DM in individuals without evidence of MTB infection (%) [95%CI] | OR (95%CI), p-value* | Z-score, p-value |
| All (n = 23,018) | 126/791 (15.9%) [13.4–18.5%] | 1158/17,856 (6.5%) [6.1–6.9%] | 206/4371 (4.7%) [4.1–5.3%] | ||
| Philippines (n = 2544) | 36/113 (31.9%) [23.3–40.5%] | 140/1815 (7.7%) [6.5–8.9%] | 41/616 (6.7%) [4.7–8.6%] | 5.59 (3.63–8.61), p<0.001 | Reference |
| China (n = 3582) | 40/233 (17.2%) [12.3–22.0%] | 159/2569 (6.2%) [5.3–7.1%] | 31/780 (4.0%) [2.6–5.4%] | 3.14 (2.16–4.58), p<0.001 | −1.974, p = 0.048 |
| Mexico (n = 2530) | 4/48 (8.3%) [0.5–16.2%] | 144/2109 (6.8%) [5.8–7.9%] | 11/373 (2.9%) [1.2–4.7%] | 1.24 (0.44–3.5), p = 0.68 | −2.627, p = 0.01 |
| US (n = 6732) | 16/173 (9.2%) [4.9–13.6%] | 350/5391 (6.5%) [5.8–7.2%] | 51/1168 (4.4%) [3.2–5.5%] | 1.47 (0.87–2.48), p = 0.15 | −3.855, p<0.001 |
*Comparison between DM in individuals with LTBI and DM in individuals with TB.
Z-score: compares odds ratio between different populations by using Philippines as a reference.
Prevalence of DM
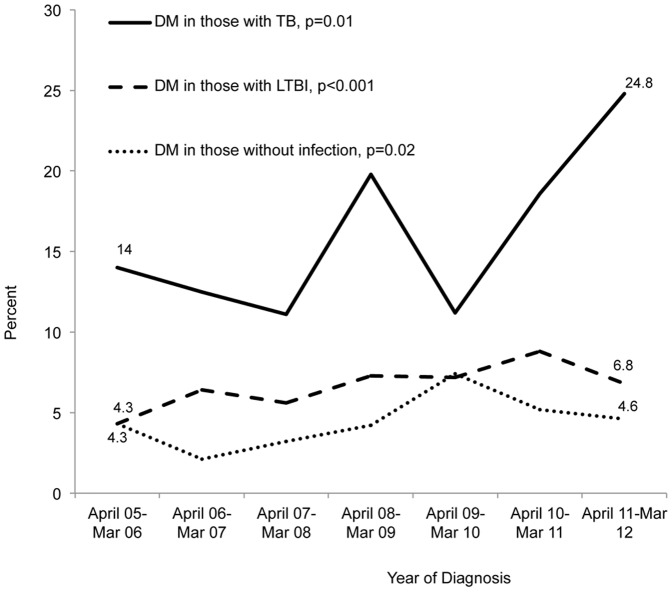
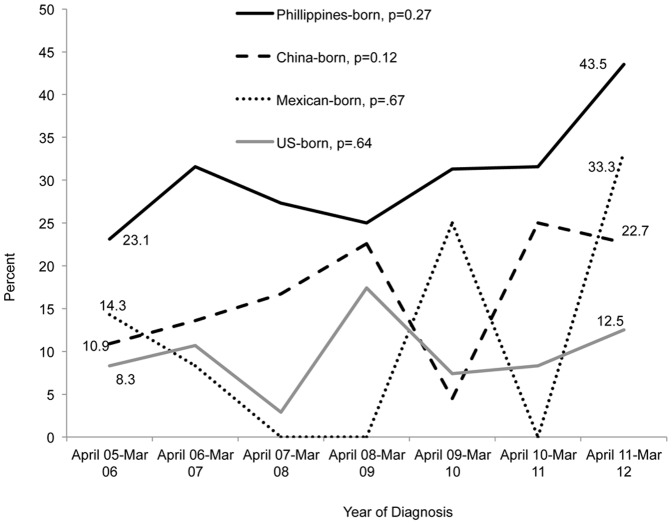
The overall prevalence of DM was highest among individuals with TB (126/791; 15.9%), followed by those with LTBI (1158/17,856; 6.5%) and by individuals without LTBI or TB (206/4371; 4.7%) (Table 1). The prevalence of DM in the different TB categories increased from April 2006 to March 2012, among patients with TB and known DM status, (p = 0.01) specifically from 16/114 (14.0%) in the period from April 2005 to March 2006 to 30/121 (24.8%) in the period of April 2011 to March 2012 (Figure 1). During the same time period, the prevalence of HIV among TB patients decreased from 12/114 (10.5%) in 2005-06 to 5/121 (4.1%) in 2011-12 (p = 0.03). The prevalence of DM in patients with TB from the Philippines increased from 23.1% to 43.5% (p = 0.27). The prevalence for the other foreign and US-born groups was more variable (Figure 2).
Figure 1. Prevalence of DM in the different TB categories in all study subjects: April 2005- March 2012.
The temporal trend of DM was assessed using Cochran-Armitage test for trends.
Figure 2. Prevalence of DM among TB patients in the US and foreign-born populations: April 2005- March 2012.
The temporal trend of DM prevalence was assessed using Cochran-Armitage test for trends.
The prevalence of DM in patients with TB varied from 31.9% (36/113) in individuals born in the Philippines to 8.3% (4/48) in those born in Mexico (Table 1). The prevalence of DM in individuals with LTBI varied from 7.7% (140/1,815) for those born in the Philippines to 6.2% (159/2,569) for those born in China; and the prevalence of DM in individuals without LTBI or TB varied from 6.7% (41/616) for those born in the Philippines to 2.9% (11/373) for those born in Mexico (Table 1). Among the US-born population with TB, the prevalence of DM varied depending on race/ethnicity: 60% (3/5) for American Indian, 33.3% (1/3) for Pacific Islanders, 13.6% (9/66) for African Americans, 4.5% (1/22) for Hispanics, 4.1% (1/25) for Asians, and 1.9% (1/52) for Whites.
Because the prevalence of DM increased with age we performed a stratified analysis (Table 2). In all TB categories, Philippines-born patients older than 45 years old had the highest prevalence of DM, especially among individuals with TB. The proportion of TB with DM relative to the proportion of LTBI with DM was significantly higher among the individuals from the Philippines when compared with the other populations (vs. US-born, Z-score = −2.782, p = 0.002; vs. Mexican-born, Z-score = −1.115, p = 0.13; vs. China-born, Z-score = −2.333, p = 0.009) (Table 2).
Table 2. Prevalence of DM in the different TB categories and in the different FB and US-born populations, April 2005-March 2012 stratified by age.
| AGE ≤ 45 | |||||
| Populations | DM in individuals with TB (%) [95%CI] | DM in individuals with LTBI (%) [95%CI] | DM in individuals without evidence of MTB infection (%) [95%CI] | OR (95%CI), p-value* | Z-score, p-value |
| All | 13/302 (4.3%) [2.0–6.6%] | 301/10,867 (2.8%) [2.5–3.1%] | 59/3097 (1.9%) [1.4–2.4%] | 1.58 (0.90–2.78), p = 0.11 | |
| Philippines | 1/26 (3.9%) [0.0–11.2%] | 31/1081 (2.9%) [1.9–3.9%] | 6/387 (1.6%) [0.6–2.8%] | 1.35 (0.18–10.3), p = 0.77 | Reference |
| China | 2/46 (4.4%) [0.0–10.2%] | 19/1415 (1.3%) [0.7–1.9%] | 9/496 (1.8%) [0.6–3.0%] | 3.34 (0.75–14.8), p = 0.11 | 0.702, p = 0.24 |
| Mexico | 2/39 (5.1%) [0.0–12.1%] | 78/1697 (4.6%) [3.6–5.6%] | 9/340 (2.7%) [0.9–4.4%] | 1.12 (0.27–4.74, p = 0.88 | −0.148, p = 0.44 |
| US | 4/77 (5.2%) [0.2–10.2%] | 74/2647 (2.8%) [2.2–3.4%] | 10/754 (1.3%) [0.5–2.1%] | 1.91 (0.68–5.35), p = 0.22 | 0.293, p = 0.38 |
| AGE> 45 | |||||
| All | 113/489 (23.1%) [19.4–26.8%] | 857/6989 (12.3%) [11.5–13.0%] | 147/1274 (11.5%) [9.8–13.3%] | 2.15 (1.72–2.69), p<0.001 | |
| Philippines | 35/87 (40.2%) [29.9–50.5%] | 109/734 (14.9%) [12.3–17.4%] | 35/229 (15.3%) [10.6–19.9%] | 3.86 (2.40–6.20), p<0.001 | Reference |
| China | 38/187 (20.3%) [14.6–26.1%] | 140/1154 (12.1%) [10.3–14.0%] | 22/284 (7.8%) [4.6–10.9%] | 1.85 (1.24–2.75), p = 0.002 | −2.333, p = 0.009 |
| Mexico | 2/9 (22.2%) [0.0–49.4%] | 66/412 (16.0%) [12.5–19.6%] | 2/33 (6.1%) [0.0–14.2%] | 1.50 (0.30–7.4), p = 0.62 | −1.115, p = 0.13 |
| US | 12/96 (12.5%) [5.9–19.1%] | 276/2744 (10.1%) [8.9–11.2%] | 41/414 (9.9%) [7.0–12.8%] | 1.28 (0.69–2.37), p = 0.44 | −2.782, p = 0.002 |
*Comparison between DM in individuals with LTBI and DM in individuals with TB.
Z-score: compares odds ratio between different populations by using Philippines as a reference.
Impact of place of birth on patients with TB and DM (Table 3)
Table 3. Characteristics of tuberculosis patients based on their diabetes status.
| Characteristic | Diabetes n (%) | No Diabetes n (%) | Unadjusted odds ratio (95%CI), p-value | Adjusted* odds ratio (95%CI), p-value |
| N | 126 (15.9) | 665 (84.1) | ||
| Place of birth | ||||
| Philippines | 36 (31.9) | 77 (68.1) | 3.05 (1.94–4.81), <.001 | 2.29 (1.41–3.72), <.001 |
| Other | 90 (13.3) | 588 (86.7) | ||
| Sex | ||||
| Male | 96 (18.9) | 413 (81.1) | 1.95 (1.26–3.03), 0.002 | 1.66 (1.02–2.68), 0.04 |
| Female | 30 (10.6) | 252 (89.4) | ||
| Age | ||||
| > 45 | 113 (23.2) | 375 (76.8) | 6.72 (3.71–12.2), <.001 | 5.78 (3.13–10.7), <.001 |
| ≤ 45 | 13 (4.3) | 290 (95.7) | ||
| HIV† status | ||||
| Positive | 3 (4.9) | 58 (95.1) | 0.25 (0.08–0.83), 0.02 | 0.36 (0.11–1.21), 0.10 |
| Negative | 87 (17) | 426 (83) | Referent | Referent |
| Unknown | 36 (16.6) | 181 (83.4) | 0.97 (0.64–1.49), 0.90 | 0.98 (0.61–1.56), 0.92 |
| Sputum smear status | ||||
| Sputum smear positive | 51 (21.0) | 192 (79.0) | 1.51 (1.01–2.27), 0.04 | 1.26 (0.79–2.02), 0.33 |
| Sputum smear negative | 67 (14.9) | 382 (85.1) | Referent | Referent |
| No sputum or solely extrapulmonary | 8 (8.1) | 91 (91.9) | 0.50 (0.23–1.08), 0.08 | 0.48 (0.20–1.11), 0.09 |
| Cavities | ||||
| Pulmonary cavitary TB | 23 (21.5) | 84 (78.5) | 1.50 (0.89–2.52), 0.13 | 1.53 (0.84–2.77), 0.16 |
| Non cavitary | 78 (15.5) | 426 (84.5) | Referent | Referent |
| Solely extrapulmonary TB | 25 (13.9) | 155 (86.1) | 0.88 (0.54–1.43), 0.61 | 1.36 (0.75–2.45), 0.31 |
| Lineage‡ | ||||
| Indo Oceanic | 33 (26.6) | 91 (73.4) | Referent | |
| East Asian | 28 (16.8) | 139 (83.2) | 0.56 (0.31–0.98), 0.04 | |
| Euro American | 31 (13.6) | 197 (86.4) | 0.43 (0.25–0.75), 0.002 | |
| Lineage specific‡ | ||||
| Indo Oceanic lineage | 33 (26.6) | 91 (73.4) | 2.07 (1.27–3.35), 0.003 | |
| Non-Indo Oceanic lineage | 59 (14.9) | 336 (85.1) | ||
| Philippine-born | ||||
| Indo Oceanic | 22 (31.4) | 48 (68.6) | Incalculable | |
| East Asian | 0 (0) | 2 (100) | ||
| Euro American | 0 (0) | 3 (100) | ||
| Non-Philippine-born‡ | ||||
| Indo Oceanic | 11 (20.4) | 43 (79.6) | Referent | |
| East Asian | 28 (17) | 137 (83) | 0.80 (0.37–1.74), 0.57 | |
| Euro American | 31 (13.8) | 194 (86.2) | 0.62 (0.29–1.34), 0.23 | |
| Any isoniazid resistance | ||||
| Isoniazid resistant | 12 (18.2) | 54 (81.8) | 1.07 (0.55–2.07), 0.85 | |
| Isoniazid susceptible | 93 (17.3) | 446 (82.8) | ||
| Multi-drug resistance | ||||
| Multi-drug resistant | 0 (0) | 8 (100) | Incalculable | |
| Non-multi drug resistant | 105 (17.6) | 492 (82.4) |
*Adjusted for: sex, age>45 years, HIV status, birth in the Philippines, AFB smear status, and cavitary/pulmonary TB.
HIV = Human immunodeficiency virus.
Eight patients had East African Indian lineage, and 1 had West African-1; none of these had diabetes.
During the study period, 791 patients were diagnosed with TB. One hundred twenty-six patients reported DM (15.9%). In order to determine if the place of birth was an independent factor associated with patients with TB and DM, we performed an adjusted analysis of the characteristics of patients with TB and DM and without DM (Table 3). We found that patients with TB and DM were more likely to be born in the Philippines (OR 2.29, 95%CI 1.41–3.72, p<0.001), to be male (OR 1.66, 95%CI 1.02–2.68, p = 0.04), and older than 45 years (OR 5.78, 95%CI 3.13–10.7, p<0.001). To determine if the effect of DM on TB and LTBI was similar in all the populations we tested for interactions. We found significant interaction (p<0.001) between Philippines birth and DM such that Filipinos with DM are much more likely to have active TB versus LTBI than other-born with DM (Table 4). There was no association between DM and isoniazid resistant or multi-drug resistant TB (Table 3). Lineage 1 strains were associated with DM. However, because patients from the Philippines are more likely to have TB due to lineage 1 strains [12], we performed a stratified analysis to determine if DM was associated with lineage 1 in patients born outside the Philippines. We did not find a significant association, although this was possibly due to a small sample size.
Table 4. Interaction terms for DM and place of birth as predictors of TB and LTBI, adjusted for age.
| Characteristic | TB n (%) | LTBI n (%) | Unadjusted odds ratio (95%CI), p-value | Adjusted odds ratio (95%CI), p-value |
| N | 791 | 17,856 | ||
| Age | ||||
| > 45 | 489 (6.5) | 6989 (93.5) | 2.52 (2.17–2.92), <.001 | 2.18 (1.86–2.54), <.001 |
| ≤ 45 | 302 (2.7) | 10,867 (97.3) | ||
| Diabetes | ||||
| Present | 126 (9.8) | 1158 (90.2) | 2.73 (2.24–3.34), <.001 | 1.81 (1.37–2.39), <.001 |
| Absent | 665 (3.8) | 16,698 (96.2) | ||
| Place of birth | ||||
| Philippines | 113 (5.9) | 1815 (94.1) | Referent | Referent |
| China | 233 (8.3) | 2569 (91.7) | 1.46 (1.15–1.84), 0.001 | 1.67 (1.27–2.19), <.001 |
| Mexico | 48 (2.2) | 2109 (97.8) | 0.37 (0.26–0.52), <.001 | 0.58 (0.40–0.85), 0.005 |
| Other foreign | 224 (3.6) | 5972 (96.4) | 0.60 (0.48–0.76), <.001 | 0.81 (0.61–1.06), 0.12 |
| US | 173 (3.1) | 5391 (96.9) | 0.52 (0.40–0.66), <.001 | 0.62 (0.47–0.82), <.001 |
| Interaction terms | ||||
| Philippines x diabetes | Referent | |||
| DM | 36 (20.5) | 140 (79.6) | 5.59 (3.63–8.61), <.001 | |
| No DM | 77 (4.4) | 1675 (95.6) | ||
| China x diabetes | 0.56 (0.31–0.99), 0.05 | |||
| DM | 40 (20.1) | 159 (79.9) | 3.14 (2.16–4.58), <.001 | |
| No DM | 193 (7.4) | 2410 (92.6) | ||
| Mexico x diabetes | 0.23 (0.08–0.72), 0.01 | |||
| DM | 4 (2.7) | 144 (97.3) | 1.24 (0.44–3.50), 0.68 | |
| No DM | 44 (2.2) | 1965 (97.8) | ||
| Other x diabetes | 0.41 (0.23–0.75), 0.003 | |||
| DM | 30 (7.6) | 365 (92.4) | 2.38 (1.59–3.54), <.001 | |
| No DM | 194 (3.3) | 5607 (96.7) | ||
| US x diabetes | 0.29 (0.15–0.57), <.001 | |||
| DM | 16 (4.4) | 350 (95.6) | 1.47 (0.87–2.48), 0.15 | |
| No DM | 157 (3.0) | 5041 (96.9) |
TB treatment outcome was reported in 735 of 791 cases (92.9%) and were similar between the 116 patients with DM and 619 without DM: 97 (83.6%) and 538 (86.9%) respectively completed treatment, 4 (3.4%) and 26 (4.2%) transferred out and 1 (0.9%) and 7 (1.1%) defaulted. The frequency of death was higher among patients with DM (14 [12.1%]) than in patients without DM (48 [7.6%]) but it was not statistically significant (OR 1.63, 95%CI: 0.87–3.07, p = 0.13). There were no treatment failures. The treatment outcome was also similar between the US and different foreign-born populations (data not shown). Patients with TB and DM received longer treatment with a median of 9.0 months (interquartile range (IQR) 6.5–10.3) compared with 6.7 months (IQR 6.2–9.5) in patients without DM, (p = 0.001). Data of the time of the conversion of sputum was available only in 66 of 126 (52.4%) patients with TB and DM and in 283 of 665 (42.6%) patients with TB and without DM. The median weeks to convert to negative sputum was 6.1 weeks (IQR 4.6–9.0) for those with DM and 5 weeks (IQR 3.7–8.4) for those without DM (p = 0.04).
Discussion
Due to the parallel epidemics of TB and DM, in 2011 the World Health Organization (WHO) made the recommendation that TB surveillance should be performed among patients with DM in settings where the TB incidence was more than 100 cases per 100,000 inhabitants [6]. They recommended testing for DM among TB patients in all countries. Due to the limited resources available to most TB control programs and the increasing number of patients with DM, it is very likely that these recommendations have not been followed in most countries despite the convergence of the two epidemics.
There has not been any systematic screening strategy during the study period that could have resulted in a relatively higher proportion of patients from the Philippines in San Francisco with DM. The increase of DM in the different TB categories during the study period can be explained by the increase of DM in the overall population. In San Francisco, the overall prevalence of DM increased from 3.8% in 2001 [19] to 6.2% in 2009 [20]. An increase in the overall prevalence of DM has also being reported in Mexico from 7.4% in 2003 to 15.9% in 2012, in China from 2.7% in 2003 to 8.82% in 2012 and in the Philippines from 3% in 2003 to 9.65% in 2012 [8], [9]. The overall incidence of TB in San Francisco among the Philippine-born individuals is also higher (90 per 100,000) than among individuals born in China-, Mexico- and US (32, 23 and 2.8 per 100,000 population, respectively) [11].
The clinical and epidemiological characteristics of the patients with TB and DM varied among the different populations. Patients with TB and DM born in the Philippines and China were older than 45 years old when compared to patients with TB without DM. A similar trend was observed in patients from the US and Mexico but it was not statistically significant. The association of TB and DM was statistically significant in patients from the Philippines. In addition, we observed an interaction between DM and being born in the Philippines comparing TB to LTBI patients that was significantly greater than the interaction with any other country of birth. Although these are cross-sectional data and we therefore cannot attribute causality, the data suggest that persons with DM born in the Philippines may progress from LTBI to TB at a higher rate than persons born elsewhere. A higher progression rate could be due to biological factors, such as the lineage of TB found in patients from the Philippines, or it could be due to behavioral and social factors, such as poorer control of DM, or some combination of factors. As described before, more than 80% of the TB in patients from the Philippines was due to M. tuberculosis strains from lineage 1, one of the ancient lineages, which have a distinctive inflammatory response [13] when compared with the other lineages frequently observed in San Francisco. We are unable to test the hypothesis that M. tuberculosis lineage may be a factor associated with TB and DM because lineage is so highly correlated with place of birth in our patients that we have no power to examine it. How the bacterial factors associate with the immunopathogenesis of TB and DM will be explored in further studies. A recent publication that included insured patients with diabetes type 2 from the San Francisco Bay Area showed that patients from the Philippines (and India) had significantly worse control measured by HbA1c value in spite of access to healthcare [21].
In contrast with several reports [5], [22], [23], [24], we did not find statistically significant differences in the outcome of TB treatment in patients with and without DM, probably due to a small sample size to detect differences. However we did see a trend of higher mortality, longer time to sputum conversion and longer treatment in patients with TB and DM compared with patients with TB and without DM. The level of glycemic control was not available to determine if this was associated with the outcome of TB treatment as reported in other studies [24].
Our study has the following limitations. First, our database did not include variables associated with the prevalence of DM such as body mass index and socioeconomic status. However, based on a recent study in San Francisco, the frequency of obesity is lower among the Asian population (7.1%) when compared with the other ethnic groups: 13.3% in Whites, 33.4% in African American and 56.9% in Latino [25]. Lower socioeconomic status has been also associated with DM [26], [27], [28] and TB [29], [30]. However, based on the San Francisco Department of Public Health data individuals born in the Philippines were less likely to belong to the lower socioeconomic status (defined by frequency of unemployment, poverty status, uninsured rates and public high school graduation) than Latino or Chinese populations [25]. Second, the definition of diabetes was based on self-report and the medical record, which may underestimate the actual prevalence of DM. However, a community-based study in the US demonstrated that the prevalence of self-reported diagnosis of DM was similar to self-reported DM combined with clinical and laboratory evaluations [31], and has shown to have reasonable validity for public health surveillance [32], [33]. Third, we did not have information about the degree to which patients with DM were controlling their DM.
Conclusions
The prevalence of DM in patients with TB has increased significantly in the past 7 years in San Francisco. Data suggest that among the different populations, Filipinos older than 45 years old were more likely to have TB probably due both to a higher prevalence of DM and to a higher probability of progressing from LTBI to TB. Therefore, in San Francisco, in spite of a relatively low incidence of TB, TB screening programs and preventive therapy should be considered for individuals with DM, specifically in patients more than 45 years old and especially in older patients from the Philippines. Although we did not find a negative outcome in patients with DM and TB, DM screening programs in patients with TB will be important. Prospective studies of DM among TB patients are needed to confirm this observation.
Acknowledgments
The authors would like to express their appreciation to the San Francisco Department of Public Health, Tuberculosis Control Section staff and the Mycobacteriology Section San Francisco Department of Public Health Laboratory, especially to Anna Babst, whose high quality of service and cooperation have made this work possible.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This work was supported by the National Institutes of Health http://www.nih.gov/ (NIH AI034238) and King Chulalongkorn Memorial Hospital, Thai Red Cross Society http://www.redcross.or.th/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.U.S. Department of Health and Human Services (2011) National Diabetes Statistics.
- 2.CDC (2013) Reported Tuberculosis in the United States, 2012. Atlanta, GA: Department of Health and Human Services, CDC.
- 3.World Health Organization (2011) Tuberculosis and Diabetes. Available: http://www.who.int/tb/publications/diabetes_tb.pdf. Accessed 2013 December.
- 4. Jeon CY, Murray MB (2008) Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 5:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, et al. (2011) The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med 9:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization (2011) Collaborative framework for care and control of tuberculosis and diabetes. WHO/HTM/TB/2011.15 WHO/HTM/TB/2011.15. [PubMed]
- 7. Kampoli AM, Tousoulis D, Briasoulis A, Latsios G, Papageorgiou N, et al. (2011) Potential pathogenic inflammatory mechanisms of endothelial dysfunction induced by type 2 diabetes mellitus. Curr Pharm Des 17:4147–4158. [DOI] [PubMed] [Google Scholar]
- 8.R Sicree JS, Zimmet P (2003) The Global Burden of Diabetes. In:Gan Deditor. second ed.
- 9.International Diabetes Federation (2012) Diabetes Atlas Update. fifth ed.
- 10. Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87:4–14. [DOI] [PubMed] [Google Scholar]
- 11. Suwanpimolkul G, Jarlsberg LG, Grinsdale JA, Osmond D, Kawamura LM, et al. (2013) Molecular epidemiology of tuberculosis in foreign-born persons living in San Francisco. Am J Respir Crit Care Med 187:998–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gagneux S, DeRiemer K, Van T, Kato-Maeda M, de Jong BC, et al. (2006) Variable host-pathogen compatibility in Mycobacterium tuberculosis . Proc Natl Acad Sci U S A 103:2869–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Portevin D, Gagneux S, Comas I, Young D (2011) Human macrophage responses to clinical isolates from the Mycobacterium tuberculosis complex discriminate between ancient and modern lineages. PLoS Pathog 7:e1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suwanpimolkul GJL, Osmond D, Grinsdale J, Higashi J, Hopewell PC, et al. (2013) Association Of Diabetes Mellitus And Tuberculosis In San Francisco. Am J Respir Crit Care Med: A1647.
- 15. Firdessa R, Berg S, Hailu E, Schelling E, Gumi B, et al. (2013) Mycobacterial lineages causing pulmonary and extrapulmonary tuberculosis, Ethiopia. Emerg Infect Dis 19:460–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(2000) Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med 161:1376–1395. [DOI] [PubMed] [Google Scholar]
- 17. American Thoracic Society, CDC, and Infectious Diseases Society of America (2003) Treatment of Tuberculosis MMWR Recomm Rep. 52:1–77. [PubMed] [Google Scholar]
- 18. World Health Organization (2011) Global tuberculosis control. WHO/HTM/TB/2011 (16):WHO/HTM/TB/2011.16. [Google Scholar]
- 19.Gary He AA, Karen Black, Susan Lopez-Payan, (2005) Diabetes in California Countries: Prevalence, Risk Factors and Resources. Available: http://www.caldiabetes.org/content_display.cfm?contentID=1160. Accessed 2014 January.
- 20.Guozhong He KB, Lopez-Payan S, Omark K, Schillinger D (2009) Diabetes in California Countries.
- 21. Holland AT, Zhao B, Wong EC, Choi SE, Wong ND, et al. (2013) Racial/ethnic differences in control of cardiovascular risk factors among type 2 diabetes patients in an insured, ambulatory care population. J Diabetes Complications 27:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alisjahbana B, Sahiratmadja E, Nelwan EJ, Purwa AM, Ahmad Y, et al. (2007) The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin Infect Dis 45:428–435. [DOI] [PubMed] [Google Scholar]
- 23. Dooley KE, Chaisson RE (2009) Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis 9:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park SW, Shin JW, Kim JY, Park IW, Choi BW, et al. (2012) The effect of diabetic control status on the clinical features of pulmonary tuberculosis. Eur J Clin Microbiol Infect Dis 31:1305–1310. [DOI] [PubMed] [Google Scholar]
- 25.The San Francisco Department of Public Health (2012) Community Health Status Assement: City and Country of San Francisco. Available: http://www.sfdph.org/dph/files/hc/HCAgen/HCAgen2012/Oct%2016/San%20Francisco%20CHSA_Final.pdf. Accessed 2014 February.
- 26. Connolly V, Unwin N, Sherriff P, Bilous R, Kelly W (2000) Diabetes prevalence and socioeconomic status: a population based study showing increased prevalence of type 2 diabetes mellitus in deprived areas. J Epidemiol Community Health 54:173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sims M, Diez Roux AV, Boykin S, Sarpong D, Gebreab SY, et al. (2011) The socioeconomic gradient of diabetes prevalence, awareness, treatment, and control among African Americans in the Jackson Heart Study. Ann Epidemiol 21:892–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Agardh E, Allebeck P, Hallqvist J, Moradi T, Sidorchuk A (2011) Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. Int J Epidemiol 40:804–818. [DOI] [PubMed] [Google Scholar]
- 29. Olson NA, Davidow AL, Winston CA, Chen MP, Gazmararian JA, et al. (2012) A national study of socioeconomic status and tuberculosis rates by country of birth, United States, 1996–2005. BMC Public Health 12:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oxlade O, Murray M (2012) Tuberculosis and poverty: why are the poor at greater risk in India? PLoS One 7:e47533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bays HE, Bazata DD, Clark NG, Gavin JR 3rd, Green AJ, et al. (2007) Prevalence of self-reported diagnosis of diabetes mellitus and associated risk factors in a national survey in the US population: SHIELD (Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes). BMC Public Health 7:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Margolis KL, Lihong Q, Brzyski R, Bonds DE, Howard BV, et al. (2008) Validity of diabetes self-reports in the Women's Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials 5:240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martin LM, Leff M, Calonge N, Garrett C, Nelson DE (2000) Validation of self-reported chronic conditions and health services in a managed care population. Am J Prev Med 18:215–218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.