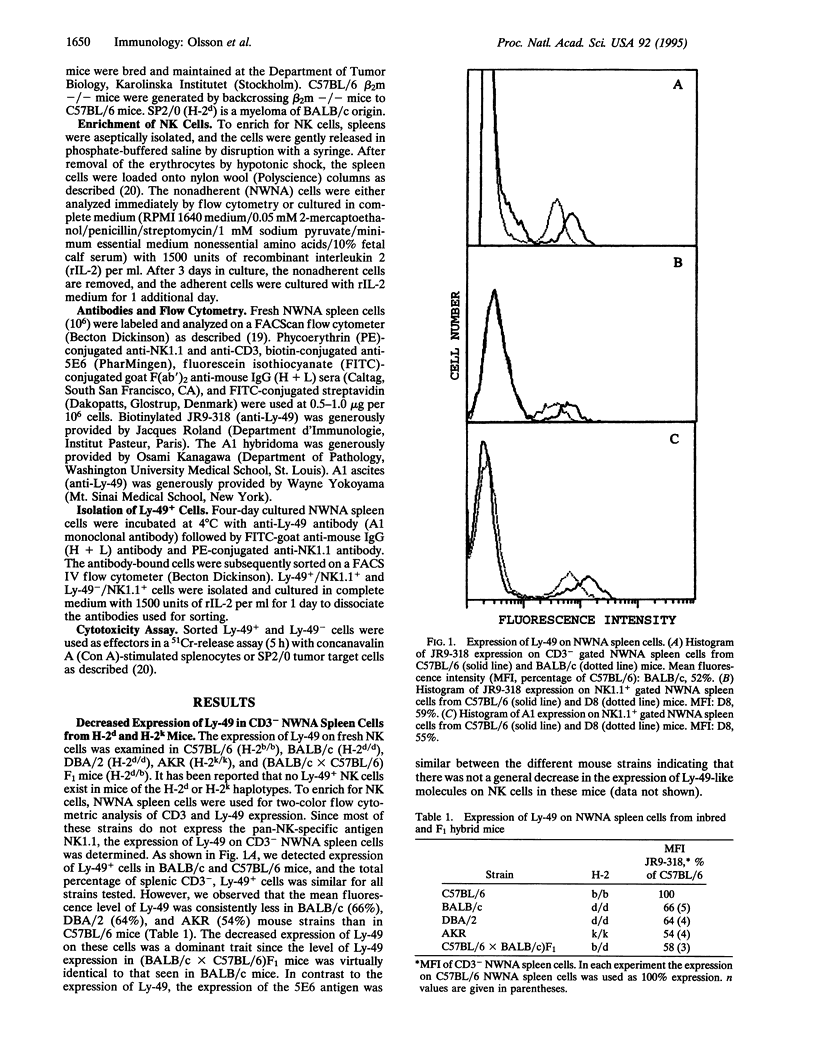
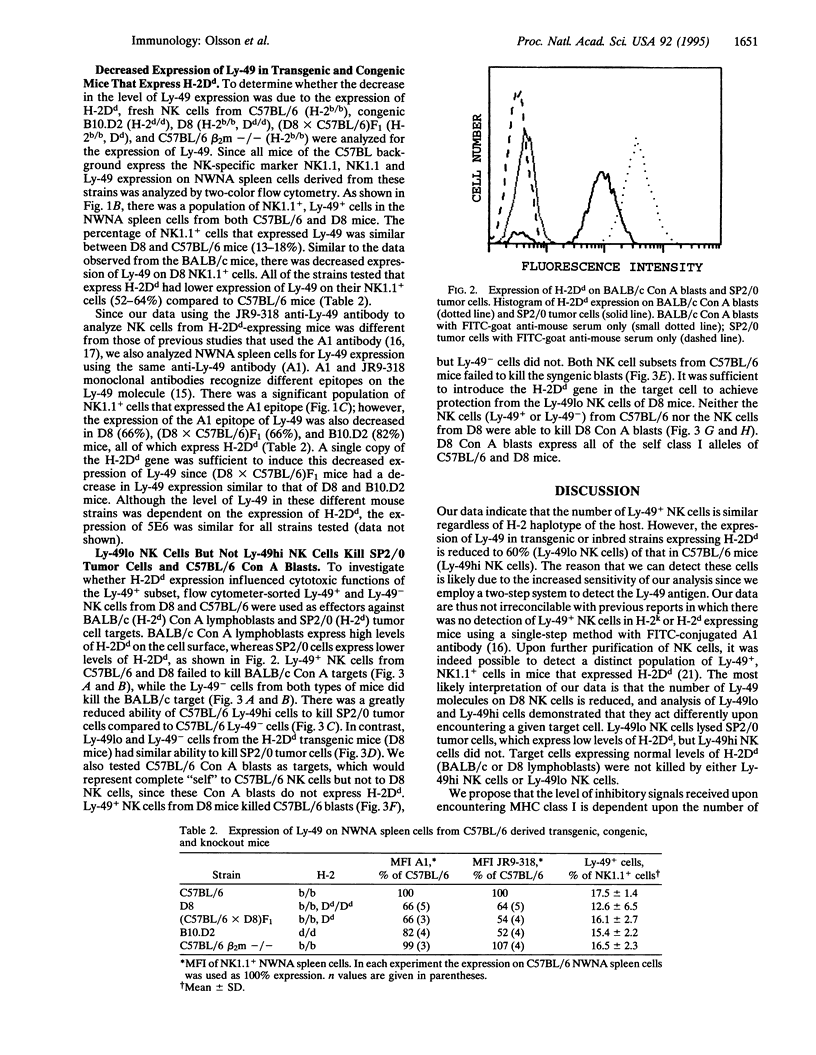
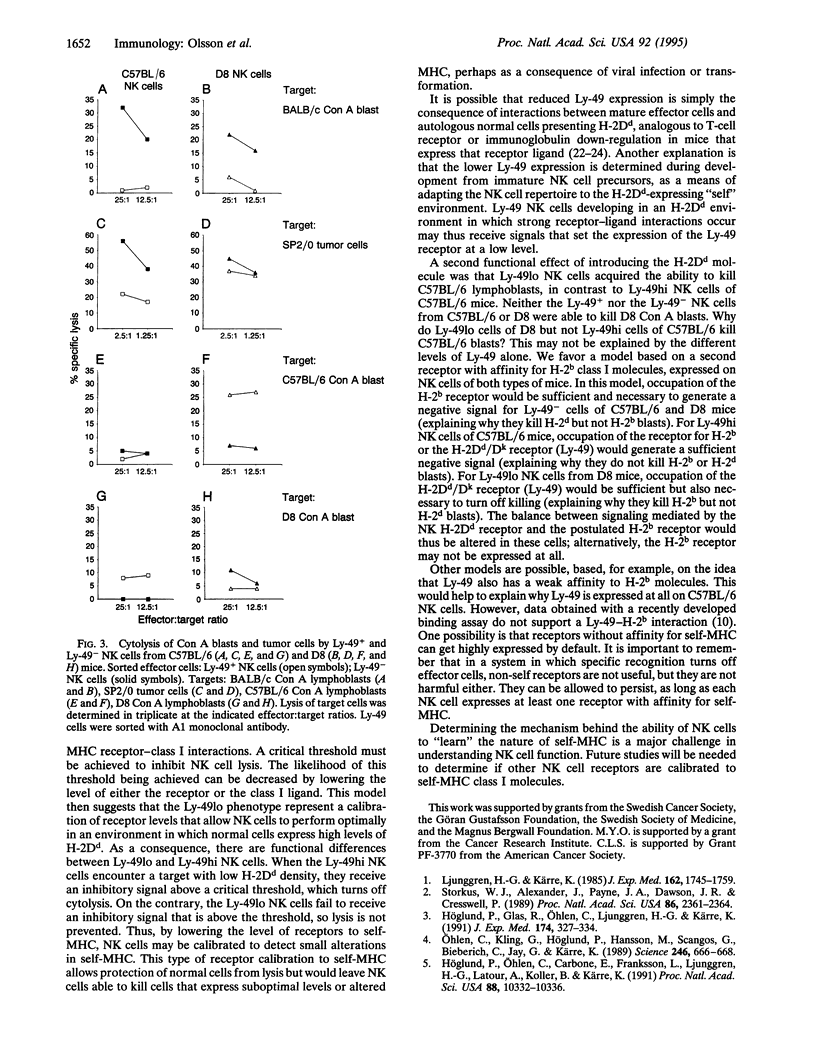
Abstract
The Ly-49 molecule has been shown to interact with major histocompatibility complex (MHC) class I molecules, and the lytic function of Ly-49+ natural killer (NK) cells from C57BL/6 (H-2b) mice is inhibited by the recognition of H-2Dd on tumor target cells. Introduction of a Ly-49 ligand, H-2Dd, into C57BL/6 mice did not alter the percentage of Ly-49+ NK cells (13-18%), but it led to three functional effects on this subset. (i) The Ly-49 expression in the positive population was reduced by 30-50% compared to C57BL/6 control mice. (ii) While this Ly-49+ subset (Ly-49lo) in the transgenic mice failed to kill BALB/c concanavalin A (Con A) blasts, which have high H-2Dd expression, it was capable of killing SP2/0 tumor cells, which have low H-2Dd expression. Ly-49+ NK cells (Ly-49hi) from nontransgenic mice failed to kill both of these H-2Dd-expressing target cells. (iii) In the transgenic mice, the Ly-49+ subset acquired the ability to kill C57BL/6 Con A blasts, in contrast to the Ly-49+ NK cells of C57BL/6 mice. We propose a "receptor-calibration" hypothesis, where low receptor density on the effector cells imposed by selection or adaptation to the environment allows higher sensitivity for detection of reduced self-MHC ligands on potential target cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daniels B. F., Karlhofer F. M., Seaman W. E., Yokoyama W. M. A natural killer cell receptor specific for a major histocompatibility complex class I molecule. J Exp Med. 1994 Aug 1;180(2):687–692. doi: 10.1084/jem.180.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow C. C., Crosbie J., Jorgensen H., Brink R. A., Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989 Nov 23;342(6248):385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- Höglund P., Glas R., Ohlén C., Ljunggren H. G., Kärre K. Alteration of the natural killer repertoire in H-2 transgenic mice: specificity of rapid lymphoma cell clearance determined by the H-2 phenotype of the target. J Exp Med. 1991 Aug 1;174(2):327–334. doi: 10.1084/jem.174.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglund P., Ohlén C., Carbone E., Franksson L., Ljunggren H. G., Latour A., Koller B., Kärre K. Recognition of beta 2-microglobulin-negative (beta 2m-) T-cell blasts by natural killer cells from normal but not from beta 2m- mice: nonresponsiveness controlled by beta 2m- bone marrow in chimeric mice. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10332–10336. doi: 10.1073/pnas.88.22.10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane K. P. Ly-49 mediates EL4 lymphoma adhesion to isolated class I major histocompatibility complex molecules. J Exp Med. 1994 Mar 1;179(3):1011–1015. doi: 10.1084/jem.179.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlhofer F. M., Hunziker R., Reichlin A., Margulies D. H., Yokoyama W. M. Host MHC class I molecules modulate in vivo expression of a NK cell receptor. J Immunol. 1994 Sep 15;153(6):2407–2416. [PubMed] [Google Scholar]
- Karlhofer F. M., Ribaudo R. K., Yokoyama W. M. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992 Jul 2;358(6381):66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- Liao N. S., Bix M., Zijlstra M., Jaenisch R., Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991 Jul 12;253(5016):199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Kärre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med. 1985 Dec 1;162(6):1745–1759. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren H. G., Kärre K. In search of the 'missing self': MHC molecules and NK cell recognition. Immunol Today. 1990 Jul;11(7):237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- Ohlén C., Kling G., Höglund P., Hansson M., Scangos G., Bieberich C., Jay G., Kärre K. Prevention of allogeneic bone marrow graft rejection by H-2 transgene in donor mice. Science. 1989 Nov 3;246(4930):666–668. doi: 10.1126/science.2814488. [DOI] [PubMed] [Google Scholar]
- Raulet D. H. Immunology. A sense of something missing. Nature. 1992 Jul 2;358(6381):21–22. doi: 10.1038/358021a0. [DOI] [PubMed] [Google Scholar]
- Rocha B., von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991 Mar 8;251(4998):1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- Roland J., Cazenave P. A. Ly-49 antigen defines an alpha beta TCR population in i-IEL with an extrathymic maturation. Int Immunol. 1992 Jun;4(6):699–706. doi: 10.1093/intimm/4.6.699. [DOI] [PubMed] [Google Scholar]
- Schönrich G., Kalinke U., Momburg F., Malissen M., Schmitt-Verhulst A. M., Malissen B., Hämmerling G. J., Arnold B. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell. 1991 Apr 19;65(2):293–304. doi: 10.1016/0092-8674(91)90163-s. [DOI] [PubMed] [Google Scholar]
- Sentman C. L., Hackett J., Jr, Kumar V., Bennett M. Identification of a subset of murine natural killer cells that mediates rejection of Hh-1d but not Hh-1b bone marrow grafts. J Exp Med. 1989 Jul 1;170(1):191–202. doi: 10.1084/jem.170.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentman C. L., Kumar V., Bennett M. Rejection of bone marrow cell allografts by natural killer cell subsets: 5E6+ cell specificity for Hh-1 determinant 2 shared by H-2d and H-2f. Eur J Immunol. 1991 Nov;21(11):2821–2828. doi: 10.1002/eji.1830211125. [DOI] [PubMed] [Google Scholar]
- Storkus W. J., Alexander J., Payne J. A., Dawson J. R., Cresswell P. Reversal of natural killing susceptibility in target cells expressing transfected class I HLA genes. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2361–2364. doi: 10.1073/pnas.86.7.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes M., Harty M. W., Karlhofer F. M., Pearson D. A., Szot G., Yokoyama W. Hematopoietic cells and radioresistant host elements influence natural killer cell differentiation. J Exp Med. 1993 Jul 1;178(1):223–229. doi: 10.1084/jem.178.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama W. M., Seaman W. E. The Ly-49 and NKR-P1 gene families encoding lectin-like receptors on natural killer cells: the NK gene complex. Annu Rev Immunol. 1993;11:613–635. doi: 10.1146/annurev.iy.11.040193.003145. [DOI] [PubMed] [Google Scholar]



