This review evaluates the basis for the effects of prednisone on mineralocorticoid-related adverse events that arise because of CYP17A1 inhibition with abiraterone for metastatic castration-resistant prostate cancer (mCRPC). Glucocorticoids are often used at higher doses than used with abiraterone acetate to manage tumor-related symptoms or to prevent treatment-related toxicity, but side effects are common at these higher doses. Given recent improvements in survival achieved for mCRPC with novel agents in combination with prednisone, the risks of recommended glucocorticoid doses must be balanced with the benefits shown for these regimens.
Keywords: Adrenal cortex hormones; 17-(3-Pyridyl)-5,16-androstadien-3β-acetate; Steroid 17-α-hydroxylase; Prednisone; Prostatic neoplasms
Abstract
Abiraterone acetate, a prodrug of the CYP17A1 inhibitor abiraterone that blocks androgen biosynthesis, is approved for treatment of patients with metastatic castration-resistant prostate cancer (mCRPC) in combination with prednisone or prednisolone 5 mg twice daily. This review evaluates the basis for the effects of prednisone on mineralocorticoid-related adverse events that arise because of CYP17A1 inhibition with abiraterone. Coadministration with the recommended dose of glucocorticoid compensates for abiraterone-induced reductions in serum cortisol and blocks the compensatory increase in adrenocorticotropic hormone seen with abiraterone. Consequently, 5 mg prednisone twice daily serves as a glucocorticoid replacement therapy when coadministered with abiraterone acetate, analogous to use of glucocorticoid replacement therapy for certain endocrine disorders. We searched PubMed to identify safety concerns regarding glucocorticoid use, placing a focus on longitudinal studies in autoimmune and inflammatory diseases and cancer. In general, glucocorticoid-related adverse events, including bone loss, immunosuppression, hyperglycemia, mood and cognitive alterations, and myopathy, appear dose related and tend to occur at doses and/or treatment durations greater than the low dose of glucocorticoid approved in combination with abiraterone acetate for the treatment of mCRPC. Although glucocorticoids are often used to manage tumor-related symptoms or to prevent treatment-related toxicity, available evidence suggests that prednisone and dexamethasone might also offer modest therapeutic benefit in mCRPC. Given recent improvements in survival achieved for mCRPC with novel agents in combination with prednisone, the risks of these recommended glucocorticoid doses must be balanced with the benefits shown for these regimens.
Implications for Practice:
Abiraterone acetate, a prodrug of the CYP17A1 inhibitor abiraterone, suppresses testosterone and cortisol production in patients with metastatic castration-resistant prostate cancer (mCRPC), but cortisol precursors with mineralocorticoid activity rise during abiraterone acetate monotherapy. Low-dose prednisone (5 mg twice daily) coadministration serves as glucocorticoid replacement therapy, lowers adrenocorticotropic hormone, and reduces the incidence and severity of mineralocorticoid-related adverse events. In contrast, pharmacologic glucocorticoid doses used to treat other malignancies and autoimmune disorders are typically ≥20 mg/day prednisone equivalence, and these higher doses are associated with an adverse safety profile. The safety profile of low-dose glucocorticoid use in mCRPC deserves further study.
Introduction
The progression of metastatic castration-resistant prostate cancer (mCRPC) despite androgen deprivation therapy and castrate testosterone levels frequently reflects the continued production of androgens in the adrenal glands and within prostate tumor tissue [1, 2]. Abiraterone acetate is the prodrug of abiraterone, which blocks androgen biosynthesis via inhibition of steroid 17-hydroxylase/17,20-lyase (cytochrome P450c17 [CYP17A1]) [3]. Abiraterone acetate in combination with prednisone or prednisolone at a low dose of 5 mg twice daily has been shown to improve survival of mCRPC patients previously treated with docetaxel and those who had not received prior chemotherapy [4, 5]. The administration of abiraterone acetate with glucocorticoids is necessary to manage adverse events related to mineralocorticoid excess, such as hypokalemia, hypertension, and fluid retention, which can occur as a result of CYP17A1 inhibition [6–8]. This review evaluates the basis for the remedial effects of low-dose prednisone to prevent mineralocorticoid excess-related adverse events anticipated with abiraterone acetate therapy and assesses safety concerns about glucocorticoid therapy based on longitudinal studies conducted in autoimmune and inflammatory diseases and cancer.
Materials and Methods
We searched PubMed using the terms corticosteroids, glucocorticoids, steroids, abiraterone, prostate cancer, and metastatic castrate-resistant prostate cancer for clinical trials, reviews, and case reports published in English without filtering for dates. Information selected for this article was obtained from preclinical investigations, early clinical trials, and phase III studies that served as the basis for approval of prednisone with abiraterone acetate in patients progressing after docetaxel and without prior chemotherapy. A focus was placed on the glucocorticoid literature with filters of clinical trial, review, case reports, and English describing longitudinal follow-up of the prolonged therapeutic use of prednisone and its association with autoimmune and inflammatory disease, malignant disease, bone abnormalities, hyperglycemia and diabetes, mood and cognitive function, fatigue, and myopathy. Also included were the effects of prednisone on immune function and the therapeutic benefits and disadvantages of prednisone coadministration in prostate cancer. Data from recent congress presentations and publications known to the authors independent of this literature search were also incorporated.
We did not include in vitro data showing glucocorticoid receptor stimulation of cancer growth or genes overlapping with androgen receptor targets in CRPC because this mechanism of driving disease progression has not been established beyond preclinical models. AFFIRM data showing increased risk of death and progression with baseline glucocorticoid use independent of other prognostic factors also were not included [9] because prognostic models from the COU-AA-301 trial did not validate this result. The latter information is the subject of a separate line of investigation which was published separately [10].
Impact of Blocking CYP17A1 on Synthesis of Adrenal Steroids Beyond Androgens
Abiraterone is a potent, selective, and irreversible inhibitor of CYP17A1, a microsomal enzyme with 17α-hydroxylase and C17,20-lyase activities that are required for androgen biosynthesis via both classic and backdoor pathways (Fig. 1) [3, 12]. Whereas androgen biosynthesis requires both of these CYP17A1 activities, cortisol biosynthesis requires only the 17α-hydroxylase activity of CYP17A1. The efficacy of abiraterone in blocking androgen biosynthesis is shown by substantial reductions in serum androgen levels (Fig. 2) [8, 13]. Use of abiraterone to inhibit androgen synthesis, however, is associated with several undesired physiologic changes, including a decrease in cortisol levels and a compensatory increase in adrenocorticotropic hormone (ACTH) [8, 13, 14]. This rise in ACTH leads to accumulation of steroids with mineralocorticoid properties upstream of CYP17A1 in the cortisol biosynthetic pathway (Fig. 3) and, ultimately, to mineralocorticoid-related adverse events, including hypertension, hypokalemia, and fluid retention [12].
Figure 1.
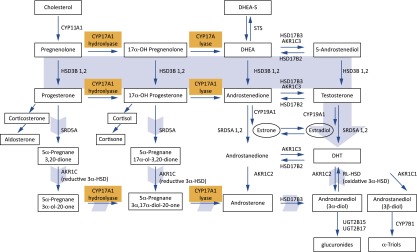
Abiraterone inhibits CYP17A1, which acts at two key synthetic steps in androgen biosynthesis. Precursors upstream of CYP17A1-catalyzed steps accumulate, resulting in mineralocorticoid excess [11].
Abbreviations: DHEA[-S], dehydroepiandrosterone [sulfate]; DHT, dihydrotestosterone.
Figure 2.
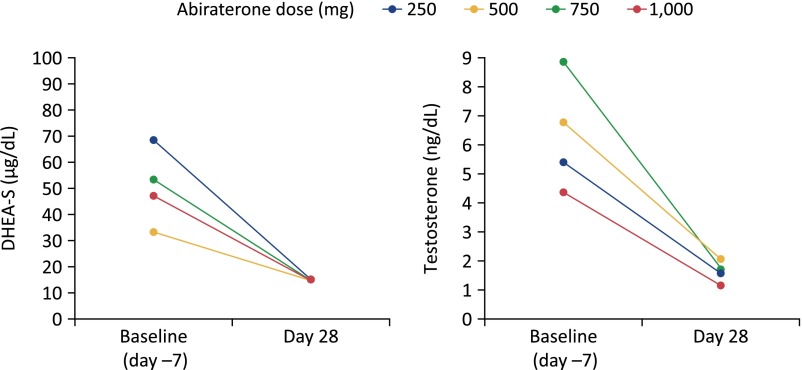
Abiraterone acetate reduces serum androgens and serum cortisol as a result of blocking CYP17A1. Shown are the changes in mean steroid levels from baseline to day 28 in patients with metastatic castration-resistant prostate cancer who received abiraterone acetate at doses of 250, 500, 750, or 1,000 mg daily [8].
Abbreviation: DHEA-S, dehydroepiandrosterone sulfate.
Figure 3.
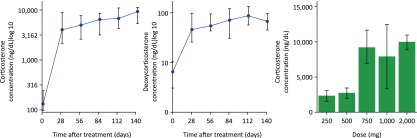
Abiraterone increases steroids with mineralocorticoid activity upstream of CYP17A1. Shown are median serum levels of corticosterone and deoxycorticosterone over time in patients with metastatic castration-resistant prostate cancer who received abiraterone acetate at doses of 250 to 2,000 mg daily. The right panel shows mean corticosterone levels at day 28 by abiraterone acetate dose [12].
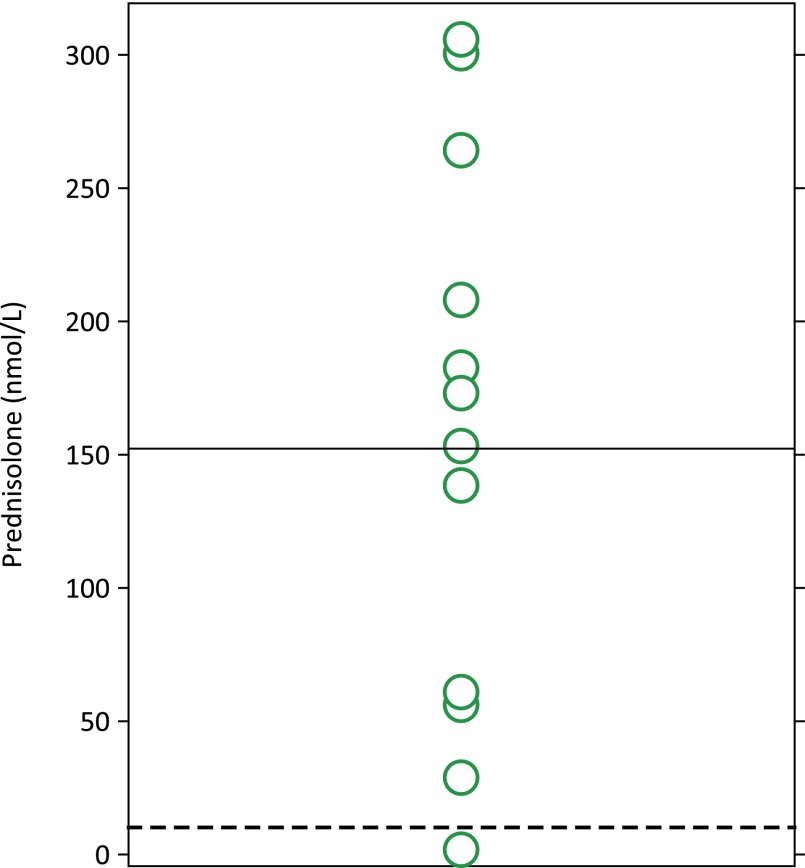
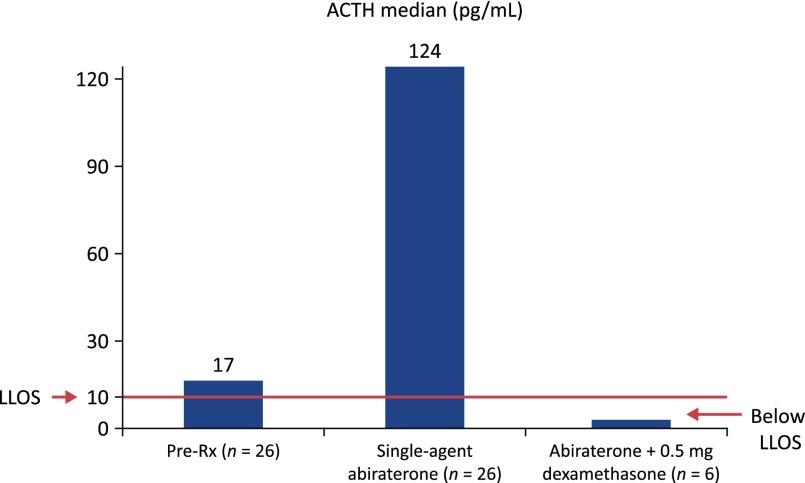
Cortisol levels follow a circadian rhythm in which levels are lowest at midnight, begin rising around 2–4 am, peak after waking, and then slowly return to their nadir [14]. Serum cortisol is regulated by its negative feedback on the hypothalamic-pituitary axis: low cortisol stimulates release of corticotropin-releasing hormone from the paraventricular nucleus of the hypothalamus, which triggers ACTH release from the anterior pituitary and, in turn, cortisol production in the adrenal cortex. Healthy adults have a morning serum cortisol level in the range of 5–23 μg/dL (138–635 nmol/L) and midnight serum cortisol <5 μg/dL (<138 nmol/L). In studies of mCRPC patients, abiraterone reduced serum cortisol to near the lower limit of the normal range [8] and increased ACTH from a median of 17 pg/mL (range: <9–50 pg/mL) to a median of 124 pg/mL (range: 46–370 pg/mL) [13]. When coadministered with abiraterone acetate, low-dose prednisone or prednisolone substitutes for cortisol, compensating for the abiraterone-induced reduction in serum cortisol (Fig. 4) [5, 15, 16]. Following this principle, the potent glucocorticoid dexamethasone normalizes the abiraterone-induced rise in ACTH (Fig. 5) [13]. Prednisolone or its precursor prednisone is approximately four times more potent as a glucocorticoid compared with cortisol [18]. Treatment of 15 mCRPC patients with abiraterone acetate plus 10 mg prednisolone resulted in median prednisolone plasma concentrations of 152 nM—equivalent to 608 nM cortisol—thus providing physiologic glucocorticoid replacement (Fig. 4) [16]. At this dose, the mineralocorticoid activity of prednisolone is minimal [19].
Figure 4.
Low-dose prednisolone yields the equivalent of physiologic cortisol levels. Daily prednisolone (10 mg/day) with abiraterone acetate (1,000 mg/day) in 15 castration-resistant prostate cancer patients led to median prednisolone concentrations of 152 nmol/L (solid line). Given an ∼4:1 relative potency of prednisolone:hydrocortisone, 152 × 4 is equivalent to 608 nmol/L cortisol, which is within physiologic concentrations [16]. Dotted line is 10 nmol/L.
Figure 5.
Dexamethasone (0.5 mg/day) suppresses abiraterone-mediated increases in adrenocorticotropic hormone (ACTH). Abiraterone acetate treatment (n = 26) was associated with a significant increase in median plasma ACTH levels from 17 pg/mL to 124 pg/mL (660% increase). This rise in ACTH was suppressed to below the lower limit of sensitivity (10 pg/mL) after administration of oral dexamethasone 0.5 mg/day for <14 days. Normal ACTH levels in adults (mean ± SE): 28.7 ± 12 [13, 17].
Abbreviations: ACTH, adrenocorticotropic hormone; LLOS, lower limit of sensitivity; Rx, treatment.
Glucocorticoid replacement therapy, as defined in this paper, has been shown to effectively reduce the incidence of mineralocorticoid-related adverse events in patients with mCRPC treated with abiraterone acetate (discussed below) [4, 6]. Similarly, subjects with congenital CYP17A1 deficiency produce excessive mineralocorticoids and develop hypertension and hypokalemia [20], which can be mitigated by glucocorticoid replacement therapy, including low-dose prednisone or prednisolone [21]. The use of glucocorticoid replacement to correct treatment-related steroid imbalances is similar to the use of glucocorticoid replacement therapy for other forms of acute or chronic adrenal insufficiency [22]. In these settings, the main goal is to mimic normal cortisol production to restore normal physiology while minimizing adverse effects. The choice of glucocorticoid, its dose, and the treatment duration are important considerations for achieving these goals [23].Currently, abiraterone acetate is approved only for use in combination with the prednisone or prednisolone dose given orally (5 mg) in the morning and evening. Other regimens and alternative glucocorticoids might be equivalent or superior cotherapies for specific patients, but different regimens have not been compared directly. There is an ongoing phase III trial of abiraterone acetate with 5 mg/day of prednisone or prednisolone in newly diagnosed patients with metastatic, hormone-naïve prostate cancer (ClinicalTrials.gov identifier NCT01715285). In the phase I and II trials, abiraterone acetate was administered without any glucocorticoid, and hypertension and hypokalemia were successfully managed with the mineralocorticoid receptor antagonist eplerenone at an average dose of 50 mg/day, often with addition of dexamethasone 0.5 mg/day on progression [13].
Pharmacologic Effects of Long-Term Glucocorticoid Therapy
It is well recognized that glucocorticoids produce a variety of adverse events when used for prolonged periods to treat various autoimmune and inflammatory diseases, such as rheumatoid arthritis, Crohn’s disease, and asthma, and various cancers [23–26]. Glucocorticoid-related adverse events include altered bone metabolism, immunosuppression, increased risk of hyperglycemia and diabetes, adverse impact on mood and cognitive function, and muscle weakness. In general, these glucocorticoid-related adverse events are related primarily to the cumulative dose over a prolonged treatment period or to use of high doses during short-term exposure.
Glucocorticoids may induce bone mineral loss and increase risk of osteoporosis and fracture after extensive exposure. In rheumatoid arthritis, glucocorticoids increase risk of these bone abnormalities beyond the risk associated with the disease itself [26, 27]. In the British General Practice Research Database, for example, the relative risk of hip fracture was two times higher among patients with versus without rheumatoid arthritis, with the relative risk increasing to 3.4-fold among those patients with rheumatoid arthritis receiving oral glucocorticoids [26]. Based on studies in rheumatoid arthritis and asthma, risk for bone mineral density reduction and/or osteoporosis is significant with high-dose glucocorticoids (>15 mg/day) and with glucocorticoid treatment at 7.5 mg/day for longer periods (>6 months) [28]. In addition, the frequency of short-course oral glucocorticoid bursts has been associated with reductions in bone mineral density and increased risk of osteopenia in longitudinal studies in asthmatic children treated for 3 years with a total follow-up of 7 years and in adults with mean treatment for 7.7 years with additional follow-up for a median of 4 years [29, 30].
For patients who have a set of autosomal recessive diseases known as congenital adrenal hyperplasia (CAH), long-term disease management using glucocorticoid therapy often negatively affects bone health and quality of life [31]. The most common form of CAH is 21-hydroxylase deficiency (21OHD), which is characterized by cortisol and aldosterone deficiency; furthermore, the accumulating cortisol precursors are shunted to other biosynthetic pathways, leading to adrenal androgen excess [31]. These patients receive long-term glucocorticoid and mineralocorticoid therapy, not only to replace the cortisol and aldosterone deficiency but also to suppress ACTH and thus adrenal androgen production. These regimens typically consist of supraphysiologic divided doses of hydrocortisone or substitution with prednisone/prednisolone or dexamethasone. A reduction in bone mineral density and a threefold increase in osteopenia or osteoporosis was seen in adult male patients with 21OHD aged >30 years versus age-matched controls, with long-acting glucocorticoids more negatively affecting bone health compared with short-acting glucocorticoids [32]. In this study, the majority of patients with 21OHD had normal bone density, and the prevalence of diabetes mellitus was not increased. As for asthma and inflammatory diseases, bone loss in CAH patients is attributed specifically to a lifetime of prolonged exposure to supraphysiologic glucocorticoids necessary to control androgen excess. Optimized glucocorticoid therapy plus vitamin D and calcium supplementation mitigate these consequences [31, 33]. In the mCRPC setting, long-term glucocorticoid therapy warrants caution and continuous monitoring, especially in frail elderly men who may have significant comorbidities and prior cumulative steroid exposure that may adversely affect their bone health. Very frail patients with poor performance scores and short life expectancies are excluded from most clinical trials, so extrapolation of published studies to these populations should be done with caution.
Glucocorticoids produce a number of metabolic effects, most importantly hyperglycemia and increased risk of diabetes mellitus. In a cohort of patients with rheumatic disease, the development of diabetes was significantly correlated with the cumulative prednisone dose over the course of treatment [34]. The mean cumulative dose for patients with steroid-induced hyperglycemia was 26.6 g compared with 11.6 g for those without steroid-induced hyperglycemia [34]. Given the limited life expectancy of mCRPC patients, the anticipated steroid exposure at a dose of 10 mg/day would be lower than these levels (<4 g over 1 year). Moreover, the incidence of post-transplant diabetes among renal transplant recipients maintained on prednisone 5–7.5 mg/day during a median 5-year follow-up was 15%, which was significantly higher than the incidence among those who did not have glucocorticoid maintenance (5%) [35].
Glucocorticoids exert negative effects on mood and cognitive function that, again, correlate with the dose and/or length of treatment [36]. In a cohort of 27 children (aged 8–16 years) with severe asthma treated with prednisolone for <14 days, those given high doses (mean: 62 mg/day) had increased symptoms of anxiety and depression compared with those receiving low doses (mean: 3 mg/day) [36, 37]. In a cohort of 20 adults with asthma or rheumatic disease receiving prednisone at a mean dose of 19 mg/day for a mean duration of 128 months, 12 (60%) met diagnostic criteria for a prednisone-induced mood disorder, most frequently depression, at some point during treatment [38]. Changes in cognition are often observed during glucocorticoid therapy, most commonly decreases in declarative (verbal) memory [36]. In the aforementioned study in children with severe asthma, greater decreases in declarative memory were reported with high versus low glucocorticoid doses [36, 37]. Patients with asthma often receive multiple medications in addition to glucocorticoids, and that might also contribute to effects on cognitive function.
Minimal data are available for glucocorticoid-induced myopathy in prostate cancer, but generally a low incidence is observed. Severe fatigue, myopathy, or muscle weakness were not reported in a phase III trial of low-dose prednisone with or without mitoxantrone in patients with asymptomatic CRPC [39]. In the TAX 327 study, in which low-dose prednisone was administered with either docetaxel or mitoxantrone, severe fatigue was reported in 5% of patients, yet myopathy was not reported [40]. Similarly, grade 3 fatigue was reported in 8% of men with mCRPC after chemotherapy who received thalidomide plus oral dexamethasone [41]. In the COU-AA-302 trial of chemotherapy-naïve mCRPC patients [42], muscle weakness was infrequently reported in 0.6% of patients in the abiraterone acetate-plus-prednisone arm and in 1.1% of patients in the prednisone-alone arm (data on file, Janssen Research & Development, 2012). In other disorders, glucocorticoid-induced myopathy has been associated primarily with high-dose steroid treatment, a sedentary lifestyle, and the use of fluorinated steroids (e.g., triamcinolone, dexamethasone) rather than nonfluorinated steroids (e.g., hydrocortisone, prednisone) [43, 44]. The glucocorticoid-induced myopathy is fully yet slowly reversible when the dose is reduced below 30 mg/day of hydrocortisone or its equivalent, with a rehabilitative conditioning program appearing to be the most effective treatment [44].
Taken together, these findings indicate that the incidence of glucocorticoid-induced adverse events—including bone loss, diabetes, central nervous system effects, and myopathy—are related to dose and choice of glucocorticoid, and these consequences tend to occur at doses much higher than those used in mCRPC [23]. When interpreting potential adverse effects of glucocorticoids in an elderly population of men with prostate cancer, the patients’ comorbidities, family history, and prior glucocorticoid and medication exposure should be taken into account.
Effect of Prednisone on Immune Function
Glucocorticoids have been commonly used in cancer treatment, although their immunosuppressive properties have always been of specific concern [23]; nevertheless, the immunosuppressive properties may be seen at doses of glucocorticoids above those recommended in approved therapeutic regimens for prostate cancer (e.g., prednisone 10 mg/day). In vitro, glucocorticoids stimulate macrophage function at low concentrations (e.g., 0.1 nM), including expression of proinflammatory cytokines and chemokines and production of nitric oxide, whereas they suppress these functions at high concentrations (e.g., 1 μM) [45]. In an animal model, prednisone did not affect the oxidative burst mediated by complement receptors during neutrophil phagocytosis, even when administered for 7–15 days at a dose equivalent to 90 mg/day in humans [46].
The systemic exposure attained with the recommended low-dose glucocorticoids is below the amount shown to inhibit immune cell proliferation in response to antigens. Pediatric patients treated with prednisone (2 mg/kg per day for 5 days, or ≤0.5 mg/kg per day for >6 months) for chronic inflammatory disorders, including juvenile idiopathic arthritis, systemic lupus erythematosus, or asthma, showed an appropriate immune response when immunized with influenza vaccine, successfully demonstrating a protective antibody titer against influenza A and B antigens [47, 48]. In addition, no flu-like symptoms were noted in any of the children during the 6-month evaluation period following vaccination [47]. Similarly, prednisone treatment did not influence the immunogenicity of an influenza vaccine in adults with rheumatoid arthritis [49], although a recent report indicated that prednisone doses ≥10 mg/day were associated with lower antibody responses in patients with systemic lupus erythematosus [50].
The systemic exposure attained with the recommended low-dose glucocorticoids is below the amount shown to inhibit immune cell proliferation in response to antigens.
The effect of low-dose glucocorticoids on immune responses to personalized peptide vaccination was evaluated in a study of 11 mCRPC patients [51]. Most patients, particularly when treated concurrently with dexamethasone (1 mg/day) or prednisolone (10 mg/day), were able to generate peptide-specific immunoglobulin-G antibodies and cytotoxic T-cell responses. These findings suggest that low-dose glucocorticoids in this study did not suppress immune responses to tumor-specific peptides. Moreover, recent data suggest that immune responses to sipuleucel-T were successfully produced in men with mCRPC when administered concurrently with or prior to treatment with abiraterone acetate plus prednisone [52]. Thus, although the immunosuppressive effects of glucocorticoids have the potential to interfere with treatment effects of immunotherapeutic agents, low doses of glucocorticoids do not appear to reduce immune responses to vaccination substantially. Nevertheless, it is essential to consider the patient population (e.g., age, frailty, previous treatments) when interpreting the effects of glucocorticoids on immune function.
Safety Profile of Abiraterone Acetate Coadministered With Prednisone
Abiraterone acetate (1,000 mg) coadministered with prednisone (5 mg twice daily) was compared with placebo plus prednisone in patients with mCRPC in two phase III, multinational, double-blind, randomized, placebo-controlled trials. Study COU-AA-301 comprised 1,195 patients with mCRPC who had progressed after docetaxel treatment [4], and study COU-AA-302 involved 1,088 patients with mCRPC who had not received chemotherapy and who did not have clinically significant cancer-related symptoms (i.e., asymptomatic or minimally symptomatic patients) [5]. Both studies evaluated treatment effects on survival and disease progression, showing clinically meaningful and significant benefits in favor of abiraterone acetate plus prednisone [5, 15]. On the basis of the results from these studies, abiraterone acetate in combination with prednisone was approved for the treatment of patients with mCRPC.
COU-AA-301 and COU-AA-302 also thoroughly assessed safety and tolerability, and these studies demonstrated that the safety profile of abiraterone acetate plus prednisone was comparable to that observed in earlier clinical studies [6, 8]. In both studies, adverse events associated with mineralocorticoid activity were more common for abiraterone acetate plus prednisone than for prednisone alone (Fig. 6) [4, 5], but their incidence was largely abrogated by low-dose prednisone when compared with earlier studies of abiraterone acetate monotherapy [8, 12, 53]. Notably, the majority of mineralocorticoid-related adverse events were grade 1 or 2 in severity. With coadministration of abiraterone acetate and prednisone in COU-AA-301, the incidence of mineralocorticoid excess-related severe adverse events, including hypertension (1.3% vs. 0.3%), hypokalemia (4.4% vs. 0.8%), and fluid retention or edema (2.5% vs. 1.0%), was low and manageable [15]. The incidence of these severe adverse events in COU-AA-302 was also low, and the difference between treatment arms was even less apparent (hypertension, 3.9% vs. 3.0%; hypokalemia, 2.4% vs. 1.9%; fluid retention or edema, 0.7% vs. 1.7%) [5].
Figure 6.
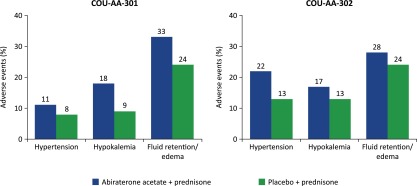
Incidence of mineralocorticoid-related adverse events in metastatic castration-resistant prostate cancer patients treated with abiraterone acetate plus prednisone compared with placebo plus prednisone in the COU-AA-301 and COU-AA-302 phase III randomized controlled studies [5, 15].
The discontinuation rate for abiraterone acetate and prednisone in COU-AA-301 and COU-AA-302 was low, and side effects were easily manageable and reversible, despite the advanced age and advanced disease states of the study populations [5, 15]. Exposure to prednisone across treatment groups was relatively short, with a median of 7.4 months (range: 0.2–25.6 months) in COU-AA-301 [15]. In COU-AA-302, no glucocorticoid-related adverse events greater than those in COU-AA-301 were observed despite a longer median duration (13.8 months) of abiraterone acetate-prednisone cotreatment [15, 42]. In the latter study, no new safety signals were observed in the subset of patients who received abiraterone acetate plus prednisone or prednisone alone for ≥24 months, with cumulative incidence rates of selected adverse events including fatigue, hypertension, osteoporosis, and hyperglycemia remaining similar between treatment groups [42]. Rates of infection were comparable in both groups during long-term treatment. Taken together, results from COU-AA-301 and COU-AA-302 provide proof of principle that low-dose prednisone can be delivered safely without any consistent additional serious adverse effects while adequately managing mineralocorticoid-related adverse events resulting from CYP17A1 inhibition by abiraterone.
Therapeutic or Adjunctive Use of Glucocorticoids in mCRPC
Glucocorticoids are often given to cancer patients to manage tumor-related symptoms, such as bone pain or weight loss, and to alleviate toxic effects associated with specific cancer treatments, such as nausea, vomiting, edema, and hypersensitivity reactions related to chemotherapy [23]. Glucocorticoids are also used in specific situations, such as pain palliation and to reduce swelling from cord compression or radiation of brain metastases. Tumor-related cachexia was infrequent in the abiraterone acetate studies. Although megestrol acetate is better tolerated than dexamethasone [54], megestrol acetate at high doses acts as a glucocorticoid receptor modulator and causes adrenal axis suppression [54], which necessitates glucocorticoid treatment if patients undergo a stress-related condition, including trauma, infection, or surgery. Glucocorticoids are a viable and less expensive alternative to megestrol acetate for tumor-related cachexia in those with a history of thromboembolic complications or other contraindication.
Low-dose glucocorticoids might also offer therapeutic benefits, albeit relatively modest, as demonstrated by results of several retrospective analyses and a randomized trial showing prostate-specific antigen response rates of 22%–61% [56–58] and by results from the placebo-prednisone arms of COU-AA-301 and COU-AA-302 [4, 5]. Particularly in COU-AA-302, low-dose prednisone behaved as an active comparator, producing objective responses defined by Response Evaluation Criteria in Solid Tumors in 16% of patients and a ≥50% decline in prostate-specific antigen levels in 24% of patients [5]. Although not the subject of this review, the observations of therapeutic benefit in mCRPC contradict results of preclinical studies that suggest glucocorticoids may have the potential to drive prostate cancer progression [59]. Glucocorticoids might also drive tumor growth via mutant androgen receptors, based on preclinical data showing that the H874Y and T877S androgen receptor mutations are weakly responsive to cortisol and dexamethasone at micromolar concentrations [60].
Additional Practical Caveats and Considerations for Glucocorticoid Use
Decisions regarding which glucocorticoid to use at what dose and for how long should take into consideration several physiologic factors. When personalizing glucocorticoid replacement therapy, it is important to recognize how differences in glucocorticoid metabolism might affect individual patient responses. Hepatic 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) is essential for the conversion of inactive oral glucocorticoid prodrugs like prednisone and cortisone to their active 11β-hydroxy derivatives prednisolone and cortisol [61]. Glucocorticoid conversion catalyzed by 11β-HSD1 is bidirectional, but its predominant action in vivo is conversion of inactive to active glucocorticoids [62]. Patients who have decreased 11β-HSD1 enzymatic activity, and in rare cases lack the 11β-HSD1 enzyme, might not respond to prednisone because they are unable to generate a substantial amount of prednisolone [63]. In such cases, in patients with liver metastases or impaired hepatic function, prednisolone might be the better alternative for glucocorticoid replacement therapy because it does not depend on metabolic conversion.
Patients who have decreased 11β-HSD1 enzymatic activity, and in rare cases lack the 11β-HSD1 enzyme, might not respond to prednisone because they are unable to generate a substantial amount of prednisolone.
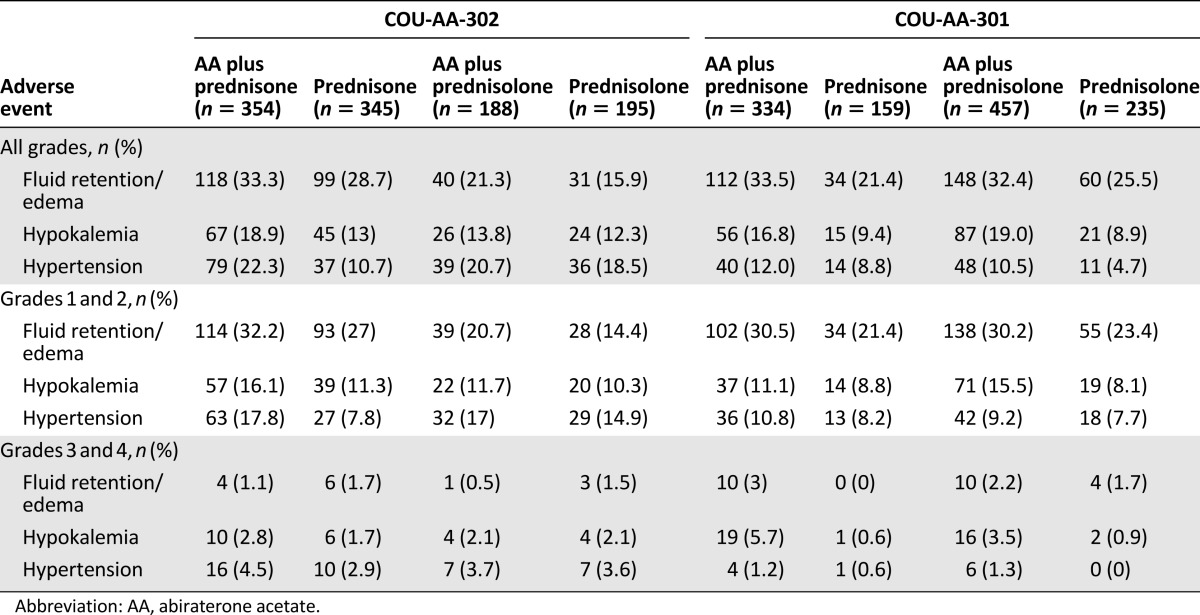
To indirectly compare the use of prednisone or prednisolone in combination with abiraterone acetate, we compared the safety profile related to mineralocorticoid excess in COU-AA-301 or COU-AA-302 among patients enrolled at North American versus European Union sites. Prednisone and prednisolone were coadministered with abiraterone acetate at the North American and European Union sites, respectively. The data suggest a lower incidence of mineralocorticoid-related adverse events with prednisolone (10 mg) versus prednisone (10 mg) coadministered with abiraterone acetate (Table 1). This apparently lower incidence of events with prednisolone compared with prednisone was observed for grade 3 or 4 fluid retention or edema and hypokalemia in both studies and for grade 3 or 4 hypertension in COU-AA-302 but not in COU-AA-301. These data suggest that switching from prednisone to prednisolone might be a beneficial approach in patients who are more sensitive to abiraterone acetate-induced mineralocorticoid-related events as a consequence of deficient 11β-HSD1 activity, such as patients with concomitant liver disease. Additional studies would be required to test this hypothesis directly.
Table 1.
Mineralocorticoid-related adverse events in abiraterone acetate-treated patients in the COU-AA-302 and COU-AA-301 registrational trials with coadministered prednisone (North American sites) versus prednisolone (European Union sites).
Some glucocorticoid-related adverse events might be of lesser concern in patients with mCRPC compared with the general population; this reflects disease consequences or previous therapeutic interventions. Patients with mCRPC also are not likely to have received pancreatic β-cell-damaging chemotherapeutic regimens, which could influence the incidence and magnitude of hyperglycemia when glucocorticoids are used [64]. In addition, consequences of treatment-induced hyperglycemia need to be placed in the context of the reduced life expectancy of the mCRPC population [65]. Finally, patients with mCRPC, a population more prone to fractures due to prior androgen deprivation therapy and increased risk of bone metastasis, are often given prophylactic vitamin D and/or calcium supplements and bisphosphonates, and these treatments attenuate bone loss in this setting [66].
Most patients with mCRPC, particularly those with disease progression following docetaxel or hormone suppression therapy, already receive glucocorticoid therapy to manage disease-related symptoms [40, 67, 68]. In cases for which glucocorticoids are not being used, physicians and patients should discuss the benefits versus the risks of glucocorticoid use, including potential short-term and long-term effects. If a decision is made to start glucocorticoid therapy, patients should be monitored regularly to determine whether treatment is providing the desired effects (e.g., minimizing mineralocorticoid-related adverse events associated with abiraterone acetate) and to monitor for glucocorticoid-related adverse events. Although such adverse events have been observed in some patients receiving low-dose glucocorticoids, these consequences are typically associated with higher exposure of cumulative long-term dosing.
Conclusion
Glucocorticoid replacement therapy with low-dose prednisone substitutes for reduced cortisol levels, lowers ACTH levels, and in turn reduces the incidence and severity of mineralocorticoid-related adverse events in mCRPC patients treated with abiraterone acetate. The dose of prednisone indicated with abiraterone acetate and the glucocorticoid doses used in other treatment regimens for mCRPC are low (10 mg/day prednisone equivalence) compared with the therapeutic glucocorticoid doses used to treat other malignancies and in autoimmune disorders (typically ≥20 mg/day prednisone equivalence). It is these latter higher doses that have been consistently associated with an adverse safety profile. Available data suggest that prednisone, when given in low doses, exerts little effect on immune function and that safe and efficacious coadministration with immunomodulatory agents is possible. Nevertheless, further evaluation of the long-term safety profile of low-dose glucocorticoids for patients with mCRPC is warranted, given the improvements in survival achieved with progressive lines of glucocorticoid-containing regimens. These evaluations need to take into consideration the patient-specific factors such as physiologic status, including ability to metabolize prednisone; comorbidities; and length of androgen suppression. Simultaneously, the risks associated with the adverse effects of glucocorticoids in mCRPC must be balanced by considering all potential benefits, including pain relief and quality of life.
Acknowledgments
The analyses and studies described in this report were funded by Janssen Research & Development (formerly Ortho Biotech Oncology Research & Development, unit of Cougar Biotechnology). Writing assistance was provided by Shala Thomas and Ira Mills of PAREXEL and was funded by Janssen Global Services, LLC.
Author Contributions
Conception/Design: Richard J. Auchus, Margaret K. Yu, Suzanne Nguyen, Suneel D. Mundle
Collection and/or assembly of data: Richard J. Auchus, Margaret K. Yu, Suzanne Nguyen, Suneel D. Mundle
Data analysis and interpretation: Richard J. Auchus, Margaret K. Yu, Suzanne Nguyen, Suneel D. Mundle
Manuscript writing: Richard J. Auchus, Margaret K. Yu, Suzanne Nguyen, Suneel D. Mundle
Final approval of manuscript: Richard J. Auchus, Margaret K. Yu, Suzanne Nguyen, Suneel D. Mundle
Disclosures
Richard J. Auchus: Janssen Research & Development (RF); Margaret K. Yu: Janssen Research & Development (E); Johnson & Johnson (OI); Suzanne Nguyen: Janssen Scientific Affairs LLC, Johnson & Johnson (E); Johnson & Johnson (OI); Suneel D. Mundle: Janssen Scientific Affairs LLC, Johnson & Johnson (E); Johnson & Johnson (OI).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Bonkhoff H, Berges R. From pathogenesis to prevention of castration resistant prostate cancer. Prostate. 2010;70:100–112. doi: 10.1002/pros.21042. [DOI] [PubMed] [Google Scholar]
- 2.Van Allen EM, Ryan CJ. Novel secondary hormonal therapy in advanced prostate cancer: An update. Curr Opin Urol. 2009;19:315–321. doi: 10.1097/MOU.0b013e328329b73a. [DOI] [PubMed] [Google Scholar]
- 3.O’Donnell A, Judson I, Dowsett M, et al. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004;90:2317–2325. doi: 10.1038/sj.bjc.6601879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danila DC, Morris MJ, de Bono JS, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496–1501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reid AH, Attard G, Danila DC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28:1489–1495. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan CJ, Smith MR, Fong L, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28:1481–1488. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scher HI, Fizazi K, Saad F, et al. Association of baseline corticosteroid with outcomes in a multivariate analysis of the phase 3 AFFIRM study of enzalutamide (ENZA), an androgen receptor signaling inhibitor (ARSI) Ann Oncol. 2012;23(suppl 9):899PDa. [Google Scholar]
- 10. doi: 10.1016/j.eururo.2014.06.042. Montgomery RB, Kheoh TS, Molina A etal. Impact of baseline corticosteroids on survival and steroid androgens in metastatic castration-resistant prostate cancer: Exploratory analysis from COU-AA-301. Eur Urol 2014 [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Mostaghel EA, Nelson PS. Intracrine androgen metabolism in prostate cancer progression: Mechanisms of castration resistance and therapeutic implications. Best Pract Res Clin Endocrinol Metab. 2008;22:243–258. doi: 10.1016/j.beem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 13.Attard G, Reid AH, Auchus RJ, et al. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab. 2012;97:507–516. doi: 10.1210/jc.2011-2189. [DOI] [PubMed] [Google Scholar]
- 14.Debono M, Ross RJ, Newell-Price J. Inadequacies of glucocorticoid replacement and improvements by physiological circadian therapy. Eur J Endocrinol. 2009;160:719–729. doi: 10.1530/EJE-08-0874. [DOI] [PubMed] [Google Scholar]
- 15.Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: Final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 16.Richards J, Lim AC, Hay CW, et al. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: A rationale for increasing abiraterone exposure or combining with MDV3100. Cancer Res. 2012;72:2176–2182. doi: 10.1158/0008-5472.CAN-11-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mishra SK, Gupta N, Goswami R. Plasma adrenocorticotropin (ACTH) values and cortisol response to 250 and 1 microg ACTH stimulation in patients with hyperthyroidism before and after carbimazole therapy: Case-control comparative study. J Clin Endocrinol Metab. 2007;92:1693–1696. doi: 10.1210/jc.2006-2090. [DOI] [PubMed] [Google Scholar]
- 18.Ward LE, Polley HF, Power MH, et al. Prednisone in rheumatoid arthritis: Metabolic and clinical effects. Ann Rheum Dis. 1958;17:145–159. doi: 10.1136/ard.17.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grossmann C, Scholz T, Rochel M, et al. Transactivation via the human glucocorticoid and mineralocorticoid receptor by therapeutically used steroids in CV-1 cells: A comparison of their glucocorticoid and mineralocorticoid properties. Eur J Endocrinol. 2004;151:397–406. doi: 10.1530/eje.0.1510397. [DOI] [PubMed] [Google Scholar]
- 20.Costa-Santos M, Kater CE, Auchus RJ. Two prevalent CYP17 mutations and genotype-phenotype correlations in 24 Brazilian patients with 17-hydroxylase deficiency. J Clin Endocrinol Metab. 2004;89:49–60. doi: 10.1210/jc.2003-031021. [DOI] [PubMed] [Google Scholar]
- 21.Costenaro F, Rodrigues TC, Kater CE, et al. Combined 17α-hydroxylase/17,20-lyase deficiency due to p.R96W mutation in the CYP17 gene in a Brazilian patient. Arq Bras Endocrinol Metabol. 2010;54:744–748. doi: 10.1590/s0004-27302010000800014. [DOI] [PubMed] [Google Scholar]
- 22.Coursin DB, Wood KE. Corticosteroid supplementation for adrenal insufficiency. JAMA. 2002;287:236–240. doi: 10.1001/jama.287.2.236. [DOI] [PubMed] [Google Scholar]
- 23.Dorff TB, Crawford ED. Management and challenges of corticosteroid therapy in men with metastatic castrate-resistant prostate cancer. Ann Oncol. 2013;24:31–38. doi: 10.1093/annonc/mds216. [DOI] [PubMed] [Google Scholar]
- 24.Dahl R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med. 2006;100:1307–1317. doi: 10.1016/j.rmed.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Katz J. The practical use of corticosteroids in the treatment of inflammatory bowel disease. Pract Gastroenterol. 2005;29:14–25. [Google Scholar]
- 26.van Staa TP, Geusens P, Bijlsma JW, et al. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:3104–3112. doi: 10.1002/art.22117. [DOI] [PubMed] [Google Scholar]
- 27.Coulson KA, Reed G, Gilliam BE, et al. Factors influencing fracture risk, T score, and management of osteoporosis in patients with rheumatoid arthritis in the Consortium of Rheumatology Researchers of North America (CORRONA) registry. J Clin Rheumatol. 2009;15:155–160. doi: 10.1097/RHU.0b013e3181a5679d. [DOI] [PubMed] [Google Scholar]
- 28.Yeap SS, Hosking DJ. Management of corticosteroid-induced osteoporosis. Rheumatology (Oxford) 2002;41:1088–1094. doi: 10.1093/rheumatology/41.10.1088. [DOI] [PubMed] [Google Scholar]
- 29.Kelly HW, Van Natta ML, Covar RA, et al. Effect of long-term corticosteroid use on bone mineral density in children: A prospective longitudinal assessment in the childhood Asthma Management Program (CAMP) study. Pediatrics. 2008;122:e53–e61. doi: 10.1542/peds.2007-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumoto H, Ishihara K, Hasegawa T, et al. Effects of inhaled corticosteroid and short courses of oral corticosteroids on bone mineral density in asthmatic patients: A 4-year longitudinal study. Chest. 2001;120:1468–1473. doi: 10.1378/chest.120.5.1468. [DOI] [PubMed] [Google Scholar]
- 31.Speiser PW, White PC. Congenital adrenal hyperplasia. N Engl J Med. 2003;349:776–788. doi: 10.1056/NEJMra021561. [DOI] [PubMed] [Google Scholar]
- 32.Falhammar H, Filipsson Nyström H, Wedell A, et al. Bone mineral density, bone markers, and fractures in adult males with congenital adrenal hyperplasia. Eur J Endocrinol. 2013;168:331–341. doi: 10.1530/EJE-12-0865. [DOI] [PubMed] [Google Scholar]
- 33.Speiser PW, Azziz R, Baskin LS, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4133–4160. doi: 10.1210/jc.2009-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raúl Ariza-Andraca C, Barile-Fabris LA, Frati-Munari AC, et al. Risk factors for steroid diabetes in rheumatic patients. Arch Med Res. 1998;29:259–262. [PubMed] [Google Scholar]
- 35.Gallon LG, Winoto J, Leventhal JR, et al. Effect of prednisone versus no prednisone as part of maintenance immunosuppression on long-term renal transplant function. Clin J Am Soc Nephrol. 2006;1:1029–1038. doi: 10.2215/CJN.00790306. [DOI] [PubMed] [Google Scholar]
- 36.Brown ES. Effects of glucocorticoids on mood, memory, and the hippocampus. Treatment and preventive therapy. Ann N Y Acad Sci. 2009;1179:41–55. doi: 10.1111/j.1749-6632.2009.04981.x. [DOI] [PubMed] [Google Scholar]
- 37.Bender BG, Lerner JA, Kollasch E. Mood and memory changes in asthmatic children receiving corticosteroids. J Am Acad Child Adolesc Psychiatry. 1988;27:720–725. doi: 10.1097/00004583-198811000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Bolanos SH, Khan DA, Hanczyc M, et al. Assessment of mood states in patients receiving long-term corticosteroid therapy and in controls with patient-rated and clinician-rated scales. Ann Allergy Asthma Immunol. 2004;92:500–505. doi: 10.1016/S1081-1206(10)61756-5. [DOI] [PubMed] [Google Scholar]
- 39.Berry W, Dakhil S, Modiano M, et al. Phase III study of mitoxantrone plus low dose prednisone versus low dose prednisone alone in patients with asymptomatic hormone refractory prostate cancer. J Urol. 2002;168:2439–2443. doi: 10.1016/S0022-5347(05)64163-8. [DOI] [PubMed] [Google Scholar]
- 40.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 41.Romero S, Stanton G, DeFelice J, et al. Phase II trial of thalidomide and daily oral dexamethasone for treatment of hormone refractory prostate cancer progressing after chemotherapy. Urol Oncol. 2007;25:284–290. doi: 10.1016/j.urolonc.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 42.Rathkopf DE, Smith MR, de Bono JS, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302) Eur Urol. 2014 doi: 10.1016/j.eururo.2014.02.056. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Owczarek J, Jasińska M, Orszulak-Michalak D. Drug-induced myopathies. An overview of the possible mechanisms. Pharmacol Rep. 2005;57:23–34. [PubMed] [Google Scholar]
- 44.Hollister JR. The untoward effects of steroid treatment on the musculoskeletal system and what to do about them. J Asthma. 1992;29:363–368. doi: 10.3109/02770909209044799. [DOI] [PubMed] [Google Scholar]
- 45.Lim HY, Müller N, Herold MJ, et al. Glucocorticoids exert opposing effects on macrophage function dependent on their concentration. Immunology. 2007;122:47–53. doi: 10.1111/j.1365-2567.2007.02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marzocchi-Machado CM, Russo EM, Alves CM, et al. Effect of low-dose prednisone in vivo on the ability of complement receptor to mediate an oxidative burst in rat neutrophils. Immunopharmacology. 2000;49:247–254. doi: 10.1016/s0162-3109(00)00204-6. [DOI] [PubMed] [Google Scholar]
- 47.Kanakoudi-Tsakalidou F, Trachana M, Pratsidou-Gertsi P, et al. Influenza vaccination in children with chronic rheumatic diseases and long-term immunosuppressive therapy. Clin Exp Rheumatol. 2001;19:589–594. [PubMed] [Google Scholar]
- 48.Park CL, Frank AL, Sullivan M, et al. Influenza vaccination of children during acute asthma exacerbation and concurrent prednisone therapy. Pediatrics. 1996;98:196–200. [PubMed] [Google Scholar]
- 49.Chalmers A, Scheifele D, Patterson C, et al. Immunization of patients with rheumatoid arthritis against influenza: A study of vaccine safety and immunogenicity. J Rheumatol. 1994;21:1203–1206. [PubMed] [Google Scholar]
- 50.Crowe SR, Merrill JT, Vista ES, et al. Influenza vaccination responses in human systemic lupus erythematosus: Impact of clinical and demographic features. Arthritis Rheum. 2011;63:2396–2406. doi: 10.1002/art.30388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naito M, Itoh K, Komatsu N, et al. Dexamethasone did not suppress immune boosting by personalized peptide vaccination for advanced prostate cancer patients. Prostate. 2008;68:1753–1762. doi: 10.1002/pros.20847. [DOI] [PubMed] [Google Scholar]
- 52.Small EJ, Lance RS, Redfern CH, et al. A randomized phase II trial of sipuleucel-T with concurrent or sequential abiraterone acetate (AA) plus prednisone (P) in metastatic castrate-resistant prostate cancer (mCRPC) J Clin Oncol. 2013;31(suppl):5047a. doi: 10.1158/1078-0432.CCR-15-0079. [DOI] [PubMed] [Google Scholar]
- 53.Attard G, Reid AH, A’Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–3748. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loprinzi CL, Kugler JW, Sloan JA, et al. Randomized comparison of megestrol acetate versus dexamethasone versus fluoxymesterone for the treatment of cancer anorexia/cachexia. J Clin Oncol. 1999;17:3299–3306. doi: 10.1200/JCO.1999.17.10.3299. [DOI] [PubMed] [Google Scholar]
- 55.Zapanti E, Terzidis K, Chrousos G. Dysfunction of the hypothalamic-pituitary-adrenal axis in HIV infection and disease. Hormones (Athens) 2008;7:205–216. doi: 10.14310/horm.2002.1200. [DOI] [PubMed] [Google Scholar]
- 56.Storlie JA, Buckner JC, Wiseman GA, et al. Prostate specific antigen levels and clinical response to low dose dexamethasone for hormone-refractory metastatic prostate carcinoma. Cancer. 1995;76:96–100. doi: 10.1002/1097-0142(19950701)76:1<96::aid-cncr2820760114>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 57.Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: A Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14:1756–1764. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 58.Venkitaraman R, Thomas K, Huddart RA, et al. Efficacy of low-dose dexamethasone in castration-refractory prostate cancer. BJU Int. 2008;101:440–443. doi: 10.1111/j.1464-410X.2007.07261.x. [DOI] [PubMed] [Google Scholar]
- 59.Arora VK, Schenkein E, Murali R, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155:1309–1322. doi: 10.1016/j.cell.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steketee K, Timmerman L, Ziel-van der Made AC, et al. Broadened ligand responsiveness of androgen receptor mutants obtained by random amino acid substitution of H874 and mutation hot spot T877 in prostate cancer. Int J Cancer. 2002;100:309–317. doi: 10.1002/ijc.10495. [DOI] [PubMed] [Google Scholar]
- 61.Diederich S, Eigendorff E, Burkhardt P, et al. 11Beta-hydroxysteroid dehydrogenase types 1 and 2: An important pharmacokinetic determinant for the activity of synthetic mineralo- and glucocorticoids. J Clin Endocrinol Metab. 2002;87:5695–5701. doi: 10.1210/jc.2002-020970. [DOI] [PubMed] [Google Scholar]
- 62.Hardy R, Rabbitt EH, Filer A, et al. Local and systemic glucocorticoid metabolism in inflammatory arthritis. Ann Rheum Dis. 2008;67:1204–1210. doi: 10.1136/ard.2008.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lawson AJ, Walker EA, Lavery GG, et al. Cortisone-reductase deficiency associated with heterozygous mutations in 11beta-hydroxysteroid dehydrogenase type 1. Proc Natl Acad Sci USA. 2011;108:4111–4116. doi: 10.1073/pnas.1014934108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brunello A, Kapoor R, Extermann M. Hyperglycemia during chemotherapy for hematologic and solid tumors is correlated with increased toxicity. Am J Clin Oncol. 2011;34:292–296. doi: 10.1097/COC.0b013e3181e1d0c0. [DOI] [PubMed] [Google Scholar]
- 65.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: A patient-centered approach: Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Droz JP, Balducci L, Bolla M, et al. Background for the proposal of SIOG guidelines for the management of prostate cancer in senior adults. Crit Rev Oncol Hematol. 2010;73:68–91. doi: 10.1016/j.critrevonc.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 67.Second-line treatment of metastatic prostate cancer. Prednisone and radiotherapy for symptom relief. Prescrire Int. 2013;22:74–78. [PubMed] [Google Scholar]
- 68.Heng DY, Chi KN. Prednisone monotherapy in asymptomatic hormone refractory prostate cancer. Can J Urol. 2006;13:3335–3339. [PubMed] [Google Scholar]