Abstract
A major frontier in global change research is predicting how multiple agents of global change will alter plant productivity, a critical component of the carbon cycle. Recent research has shown that plant responses to climate change are phylogenetically conserved such that species within some lineages are more productive than those within other lineages in changing environments. However, it remains unclear how phylogenetic patterns in plant responses to changing abiotic conditions may be altered by another agent of global change, the introduction of non-native species. Using a system of 28 native Tasmanian Eucalyptus species belonging to two subgenera, Symphyomyrtus and Eucalyptus, we hypothesized that productivity responses to abiotic agents of global change (elevated CO2 and increased soil N) are unique to lineages, but that novel interactions with a non-native species mediate these responses. We tested this hypothesis by examining productivity of 1) native species monocultures and 2) mixtures of native species with an introduced hardwood plantation species, Eucalyptus nitens, to experimentally manipulated soil N and atmospheric CO2. Consistent with past research, we found that N limits productivity overall, especially in elevated CO2 conditions. However, monocultures of species within the Symphyomyrtus subgenus showed the strongest response to N (gained 127% more total biomass) in elevated CO2 conditions, whereas those within the Eucalyptus subgenus did not respond to N. Root:shoot ratio (an indicator of resource use) was on average greater in species pairs containing Symphyomyrtus species, suggesting that functional traits important for resource uptake are phylogenetically conserved and explaining the phylogenetic pattern in plant response to changing environmental conditions. Yet, native species mixtures with E. nitens exhibited responses to CO2 and N that differed from those of monocultures, supporting our hypothesis and highlighting that both plant evolutionary history and introduced species will shape community productivity in a changing world.
Introduction
Several analyses of primary productivity at community, biome and global levels have indicated that soil nitrogen (N) generally limits carbon sequestration, but have failed to address whether individual species respond differently to increased soil N [1]–[4]. In contrast to the implied paradigm that all plants should produce more biomass in response to increased soil N, a growing body of research shows that not all species respond positively, or even at all, to increased soil N, especially in elevated CO2 conditions [5]–[10]. This is likely because plants have evolved different capacities to compete for soil resources [11]. For example, a species whose traits reflect an evolved ability to strongly compete for soil N would accumulate more biomass in response to increases in soil N, thereafter increasing in dominance over species whose traits reflect an evolved tradeoff to compete along some other niche axis [8]. Ultimately, anthropogenically increased levels of atmospheric CO2 and soil N will alter species abundance, composition and diversity, which will in turn impact many important ecosystem processes and functions [7], [12], [13]. Understanding how past evolution and contemporary biotic interactions shape plant species responses to environmental change could provide key insight into how plant diversity and function may be altered in global change scenarios.
Phylogenetic information can be used to explain patterns in species responses to global change, which in turn can explain the past evolution of species niche spaces [14], [15]. Several studies have shown that more phylogenetically diverse plant communities produce more biomass on average, indicating that species niche spaces (and, by association, traits for resource acquisition and processing) are more similar among species with more shared evolutionary history than those with less shared evolutionary history [16]–[18]. These studies provide strong evidence that niches of closely related species are phylogenetically preserved (i.e., exhibit phylogenetic niche conservatism [PNC]), reflecting ancestral traits that through various evolutionary processes [15], [19] have changed little through evolutionary time [20], [21]. Empirical evidence confirms that leaf and root traits, which are important indicators of resource uptake capacity and competitive ability, are phylogenetically conserved within, and are unique to, plant lineages [22], [23]. If this pattern is consistent across plant lineages, we should expect that more closely related plant species would respond more similarly to environmental change. In support of this prediction, Davis et al. (2010) have found that species within certain angiosperm lineages flower earlier in the year in response to a warming climate and may thus be more favored than other lineages as global temperatures rise [24]. Although PNC is certainly not universal [21], it may be strong enough in specific plant traits and lineages such that evolutionary history alone can sufficiently explain patterns in plant species productivity, especially in the context of global change [14], [15].
Despite evidence that evolutionary history can be used to predict patterns of plant responses to globally altered abiotic conditions [14], [24], it remains unclear how community responses to environmental change may be altered by introductions of non-native species [25]. A warming climate and altered soil nutrient levels will have variable effects on the productivity of plant species depending on the axes along which respective species are specialized [5], [11], as well as open niche spaces that can be filled by non-native species [26]. In particular, increased soil N can facilitate the establishment and proliferation of fast growing, nitrophilic non-natives at the expense of slow-growing natives whose capacities for N uptake and processing are more limited (i.e., whose growth strategies are more conservative) [7], [27]. Once established in a new range, non-native species can create plant-soil feedbacks that increase [28] or decrease [29] native species productivity. The consequences of these feedbacks have been shown to be greater in situations where native and introduced species share less evolutionary history (i.e., the interaction is more ecologically ‘novel’) [30], [31]. Thus, introduced species should have different influences on communities with different evolutionary backgrounds. Amidst increasing rates of non-native species across the globe [32], understanding the interacting effects of abiotic changes and species introductions on native plant productivity represents a critical challenge to address in global change research.
To determine how evolutionary history and novel biotic interactions shape plant responses to abiotic agents of global change, we analyzed biomass production of eucalypt species monocultures and mixtures with an introduced species across elevated CO2 and increased soil N conditions. Specifically, we used 28 Eucalyptus species in two subgenera, Eucalyptus and Symphyomyrtus, that are native to the island state of Tasmania, Australia, where they co-dominate sub-alpine to coastal forest and shrubland ecosystems [33]. Despite their ecological and economic prominence in Australia, the magnitude and direction in which agents of global change alter the ecology of these species has scarcely been investigated (but see [34]), thus representing an ideal system for better understanding plant responses to global change. Using subgenus as an indicator of evolutionary history, we hypothesized that more closely related species would share more similar productivity responses to experimentally manipulated atmospheric CO2 and soil N, as research shows that species with greater shared evolutionary history have more similar resource acquisition traits [22], [23], but that novel biotic interactions would alter evolutionarily-based plant productivity responses to changing abiotic conditions. Subgeneric differences in productivity responses to abiotic global change factors would suggest that species within the same lineage have inherited similar traits for resource use (i.e., support for PNC), which should be confirmed by subgeneric differences in resource-use traits (e.g., ratio of root to shoot biomass). Further, effects of species interactions on responses to abiotic changes would indicate that biotic agents of global change can also mediate patterns of plant productivity.
Methods
We used a design whereby two individuals were planted per pot, and pairs were composed of two same-species native individuals or one native individual with one introduced, non-native individual of Eucalyptus nitens. Plants were grown in a greenhouse setting (alternating high and low CO2 conditions weekly) and treated with factorial combinations of elevated vs. ambient atmospheric CO2 and high vs. low soil N. After six months of growth, plants were harvested for biomass. We analyzed patterns in total, aboveground and belowground biomass production, as well as the ratio of root to shoot biomass, as these reflect the evolutionary basis of resource acquisition strategy and ecosystem functioning.
Focal species
To determine independent and interactive effects of three global change factors (CO2, N and novel species interaction) and phylogenetic relatedness on plant productivity, we used 28 of the 30 species within the genus Eucalyptus (family Myrtaceae) that are native to Tasmania. Native individuals were paired with either a conspecific or the tree species E. nitens, which is non-native to Tasmania, and grown in varying atmospheric CO2 and soil N concentrations. The native species have been phylogenetically and morphologically separated into two subgenera: Eucalyptus (E. amygdalina, E. coccifera, E. delegatensis, E. nitidia, E. obliqua, E. pauciflora, E pulchella, E. radiata, E. regnans, E. risdonii, E. sieberi, and E. tenuiramis) and Symphyomyrtus (E. barberi, E. brookeriana, E. cordata, E. dalrympleana, E. globulus, E. gunii, E. johnstonii, E. morrisbyi, E. ovata, E. perriniana, E. rodwayi, E. rubidia, E. subcrenulata, E. urnigera, E. vernicosa and E. viminalis) [35]–[37]. Although the species used in this experiment are not representative of all species within the Symphyomyrtus and Eucalyptus subgenera (which consist of over 400 and 100 species, respectively, that inhabit Australia and exhibit overlapping ranges of nutrient uptake and growth) [38], they represent a group of sympatric species that have evolved in similar environments. Further, differences in stoichiometry, physiology, and growth strategy (e.g., available foliar N, stem volume, and biomass production) between Symphyomyrtus and Eucalyptus subgenera [39]–[41] indicate phylogenetic conservatism of resource use strategy within each subgenus. Correspondingly, recent research has shown that responses to environmental change are phylogenetically conserved such that Symphyomyrtus species tend to gain more biomass in response to increased soil N and elevated CO2 than species within the subgenus Eucalyptus [42], [43]. Eucalyptus nitens, a species within the Symphyomyrtus subgenus that is native to mainland Australia but not to Tasmania, is gradually becoming more common in Tasmania via hardwood plantations. As shown in other commercially important tree species [44], E. nitens holds the potential to disperse into native stands and alter productivity and composition of native plant communities [45]. In this system and multiple others, it remains unclear how the expected doubling of global terrestrial N deposition and nutrient eutrophication in the next half century [7], [46], [47], combined with dependence of the hardwood N fertilization [48], rapidly rising atmospheric CO2 levels [49], and the introduction of non-native species, will interact to alter the composition and function of native plant communities.
Experimental Design
Seed of 26 native Tasmanian eucalypt species was purchased from Forestry Tasmania (http://www.forestrytas.com.au/) (as such, no specific permits were required and no endangered or protected species were used). Seeds were vernalized by folding approximately a tablespoon of seed in a paper towel, soaking overnight in water plus a drop of dishwashing liquid (which acts as a surfactant and facilitates adhesion of water to seeds; Mason and Miller 1991), and refrigerating for 30 days at 4°C. Seeds were then sown into a commercial potting mix, with added macro- and micro-nutrients from Nutricote Grey (Langley Australia Pty Ltd., Welshpool, WA) at a concentration of approximately 3 kg/m3 (N∶P∶K ratio of 19∶2.6∶10) and covered with a layer of vermiculite (for water retention). After three weeks, 12 similar-sized seedlings of each species were placed into four treatments, which consisted of factorial combinations of ambient vs. elevated CO2 (420 ppm vs. 700 ppm) and low vs. high soil N (3 kg/ha/mo vs. 30 kg/ha/mo, applied as urea). The elevated CO2 and high N treatments represent levels likely to be reached by the end of the century, although increases in soil N will be spatially heterogeneous [46], [49]. We were confident that soil N concentrations in the greenhouse reflected those in the field, as plant N concentrations of species grown in a greenhouse are comparable to those of the same species grown in the field given constant fertilization regimes [50], [51]. Six of the individuals within each treatment were planted with an individual of the same species (i.e., monocultures) and the other six were planted with an E. nitens individual (i.e., mixtures). The elevated CO2 treatments were created in two greenhouse chambers: in one chamber CO2 was kept at an ambient level and in the other CO2 was elevated using compressed CO2 and a CO2 control unit (Thermoline Scientific equipment, Smithfield, Australia). To avoid greenhouse effects (pseudoreplication), the CO2 levels and their respective seedlings were exchanged between two chambers each week, and the CO2 concentrations were monitored bi-weekly with an infra-red gas analyzer (LiCor 6200, LiCor Inc., Lincoln, NE, USA). Pots were also randomly repositioned each week to avoid positional effects in the greenhouse. After six months of growth, and watering as needed, individuals from each pot were harvested and separated into aboveground and belowground biomass. Aboveground sections were weighed after 48 hours of oven-drying at 60°C, while belowground sections were weighed after careful rinsing over 2 and 0.5 mm sieves (to remove soil and retain fine root biomass) and 48 hours of oven-drying at 60°C.
Statistical Analyses
To test our hypothesis that evolutionary history and novel biotic interactions would explain patterns in plant productivity responses to abiotic agents of global change, we analyzed whole-pot biomass (total, aboveground and belowground) and root∶shoot ratios of native species monocultures and mixtures with E. nitens using mixed effects models implemented in R (version 3.1.1) [52]. These models included cube root transformed biomass and root∶shoot measurements (averaged for each species within each CO2, N, and species pair type treatment combination) as dependent variables, and CO2, soil N, species pair type (i.e., native species monoculture or mixture with E. nitens), and native species subgenus as independent variables. Species was included as a blocking factor. Analysis of Variance (ANOVA) tables were calculated using marginal sums of squares, with significance assessed using Wald χ2 statistics. Because a resolved phylogenetic reconstruction of these species is not currently available [36], [37], we were unable to use phylogenetic comparative methods to address our hypothesis. Pairs in which one or both individuals died were excluded from these analyses (N = 354 total species pairs after exclusion and 190 observations after averaging across species and treatment combinations). Additionally, to quantify the magnitude of the effects of CO2 and soil N on productivity across native species evolutionary histories and biotic interactions, we calculated z-transformed effect sizes using native species-based differences in whole-pot total biomass between elevated and ambient CO2 and between high and low N. Treatment effect sizes were calculated for each subgenus (i.e., Eucalyptus and Symphyomyrtus) and species pair type (i.e., native species monocultures and mixtures with E. nitens).
Results
Consistent with our hypothesis that plant productivity responses to abiotic agents of global change are contingent upon both evolutionary history and novel species interactions, full models of total and aboveground biomass identified a significant interaction among atmospheric CO2, soil N, species pair type, and subgenus (χ2 = 4.2151, p = 0.040; χ2 = 4.1311, p = 0.042) ( Table 1 ; Fig. 1 ). Because of this interaction, we were unable to interpret single or two-way interactive effects identified by the models [53], [54]. Thus, we ran separate models for each subgenus that included fixed effects of atmospheric CO2, soil N, and species pair type, with species as a blocking factor. If we found significant interactions among factors, we ran subsequent models to better interpret the main effects (see Tables S1 and S2).
Table 1. Linear mixed effects model results of eucalypt productivity (total, aboveground and belowground; TB, AGB, and BGB, respectively) and biomass allocation (root to shoot ratio; R∶S) across CO2, soil N, species pair type (monoculture vs. mixture with the non-native E. nitens) treatments and native species subgenus (N = 190).
| Variable | |||||||||
| δTB | AGB | BGB | R∶S | ||||||
| Treatment | Df | Chisq | p | Chisq | p | Chisq | p | Chisq | p |
| S | 1 | 2.63 | 0.105 | 2.458 | 0.117 | 3.42 | 0.064 | 3.78 | 0.052 |
| M | 1 | 19.995 | 1*10−5 | 19.428 | 1*10−5 | 17.966 | 2*10−5 | 2.851 | 0.091 |
| C | 1 | 2.191 | 0.139 | 1.78 | 0.182 | 3.518 | 0.061 | 1.789 | 0.181 |
| N | 1 | 14.749 | 1.2*10−4 | 15.782 | 7*10−5 | 7.952 | 0.005 | 0.141 | 0.707 |
| S*M | 1 | 1.377 | 0.241 | 1.102 | 0.294 | 1.686 | 0.194 | 0.084 | 0.773 |
| S*C | 1 | 0.205 | 0.65 | 0.151 | 0.698 | 0.494 | 0.482 | 0.358 | 0.55 |
| M*C | 1 | 5.207 | 0.022 | 5.722 | 0.017 | 3.375 | 0.066 | 0.616 | 0.433 |
| S*N | 1 | 9.377 | 0.002 | 10.188 | 0.001 | 5.427 | 0.02 | 0.071 | 0.79 |
| M*N | 1 | 2.4*10−4 | 0.988 | 0.002 | 0.965 | 9*10−5 | 0.992 | 0.013 | 0.908 |
| C*N | 1 | 5.513 | 0.019 | 5.234 | 0.022 | 4.803 | 0.028 | 0.915 | 0.339 |
| S*M*C | 1 | 0.781 | 0.377 | 0.813 | 0.367 | 0.533 | 0.465 | 0.125 | 0.724 |
| S*M*N | 1 | 0.075 | 0.785 | 0.115 | 0.734 | 0.006 | 0.939 | 0.005 | 0.945 |
| S*C*N | 1 | 0.284 | 0.594 | 0.384 | 0.536 | 0.015 | 0.904 | 0.878 | 0.349 |
| M*C*N | 1 | 0.719 | 0.396 | 0.869 | 0.351 | 0.169 | 0.681 | 0.296 | 0.587 |
| S*M*C*N | 1 | 4.215 | 0.04 | 4.131 | 0.042 | 3.364 | 0.067 | 0.785 | 0.376 |
In a greenhouse experiment, 28 native Tasmanian eucalypt species within two subgenera (S), Symphyomyrtus and Eucalyptus, were treated with factorial combinations of ambient or elevated CO2 (C; 420 or 700 ppm, respectively) and low or high soil N (N; 3 or 30 kg/ha/mo), and paired with a conspecific or a non-native (E. nitens) individual (M). In these models, whole-pot biomass measurements and ratios of root to shoot biomass were averaged for each native species in each treatment combination, cube root transformed, and blocked by species. P values are shown in bold and are significant at α≤0.05.
TB, total biomass; AGB, aboveground biomass; BGB, belowground biomass; R∶S, root to shoot ratio; M, species pair type (native species monoculture vs. mixture with E. nitens); C, CO2 treatment (420 or 700 ppm); N, nitrogen treatment (3 or 30 kg ha−1 mo−1).
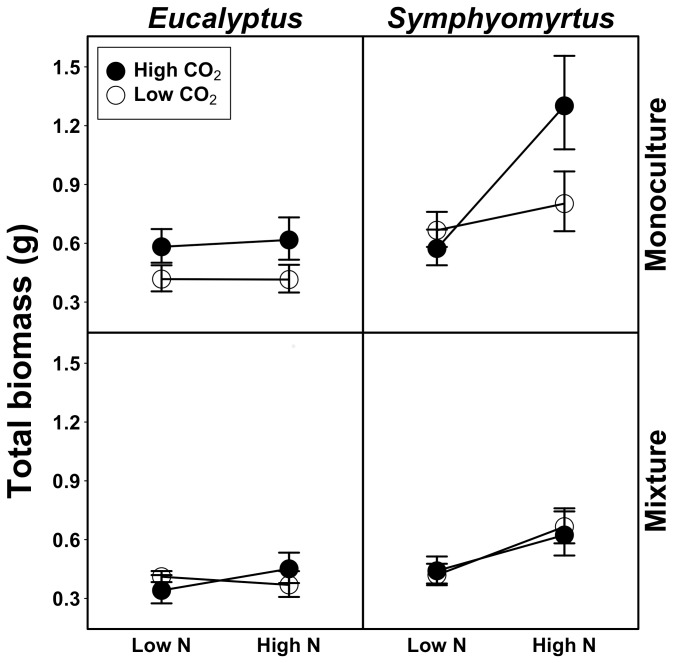
Figure 1. Productivity responses to global change scenarios are contingent upon species evolutionary history and novel biotic interactions.
Overall, monocultures (pairs of conspecific individuals) of species in the subgenus Symphyomyrtus (top right panel) in elevated CO2 conditions exhibit the strongest responses to N. On average, these monocultures produce 126% more biomass than all other species pairs in high N and elevated CO2 treatments (1.301±0.205 g and 0.576±0.061 g, respectively). Above- and belowground biomass follow similar patterns. Error bars represent ±1 SEM.
In species pairs containing subgenus Eucalyptus species (N = 82), we identified a significant interaction between species pair type and CO2 for total and aboveground biomass (χ2 = 4.2141, p = 0.040; χ2 = 4.3951, p = 0.036) ( Table 2 ). Subsequent models showed that from ambient to elevated CO2 conditions, monocultures (N = 45) gained 44, 40, and 68% more total, aboveground, and belowground biomass in response to elevated CO2 (χ2 = 5.4371, p = 0.020; χ2 = 6.3971, p = 0.011; χ2 = 5.1531, p = 0.023), whereas the biomass of mixtures (N = 37) on average did not differ between ambient and elevated CO2 conditions (χ2 = 0.1341, p = 0.714; χ2 = 0.0111, p = 0. 915; χ2 = 0. 1341, p = 0. 714) (Table S1). Moreover, we found a marginally significant interaction between CO2 and N in total and belowground biomass of mixtures (χ2 = 2.640, p = 0.104; χ2 = 3.6071, p = 0.058) (Table S1). Subsequent models showed that total and belowground biomass increased by 32 and 34% in response to soil N in elevated CO2 conditions (N = 18) (χ2 = 2.5801, p = 0.108), but decreased by 10 and 28% in response to soil N in ambient CO2 conditions (N = 19) (χ2 = 0.9981, p = 0.318) (Table S2; Fig. 1). These results indicate that CO2 stimulates growth of monocultures regardless of soil N levels, yet in mixtures CO2 stimulates growth when soil N is abundant and has a negative effect on growth when soil N is limiting.
Table 2. Linear mixed effects model results of subgenus-level eucalypt productivity (total, aboveground and belowground; TB, AGB, and BGB, respectively) and biomass allocation (root to shoot ratio; R∶S) across CO2, soil N, and species pair type (monoculture vs. mixture with the non-native E. nitens).
| Variable | ||||||||||
| δTB | AGB | BGB | R∶S | |||||||
| Treatment | Df | Chisq | p | Chisq | p | Chisq | p | Chisq | p | |
| Eucalyptus N = 82 | M | 1 | 3.808 | 0.051 | 3.821 | 0.051 | 3.318 | 0.069 | 0.71 | 0.399 |
| C | 1 | 2.136 | 0.144 | 1.686 | 0.194 | 3.845 | 0.05 | 1.762 | 0.184 | |
| N | 1 | 0.048 | 0.827 | 0.042 | 0.838 | 0.015 | 0.902 | 0.174 | 0.677 | |
| M*C | 1 | 4.214 | 0.04 | 4.395 | 0.036 | 2.897 | 0.089 | 0.337 | 0.561 | |
| M*N | 1 | 0.046 | 0.83 | 0.059 | 0.808 | 0.003 | 0.96 | 0.028 | 0.868 | |
| C*N | 1 | 1.309 | 0.253 | 1.051 | 0.305 | 1.939 | 0.164 | 1.509 | 0.219 | |
| M*C*N | 1 | 0.92 | 0.338 | 0.767 | 0.381 | 1.23 | 0.267 | 0.816 | 0.366 | |
| Symphyomyrtus N = 108 | M | 1 | 18.795 | 1.46*10−5 | 18.299 | 1.89*10−5 | 16.846 | 4.05*10−5 | 2.761 | 0.097 |
| C | 1 | 0.467 | 0.494 | 0.384 | 0.536 | 0.678 | 0.41 | 0.391 | 0.532 | |
| N | 1 | 26.338 | 2.87*10−7 | 29.428 | 5.8*10−8 | 13.666 | 2.18*10−4 | 0.003 | 0.958 | |
| M*C | 1 | 1.414 | 0.234 | 1.653 | 0.198 | 0.841 | 0.359 | 0.162 | 0.688 | |
| M*N | 1 | 0.026 | 0.872 | 0.055 | 0.815 | 0.009 | 0.924 | 0.008 | 0.927 | |
| C*N | 1 | 4.725 | 0.03 | 4.941 | 0.026 | 2.994 | 0.084 | 0.018 | 0.894 | |
| M*C*N | 1 | 4.136 | 0.042 | 4.5 | 0.034 | 2.242 | 0.134 | 0.028 | 0.867 | |
In a greenhouse experiment, 28 native Tasmanian eucalypt species within two subgenera (S), Symphyomyrtus and Eucalyptus, were treated with factorial combinations of ambient or elevated CO2 (C; 420 or 700 ppm, respectively) and low or high soil N (N; 3 or 30 kg/ha/mo), and paired with a conspecific or a non-native (E. nitens) individual (M). In these models, whole-pot biomass measurements and ratios of root to shoot biomass were averaged for each native species in each treatment combination, cube root transformed, and blocked by species. P values are shown in bold and are significant at α≤0.05.
TB, total biomass; AGB, aboveground biomass; BGB, belowground biomass; R∶S, root to shoot ratio; M, species pair type (native species monoculture vs. mixture with E. nitens); C, CO2 treatment (420 or 700 ppm); N, nitrogen treatment (3 or 30 kg ha−1 mo−1).
In contrast, analysis of species pairs containing subgenus Symphyomyrtus species (N = 108) revealed a significant interaction among CO2, N, and species pair type in total and aboveground biomass models (χ2 = 4.1361, p = 0.042; χ2 = 4.51, p = 0.034) (Table 2). Subsequent models of response to CO2 and N for each species pair type showed a significant interaction of CO2 and N in total, aboveground, and belowground biomass in monocultures (N = 53) (χ2 = 7.2731, p = 0.007; χ2 = 4.2751, p = 0.039; χ2 = 7.8211, p = 0.005), but not in mixtures (N = 55) (χ2 = 0.0171, p = 0.897; χ2 = 0.0751, p = 0.785; χ2 = 0.0061, p = 0.940) (Table S1). Whereas mixtures responded positively to N in ambient and elevated CO2 conditions (gaining 58 and 41% more total biomass, respectively) (χ2 = 6.3471, p = 0.012; χ2 = 14.2541, p = 1.60*10−4), monocultures did not respond to N in ambient CO2 conditions (χ2 = 0.0611, p = 0.805), but gained 127% more total biomass in response to N in elevated CO2 conditions (χ2 = 32.6711, p = 1.09*10−8) (Table S2; Fig. 1). Overall, monocultures of species in the subgenus Symphyomyrtus receiving high N and elevated CO2 treatments produced 126% more biomass than all other species pairs receiving the same N and CO2 treatments (1.301±0.205 g and 0.576±0.061 g, respectively; Fig. 1). These results suggest that monocultures are strongly limited by N when CO2 levels are high, but not when CO2 levels are low; on the other hand, N strongly limits mixtures regardless of CO2 levels.
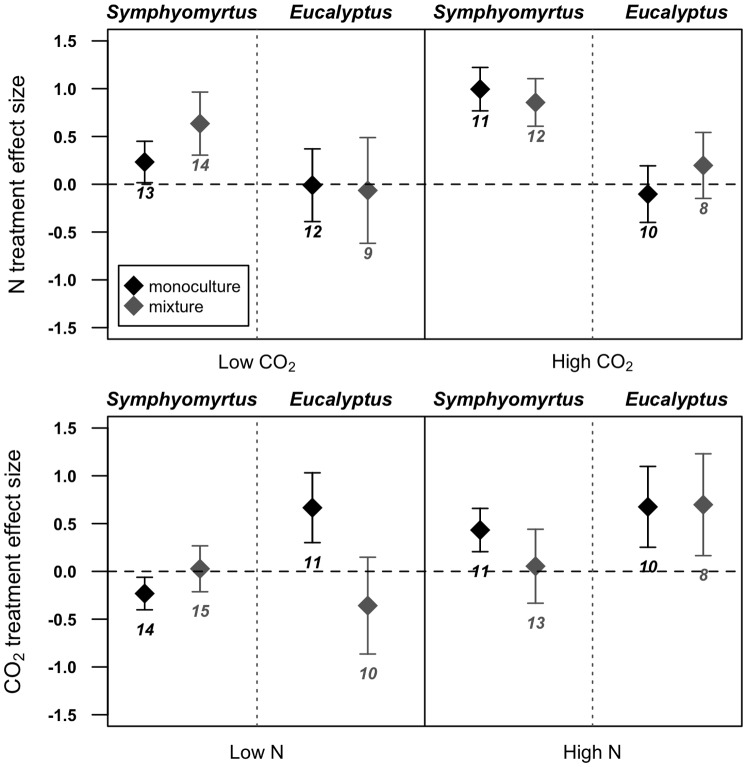
Treatment effect sizes of increased elevated CO2 and soil N ( Fig. 2 ) broadly support results of our ANOVAs. The effect of elevated CO2 was positive for subgenus Eucalyptus monocultures in both low and high soil N levels, but was positive for subgenus Eucalyptus mixtures only in high soil N. Alternatively, elevated CO2 had a opposite effects (positive and negative, respectively) on Symphyomyrtus monocultures in high and low soil N conditions, and had no effect on Symphyomyrtus mixtures with E. nitens. Neither monocultures nor mixtures of species within the subgenus Eucalyptus responded significantly positively to increased soil N in elevated or ambient CO2 conditions (although mixtures exhibited slightly higher responses to N in high CO2 conditions), whereas those within the subgenus Symphyomyrtus did (although monocultures responded less positively to N in ambient compared to elevated CO2 conditions). These differences in response to CO2 and N between native species subgenera and between monocultures and mixtures with the introduced E. nitens support our hypothesis that both native species evolutionary history and novel interactions between native and introduced individuals shape plant responses to abiotic agents of global change.
Figure 2. Effect sizes (standardized z-scores) of species total biomass responses to added soil N (30 kg/ha/month; upper panel) and elevated CO2 (700 ppm; lower panel) for native species monocultures (black) and mixtures with the non-native E. nitens (gray).
Error bars represent ±1 SEM.
Subgeneric differences in plant responses to increased soil N and elevated CO2 suggest that species within the same lineage have inherited similar traits for resource use. In support of this, we identified a marginally significant effect of subgenus on ratios root to shoot biomass in our full model (χ2 = 3.781, p = 0.052) (Table 1). Specifically, we found that species pairs containing individuals in the Symphyomyrtus subgenus had an average root to shoot ratio that was 16% greater than species pairs containing individuals in the Eucalyptus subgenus (0.182±0.009 and 0.158±0.007, respectively). This result indicates that greater allocation towards belowground biomass in species within the Symphyomyrtus subgenus could be driving greater overall responses to increased soil N than species within the Eucalyptus subgenus; alternatively, greater allocation towards aboveground biomass in species within the Eucalyptus subgenus could be driving greater overall responses to elevated CO2 than species within the Symphyomyrtus subgenus.
Discussion
The aim of this study was to address whether evolutionary history can explain responses to global change, and whether novel biotic interactions have the potential to alter evolutionarily based responses to abiotic global change factors. Drawing upon the concept of phylogenetic niche conservatism, wherein closely related species inherit the traits of their ancestors and thus occupy similar niches [20], [21], we addressed whether separate lineages respond differently to elevated atmospheric CO2 and increased soil N. Further, as introduced species have been shown to alter plant function in changing environments [28], [29], we examined how plant responses to elevated CO2 and N fertilization shift with the introduction of non-native individuals. We found that N addition increases overall productivity, especially in elevated CO2 conditions [1]–[4] (Table 1). However, further analysis showed that plant lineages respond differently to CO2 and N [8], [17], [18], [24], [42], [55] across monocultures and mixtures with an introduced species (Figs. 1 and 2). Our analysis of root∶shoot ratio across lineages indicate that this trait, and probably others important for resource acquisition and competitive ability, are evolutionarily conserved, which could explain why plant response to CO2 and N are contingent upon plant evolutionary background. We conclude that evolutionary history can be useful in predicting which species may be more productive with anthropogenically-driven environmental changes, but novel biotic interactions have the potential to alter these patterns. Ultimately, our results provide strong evidence that novel biotic interactions may drive unexpected patterns in carbon sequestration, and likely other critical ecosystem processes and functions, as environments change globally.
Responses to global change through a phylogenetic lens
Consistent with previous studies, we found a strong connection between evolutionary history and response to altered abiotic conditions [14],[24],[42], suggesting that plant ecological niche space can be predicted by species phylogenetic relatedness. In their natural environments, Tasmanian eucalypt species within the Symphyomyrtus and Eucalyptus subgenera tend to co-dominate natural eucalypt stands [41], niche partitioning is a likely mechanism that has driven observed phylogenetic effects on their productivity in global change scenarios. Specifically, competitive interactions among species in the same subgenus and facilitative interactions among species in separate subgenera could have maintained unique traits such that co-occurring species would not compete for the same resources [55]. We have found that allocation of biomass above- versus belowground is an evolutionarily conserved trait that could be a key indicator of niche partitioning and response to above- and belowground environmental change. Overall, our work emphasizes that plant species responses to global change are not idiosyncratic but largely contingent upon phylogenetic relatedness; moreover, phylogenetic patterns in a group of plant species can be combined with knowledge about the ecology of those species to develop and test hypotheses about past processes (i.e., niche partitioning) that have driven their evolution.
Given that the evolution of traits involved in resource use are shaped by tradeoffs between competitive and conservative growth strategies [56], greater belowground allocation in Symphyomyrtus species may be accompanied by a suite of traits (e.g., lower xylem density for increased water conductance and greater specific leaf area for increased CO2 acquisition) that increase acquisition and rates of resource use. Ultimately, species with competitive growth strategies (in other words, greater ability to compete for and acquire resources, as exhibited in the Symphyomyrtus species) may be more favored by N addition and elevated CO2 than species with conservative growth strategies (greater ability to persist in low resource environments) as global change continues. This would alter not only community composition and diversity, but also important ecosystem processes such as nutrient cycling [57]. Thus, information about the plant species evolutionary relatedness and functional traits can potentially be useful in refining our current understanding of how plant diversity and ecosystem processes might be altered by future global change.
Novel biotic interactions as a critical agent of global change
Human-assisted species introductions will continue to present novel plant interactions in terrestrial communities [31], [58] and our results indicate that these interactions can alter native community responses to atmospheric CO2 and soil N. Yet, the effects of novel biotic interactions may be unique to native evolutionary lineages. Our analysis reveals that mixtures between eucalypt species that are native to Tasmania and the introduced E. nitens exhibit differing responses to elevated CO2 and added soil N depending on the subgeneric identity of the native species (Fig. 2). Thus, the establishment of E. nitens individuals in native eucalypt forests may reflect a combination of abiotic agents of global change [59] as well as biotic interactions with already established species. Further, our results parallel previous studies that, in certain global change scenarios, ecological consequences are more negative for novel biotic interactions between distant relatives than those between close relatives [30], [31]. For example, in low soil N conditions, mixtures between native species within the subgenus Eucalyptus species (to which E. nitens, a symphyomyrt, is less closely related) and E. nitens respond less positively to elevated CO2 than subgenus Eucalyptus monocultures, whereas mixtures between native species within the subgenus Symphyomyrtus species and E. nitens respond more positively to elevated CO2 than subgenus Symphyomyrtus monocultures (Fig. 2). However, our results reveal that this pattern does not always hold across all global change scenarios. For example, in high soil N conditions, mixtures between native species within the subgenus Eucalyptus species and E. nitens respond no differently to elevated CO2 than subgenus Eucalyptus monocultures, whereas mixtures between native species within the subgenus Symphyomyrtus species and E. nitens respond less positively to elevated CO2 than subgenus Symphyomyrtus monocultures. Given these observed effects of increased soil N and elevated CO2 on different species combinations, current predictions of how community and global productivity will be altered in the future may be too simplistic. Thus, it is critical that both plant evolutionary history and opportunities for introduced species establishment be considered as important drivers of future carbon sequestration and multiple other important ecosystem functions [5], [7], [8], [12], [55].
The ability of introduced species to compete for resources under environmental change can determine their success in novel environments, illustrating how plasticity play an important role in introduced plant response to escalating global change [60]. As one of the most widely planted tree species in the hardwood industry, non-native E. nitens are high-yielding, fast growing and phenotypically adaptable to a range of environments [48]. Our results support evidence suggesting that introduced plant species have the potential to grow aggressively in novel soils [61], [62] and atmospheres, such that dispersal of E. nitens into native eucalypt forests may facilitate carbon sequestration as levels of soil N and atmospheric CO2 increase (Fig. 2). However, the ability of species to capitalize on resources may vary locally and is highly contingent upon abiotic and biotic components of destination environments [63], [64]. Though our study advances current knowledge about effects of non-native species introductions on community productivity in different global change scenarios, further research might focus on landscape-level variation in the interactions among biotic and abiotic forms of global change and the mechanisms that drive this variation. Ultimately, mosaics of past evolutionary history and contemporary abiotic and biotic interactions are fundamental to how we understand productivity across species and communities with escalating global change.
Conclusions
The earth is experiencing an increasingly dynamic interplay of atmospheric, edaphic, and biotic changes that are already influencing plant species performance and patterns of biodiversity. Although recent research has shown that plant species evolutionary history can predict responses to one or two global change factors, outcomes of synergistically acting agents of global change are not well understood. We show that species phylogenetic relatedness and responses to multiple global change drivers can be strongly interconnected, indicating that functional traits and nutrient uptake strategies are unique to phylogenetic groups. However, given that plant communities differ in species composition and the magnitude of global change may differ across communities, collaboration among evolutionary biologists, global change biologists, ecologists and agricultural managers is critical to understand large-scale patterns in plant community responses to global change. We posit that conclusions about the future of biodiversity, composition, and function of plant communities in a rapidly changing world could be misleading if phylogenetic information and multiple agents of global change are not accounted for.
Supporting Information
Models of biomass (total, aboveground and belowground) and ratio of root to shoot biomass in changing to abiotic (atmospheric CO2 and soil N) conditions show that the evolutionary history of native species and novel interaction with an introduced species mediate plant response to abiotic agents of global change.
(DOCX)
Models of biomass (total, aboveground and belowground) and root to shoot ratio in changing soil N conditions show that species pairs respond differently to increased soil N depending on evolutionary background, interaction with an introduced species, and atmospheric CO2 level.
(DOCX)
Acknowledgments
We thank the staff at the Central Science Laboratory at UTAS for use of molecular laboratory facilities. We also thank B. Potts, R. Vaillancourt, M. Hovenden, M. Genung, M. Van Nuland, Z. Marion, A. Pfennigwerth, and anonymous reviewers for helpful guidance and feedback. Thanks to M. Hovenden for his expertise in climate change experiments and design; T. Winterbottom for her expertise in the greenhouse; C. and J. Bloomfield and D. Lusk for their greenhouse and lab assistance.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Vitousek P, Howarth R (1991) Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 13:87–115. [Google Scholar]
- 2. Reich PB, Hobbie SE, Lee T, Ellsworth DS, West JB, et al. (2006) Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nat London 440:922–925. [DOI] [PubMed] [Google Scholar]
- 3. Churkina G, Brovkin V, von Bloh W, Trusilova K, Jung M, et al. (2009) Synergy of rising nitrogen depositions and atmospheric CO2 on land carbon uptake moderately offsets global warming. Global Biogeochem Cycles 23: doi:10.1029/2008GB003291 [Google Scholar]
- 4. Norby RJ, Warren JM, Iversen CM, Medlyn BE, Mcmurtrie RE (2010) CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proc Natl Acad Sci U S A 107:19368–19373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zavaleta ES, Shaw MR, Chiariello NR, Mooney HA, Field CB (2003) Additive effects of simulated climate changes, elevated CO2, and nitrogen deposition on grassland diversity. Proc Natl Acad Sci U S A 100:7650–7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kulmatiski A, Vogt KA, Vogt DJ, Wargo PM, Tilley JP, et al. (2007) Nitrogen and calcium additions increase forest growth in northeastern USA spruce–fir forests. Can J For Res 37:1574–1585. [Google Scholar]
- 7. Bobbink R, Hicks K, Galloway J, Spranger T, Alkemade R, et al. (2010) Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol Appl 20:30–59. [DOI] [PubMed] [Google Scholar]
- 8. Cleland EE, Harpole WS (2010) Nitrogen enrichment and plant communities. Ann N Y Acad Sci 1195:46–61. [DOI] [PubMed] [Google Scholar]
- 9. Elser JJ, Fagan WF, Kerkhoff AJ, Swenson NG, Enquist BJ (2010) Biological stoichiometry of plant production: metabolism, scaling and ecological response to global change. New Phytol 186:593–608. [DOI] [PubMed] [Google Scholar]
- 10. Langley JA, Megonigal JP (2010) Ecosystem response to elevated CO(2) levels limited by nitrogen-induced plant species shift. Nature 466:013–99 [DOI] [PubMed] [Google Scholar]
- 11. Harpole WS, Suding KN (2010) A test of the niche dimension hypothesis in an arid annual grassland. Oecologia 166:197–205. [DOI] [PubMed] [Google Scholar]
- 12. Sala OE (2000) Global Biodiversity Scenarios for the Year 2100. Science 287:1770–1774. [DOI] [PubMed] [Google Scholar]
- 13. Chapin FS, Zavaleta ES, Eviner VT, Naylor RL, Vitousek PM, et al. (2000) Consequences of changing biodiversity. Nature 405:234–242. [DOI] [PubMed] [Google Scholar]
- 14. Edwards EJ, Still CJ, Donoghue MJ (2007) The relevance of phylogeny to studies of global change. Trends Ecol Evol 22:243–249. [DOI] [PubMed] [Google Scholar]
- 15. Crisp MD, Cook LG (2012) Phylogenetic niche conservatism: what are the underlying evolutionary and ecological causes? New Phytol 196:681–694. [DOI] [PubMed] [Google Scholar]
- 16. Cadotte MW, Cardinale BJ, Oakley TH (2008) Evolutionary history and the effect of biodiversity on plant productivity. Proc Natl Acad Sci U S A 105:17012–17017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Srivastava DS, Cadotte MW, MacDonald AAM, Marushia RG, Mirotchnick N (2012) Phylogenetic diversity and the functioning of ecosystems. Ecol Lett 15:637–648. [DOI] [PubMed] [Google Scholar]
- 18. Cadotte MW, Cavender-Bares J, Tilman D, Oakley TH (2009) Using phylogenetic, functional and trait diversity to understand patterns of plant community productivity. PLoS One 4:e5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Revell LJ, Harmon LJ, Collar DC (2008) Phylogenetic signal, evolutionary process, and rate. Syst Biol 57:591–601. [DOI] [PubMed] [Google Scholar]
- 20. Wiens J, Graham C (2005) Niche conservatism: Integrating evolution, ecology, and conservation biology. Annu Rev Ecol Syst 36:519–539. [Google Scholar]
- 21. Losos JB (2008) Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol Lett 11:995–1003. [DOI] [PubMed] [Google Scholar]
- 22. Kembel SW, Cahill JF (2011) Independent evolution of leaf and root traits within and among temperate grassland plant communities. PLoS One 6:e19992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kraft NJB, Ackerly DD (2010) Functional trait and phylogenetic tests of community assembly across spatial scales in an Amazonian forest. Ecol Monogr 80:401–422. [Google Scholar]
- 24. Davis CC, Willis CG, Primack RB, Miller-Rushing AJ (2010) The importance of phylogeny to the study of phenological response to global climate change. Philos Trans R Soc Lond B Biol Sci 365:3201–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sax DF, Stachowicz JJ, Brown JH, Bruno JF, Dawson MN, et al. (2007) Ecological and evolutionary insights from species invasions. Trends Ecol Evol 22:465–471. [DOI] [PubMed] [Google Scholar]
- 26. Bradley BA, Blumenthal DM, Wilcove DS, Ziska LH (2010) Predicting plant invasions in an era of global change. Trends Ecol Evol 25:310–318. [DOI] [PubMed] [Google Scholar]
- 27. Dukes JS, Mooney HA (1999) Does global change increase the success of biological invaders? Trends Ecol Evol 14:135–139. [DOI] [PubMed] [Google Scholar]
- 28. Lankau RA (2013) Species invasion alters local adaptation to soil communities in a native plant. Ecology 94:32–40. [DOI] [PubMed] [Google Scholar]
- 29. Stinson KA, Campbell SA, Powell JR, Wolfe BE, Callaway RM, et al. (2006) Invasive Plant Suppresses the Growth of Native Tree Seedlings by Disrupting Belowground Mutualisms. PLoS Biol 4:e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hierro JL, Maron JL, Callaway RM (2005) A biogeographical approach to plant invasions: the importance of studying exotics in their introduced and native range. J Ecol 93:5–15. [Google Scholar]
- 31. Strauss SY, Lau JA, Carroll SP (2006) Evolutionary responses of natives to introduced species: what do introductions tell us about natural communities? Ecol Lett 9:357–374. [DOI] [PubMed] [Google Scholar]
- 32. Laikre L, Schwartz MK, Waples RS, Ryman N (2010) Compromising genetic diversity in the wild: unmonitored large-scale release of plants and animals. Trends Ecol Evol 25:520–529. [DOI] [PubMed] [Google Scholar]
- 33. Williams KJ, Potts BM (1996) The natural distribution of Eucalyptus species in Tasmania. Tasforests 8:39–149. [Google Scholar]
- 34. Hovenden MJ, Williams AL (2010) The impacts of rising CO2 concentrations on Australian terrestrial species and ecosystems. Austral Ecol 35:665–684. [Google Scholar]
- 35. Brooker M (2000) A new classification of the genus Eucalyptus L'Her. (Myrtaceae). Aust Syst Bot 13:79–148. [Google Scholar]
- 36. McKinnon GE, Vaillancourt RE, Steane DA, Potts BM (2008) An AFLP marker approach to lower-level systematics in Eucalyptus (Myrtaceae). Am J Bot 95:368–380. [DOI] [PubMed] [Google Scholar]
- 37. Steane DA, Nicolle D, Sansaloni CP, Petroli CD, Carling J, et al. (2011) Population genetic analysis and phylogeny reconstruction in Eucalyptus (Myrtaceae) using high-throughput, genome-wide genotyping. Mol Phylogenet Evol 59:206–224. [DOI] [PubMed] [Google Scholar]
- 38. Judd TS, Bennett LT, Weston CJ, Attiwill PM, Whiteman PH (1996) The response of growth and foliar nutrients to fertilizers in young Eucalyptus globulus (Labill.) plantations in Gippsland, southeastern Australia. For Ecol Manage 82:87–101. [Google Scholar]
- 39. Wallis IR, Nicolle D, Foley WJ (2010) Available and not total nitrogen in leaves explains key chemical differences between the eucalypt subgenera. For Ecol Manage 260:814–821. [Google Scholar]
- 40. Anekonda TS, Criddle RS, Bacca M, Hansen LD (1999) Contrasting adaptation of two Eucalyptus subgenera is related to differences in respiratory metabolism. Funct Ecol 13:675–682. [Google Scholar]
- 41. Noble I (1989) Ecological Traits of the Eucalyptus L'hérit Subgenera Monocalyptus and Symphyomyrtus. Aust J Bot 37:207. [Google Scholar]
- 42. Senior JK, Schweitzer JA, O'Reilly-Wapstra J, Chapman SK, Steane D, et al. (2013) Phylogenetic responses of forest trees to global change. PLoS One 8:e60088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Genung MA, Schweitzer JA, Bailey JK (2014) Evolutionary history determines how plant productivity responds to phylogenetic diversity and species richness. PeerJ 2:e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Simberloff D, Nuñez MA, Ledgard NJ, Pauchard A, Richardson DM, et al. (2009) Spread and impact of introduced conifers in South America: Lessons from other southern hemisphere regions. Austral Ecol 35:489–504. [Google Scholar]
- 45. Barbour RC, Wise SL, McKinnon GE, Vaillancourt RE, Williamson GJ, et al. (2010) The potential for gene flow from exotic eucalypt plantations into Australia's rare native eucalypts. For Ecol Manage 260:2079–2087. [Google Scholar]
- 46. Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, et al. (2004) Nitrogen Cycles: Past, Present, and Future. Biogeochemistry 70:153–226. [Google Scholar]
- 47. Phoenix GK, Emmett BA, Britton AJ, Caporn SJM, Dise NB, et al. (2012) Impacts of atmospheric nitrogen deposition: responses of multiple plant and soil parameters across contrasting ecosystems in long-term field experiments. Glob Chang Biol 18:1197–1215. [Google Scholar]
- 48.May B, Smethurst P, Carlyle C, Mendham D, Bruce J, et al. (2009) Review of fertiliser use in Australian forestry.
- 49.IPCC (2007) Climate Change 2007: The Physical Science Basis, Contribution of Working Group 1 to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press. [Google Scholar]
- 50. Warren CR, Adams MA (2002) Phosphorus affects growth and partitioning of nitrogen to Rubisco in Pinus pinaster. Tree Physiol 22:11–19. [DOI] [PubMed] [Google Scholar]
- 51. Amr A, Hadidi N (2001) Effect of cultivar and harvest date on nitrate (NO3) and nitrite (NO2) content of selected vegetables grown under open field and greenhouse conditions in Jordan. J Food Compos Anal 14:59–67. [Google Scholar]
- 52.Team RC (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. Available: http://www.R-project.org/.
- 53.Aiken LS, West SG (1991) Multiple regression: Testing and interpreting interactions. Thousand Oaks, California: Sage Publications, Inc. [Google Scholar]
- 54. Dimarco RD, Fordyce JA (2013) Larger clutches of chemically defended butterflies reduce egg mortality: evidence from Battus philenor. Ecol Entomol 38:535–538. [Google Scholar]
- 55. Harpole W, Tilman D (2007) Grassland species loss resulting from reduced niche dimension. Nature 446:791–793. [DOI] [PubMed] [Google Scholar]
- 56. Reich PB (2014) The world-wide “fast-slow” plant economics spectrum: a traits manifesto. J Ecol 102:275–301 Available: http://doi.wiley.com/10.1111/1365-2745.12211. [Google Scholar]
- 57. Díaz S, Hodgson JG, Thompson K, Cabido M, Cornelissen JHC, et al. (2004) The plant traits that drive ecosystems: Evidence from three continents. J Veg Sci 15:295. [Google Scholar]
- 58. Ricciardi A (2007) Are modern biological invasions an unprecedented form of global change? Conserv Biol 21:329–336. [DOI] [PubMed] [Google Scholar]
- 59. Vitousek PM, D'Antonio CM, Loope LL, Rejmanek M, Westbrooks R (1997) Introduced species: a significant component of human-caused global change. N Z J Ecol 21:1–16. [Google Scholar]
- 60. Agrawal AA (2001) Phenotypic plasticity in the interactions and evolution of species. Science 294:321–326. [DOI] [PubMed] [Google Scholar]
- 61. Schweitzer JA, Larson KC (1999) Greater morphological plasticity of exotic honeysuckle species make them better invaders than native species. J Torrey Bot Soc 126:15–23. [Google Scholar]
- 62. Burns JH, Winn AA (2006) A comparison of plastic responses to competition by invasive and non-invasive congeners in the Commelinaceae. Biol Invasions 8:797–807. [Google Scholar]
- 63. Felker-Quinn E, Bailey JK, Schweitzer JA (2011) Soil biota drive expression of genetic variation and development of population-specific feedbacks in an invasive plant. Ecology 92:1208–1214. [DOI] [PubMed] [Google Scholar]
- 64. Nuñez MA, Horton TR, Simberloff D (2009) Lack of belowground mutualisms hinders Pinaceae invasions. Ecology 90:2352–2359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Models of biomass (total, aboveground and belowground) and ratio of root to shoot biomass in changing to abiotic (atmospheric CO2 and soil N) conditions show that the evolutionary history of native species and novel interaction with an introduced species mediate plant response to abiotic agents of global change.
(DOCX)
Models of biomass (total, aboveground and belowground) and root to shoot ratio in changing soil N conditions show that species pairs respond differently to increased soil N depending on evolutionary background, interaction with an introduced species, and atmospheric CO2 level.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.