Clinical and genetic differences between patients with cancer-induced bone pain (CIBP) and patients with non-CIBP, as well as good versus poor opioid response in those with CIBP, were evaluated. No specific genetic pattern emerged for CIBP versus non-CIBP and for responsive versus nonresponsive patients with CIBP.
Keywords: Cancer-induced bone pain, Haplotypes, Opioids, Palliative care, Assessment, Genetic factors
Abstract
Objective.
The study objective was to evaluate whether there are clinical or genetic differences between patients with cancer-induced bone pain (CIBP) and patients with non-CIBP, and, in the CIBP group, in those with good versus poor opioid response.
Materials and Methods.
A total of 2,294 adult patients with cancer who were receiving opioids for moderate or severe pain were included in the European Pharmacogenetic Opioid Study. Pain intensity and pain relief were measured using the Brief Pain Inventory. Linkage disequilibrium of 112 single nucleotide polymorphisms was evaluated in 25 candidate genes, and 43 haplotypes were assessed. Correlations among demographical factors, disease-related factors, genetic factors, CIBP, and pain relief were analyzed by logistic regression models corrected for multiple testing. Patients with bone metastases and bone/soft tissue pain were defined as having prevalent bone pain (CIBP population). This population was compared with patients who had other types of cancer pain (non-CIBP).
Results.
A total of 577 patients (26.2%) had CIBP, and 1,624 patients (73.8%) had non-CIBP. Patients with CIBP had more breakthrough cancer pain episodes (64.2% vs. 56.4%, p = .001), had significantly higher pain interference in “walking ability in the past 24 hours” (p < .0001), used more adjuvant drugs (84.1% vs. 78.3%, p = .003), and had a higher, albeit nonsignificant, median overall survival (3.8 vs. 2.9 months, p = .716) than patients with non-CIBP. None of the examined haplotypes exceeded p values corrected for multiple testing for the investigated outcomes.
Conclusion.
Patients with CIBP who were taking opioids had a clinical profile slightly different from that of the non-CIBP group. However, no specific genetic pattern emerged for CIBP versus non-CIBP or for responsive versus nonresponsive patients with CIBP.
Implications for Practice:
The objective of this study was to identify the characteristics that would make patients with bone pain “different” from those with pain of any kind. Genetic peculiarity has not emerged, and from this point of view the study is negative. However, the study has shown some important clinical features. Patients with cancer-induced bone pain (CIBP) had more breakthrough pain, had greater difficulty in walking, used more adjuvant drugs, and had a slightly longer median overall survival than patients with non-CIBP. Patients with bone pain are carriers of a major symptom burden for a long time; thus, their management is clinically complicated and requires relevant attitudes and skills.
Introduction
More than half of all patients with cancer experience pain [1]. Cancer-induced bone pain (CIBP) is a frequent cause of cancer pain [2–4]. Pecherstorfer and Vesely [5] reported that CIBP was present in 34% of hospitalized patients with cancer and 45% of patients enrolled in a palliative home-care program. In most cases, CIBP is associated with metastatic disease and caused by tumor involvement of the bone. Tumor cells can elicit bone pain by causing pathological fractures and microfractures or by invading sensory nerve endings. Tumor burden causes oxidative stress, and disruption of the normal bone homeostasis will result in an acidic microenvironment. CIBP also is associated with the release of inflammatory cytokines. These biological processes are thought to mediate pain, but the experience of pain is closely related to characteristics of the “host” [3, 6–8].
Patients with CIBP often experience a combination of constant pain and pain exacerbations, either spontaneous or episodic pain provoked by movement or weight bearing [7–11]. Because of the complex etiology and clinical fluctuations of pain intensities, treatment of CIBP often needs to be multimodal. Therapy can involve both pharmacological and nonpharmacological approaches, including the administration of various analgesics, bisphosphonates, radiation therapy, and surgery [12, 13].
Opioids represent the basis of analgesic therapy of CIBP [14–18] despite the lack of controlled clinical trials on the effectiveness of opioids in pain due to bone metastases [19]. There is a large interindividual variability in opioid efficacy. This variability has initiated numerous studies on the effect of genotype for opioid pharmacokinetics and pharmacodynamics. These studies are designed to assess the influence of single nucleotide polymorphisms (SNPs) in candidate pain genes encoding receptors and ion channels implicated in pain modulation [20] and/or the effect of genes encoding drug-metabolizing enzymes and transporters on analgesic drug pharmacokinetics [21]. A theoretical aim would be to develop “point-of-care genotyping devices” to tailor analgesic drug therapy to the individual patient [22, 23]. Some preliminary studies show promising results, but these have not been replicated across larger studies, and the ultimate aim of personalized prescribing remains elusive [24–28].
One explanation for the lack of consistent genetic association with opioid efficacy for cancer pain may be that the studies usually include unselected cohorts of patients with cancer pain. Because CIBP represents pain etiology with its own biology, important clinical appearance can be missed in a larger study including all subgroups of cancer pain. Therefore, this study represents a secondary analysis of the European Pharmacogenetic Opioid Study (EPOS), whose primary aim was to assess the influence from genetic variability on opioid use for cancer pain [27]. The study analyzed 2,294 patients with cancer in an unselected group of patients with cancer and observed no statistically significant relations between genetic variability and opioid dose. In this study, we investigated the specific characteristics of CIBP and address whether there are clinical or genetic differences between patients with CIBP and patients with non-CIBP. The study also assessed whether there is genetic variability in patients with CIBP with good versus poor opioid response.
Materials and Methods
Study Design and Patients
A total of 2,294 patients with cancer were recruited in the international, multicenter, cross-sectional EPOS [27], with the principal aim to examine the association between genetic variations and opioid efficacy on cancer pain relief. Patients were eligible if they had verified malignant disease, were aged ≥18 years, and were scheduled with opioid treatment for moderate to severe pain for at least 3 days. Patients who were not capable of speaking the language used at the study center were excluded. A total of 93 patients were excluded from the genetic association analyses (because 53 were of nonwhite ethnicity, 5 patients were Greek, 24 did not have a blood sample, and 11 withdrew from the study). Thus, 2,201 white patients were included in the final genetic analyses.
Study Procedure and Assessment
At the time of inclusion, the study collected the following information: demographic data (age, gender, ethnicity), cancer diagnosis, presence of metastasis, weight, height, place of treatment, opioid treatment details (medications and dosages, time since opioid treatment started), and use of nonopioid and adjuvant medication. Opioid doses were converted to equivalent total daily oral morphine doses using standard tables [29]. Pain mechanisms were assessed according to the Revised Edmonton Staging System for cancer pain (visceral, bone soft tissue, neuropathic, mixed, unknown pain) [30]. Breakthrough pain was evaluated as present or not present. Pain intensities were measured using the Brief Pain Inventory, an 11-point numeric rating scale from 0 (“no pain”) to 10 (“pain as bad as you can imagine”) [31]. Pain relief was evaluated in percentage from 0% (“no pain relief”) to 100% (“complete pain relief”) [31].
Cognitive function was assessed by the Mini-Mental State Examination [32, 33], scoring from 0 to 30 (0–23 definite cognitive dysfunction, 24–26 possible cognitive dysfunction, 27–30 no cognitive dysfunction). Functional status was assessed by the Karnofsky Performance Status [34], scoring from 0% to 100%, with higher scores meaning better function.
The European Organization for Research and Treatment of Cancer Quality of Life (EORTC QLQ)-C30 version 3.0 [35] was used to assess the patient’s self-reported quality of life (EORTC QLQ-C30), which consists of 30 questions comprising 5 functional scales (physical, role, cognitive, emotional, and social), 3 symptom scales (fatigue, pain, and nausea/vomiting), global health status, perceived financial impact of the disease, and single items to evaluate additional symptoms (dyspnea, loss of appetite, insomnia, constipation, and diarrhea). Scores are linearly transformed into scales of 0 to 100 (100 corresponds to high health-related quality of life for global health status and the 5 functional scales, whereas symptom burden and financial impact correspond to maximum difficulty). A validated version in the language of each study center was applied for all instruments. Data from overall survival (OS) were available for 1,879 of 2,201 patients.
Patients with bone metastases and bone/soft tissue pain were defined as patients with prevalent bone pain (CIBP population). This population was compared with patients with pain not originating from bone metastases (non-CIBP population). Patients with a pain relief score of 90% or more were defined as “good responders” to opioid therapy, and patients with a pain relief score of 40% or less were defined as “poor responders.”
The study was conducted in accordance with the Declaration of Helsinki on Biomedical Research Involving Human Subjects. Approval from the ethics committees of each participating center was obtained, and each participating subject provided written informed consent.
Polymorphism Analyses
Whole blood samples were obtained and DNA extraction and SNP analyses were performed as previously described [27]. Individuals with a minimum allele frequency of less than 5% or in violation with the Hardy-Weinberg equilibrium (chi-square test, p < .0005) were excluded [36]. The selection of genes was based on genes reported to be candidates for genetic variability related to pain or genes coding molecules with a putative role in inflammation and pain physiology. SNPs were chosen according to location and expected frequency. To evaluate the linkage disequilibrium between the SNPs, we used the haplotype analysis software Haploview 4.2 (Broad Institute, Cambridge, MA, http://www.broadinstitute.org/scientific-community/science/programs/medical-and-population-genetics/haploview/haploview) [37] using the default algorithm. In our sample set, we could predict the following haplotype blocks: OPRD1 (rs678849-rs2236857), CRP (rs1130864-rs1800947), IL10 (rs1800872-rs1800896), TGFB2 (rs1418553-rs1890995), TACR1 (rs6725334-rs2024512), IL1A (rs17561-rs1800587), IL1B (rs1143627-rs16944), CXCR7 (rs10183022-rs9287599), HRH1 (rs2606731-rs346076), DRD3 (rs3732790-rs963468-rs377367-rs1677719), DRD3 (rs6280-rs324026), HTR3D (rs939334-rs6792482-rs6443930), HTR3C (rs6766410-rs6807362), HTR3E (rs6443950-rs7627615), HTR3E (rs7432211-rs4912522), TFRC (rs2284890-rs3817672), CCKAR (rs7665027-rs2000978-rs2040342-rs3822222), TLR2 (rs4696480-rs3804100), HINT1 (rs3852209-rs2551038-rs3864283), ADRB2 (rs1042713-rs1042714-rs1042717), GABBR1 (rs740882-rs29261-rs29259), TNF (rs17999641-rs1800629), CNR1 (rs806368-rs12720071-rs1049353), OPRM1 (rs9479757-rs540825-rs562859-rs548646-rs1323042-rs618207-rs639855-rs497976), IL6 (rs1800795-rs2069835-rs1554606-rs2069845), CAK2B (rs10441113-rs4526269), ABCB1 (rs1045642-rs4437575), ABCB1 (rs2235013-rs2235033-rs1128503-rs1202170), GABBR2 (rs108187393-rs10818743), CCKBR (rs2941029-rs2880898), IL18 (rs360729-rs549908), IL18 (rs5744256-rs2043055-rs187238), DRD2 (rs1554929-rs6279-rs1125394), DRD2 (rs7131440-rs7122246), DRD2 (rs7131056-rs4648317), HTR3B (rs7103572-rs1176744), TNFRSF1A (rs767455-rs4149570), STAT6 (rs3024971-rs167769), IFNG (rs2193049-rs2069727-rs2430561), ARRB2 (rs4790693-rs3786047-rs1045280-rs2271167-rs2036657), ARRB2 (rs4329-rs4341-rs4362), COMT (rs2020917-rs5993882), and COMT (rs4646312-rs165722-rs4680).
Statistical Analysis
The comparisons between patients with CIBP and patients with non-CIBP related to clinical data or haplotypes were analyzed by logistic regression models. Odds ratios and 95% confidence intervals (CIs) were adjusted for gender and country of origin. To mitigate the issue of multiple testing, a false discovery rate (FDR) of less than 10% was used to determine haplotypes associated with the dependent variable bone pain. FDR was controlled using the Benjamini-Hochberg step-up procedure [38], on the basis of all tests for significance of covariates for the regression models. We examined models in which haplotypes were given dominant, codominant, or recessive effects.
Overall survival was calculated from the date of inclusion in the study to the date of death as a result of any reason. OS was estimated using the Kaplan-Meier life-table method and compared by a 2-sided log-rank test. All statistical analyses were carried out with SAS software (version 9.3, SAS Institute, Inc., Cary, NC, http://www.sas.com).
Results
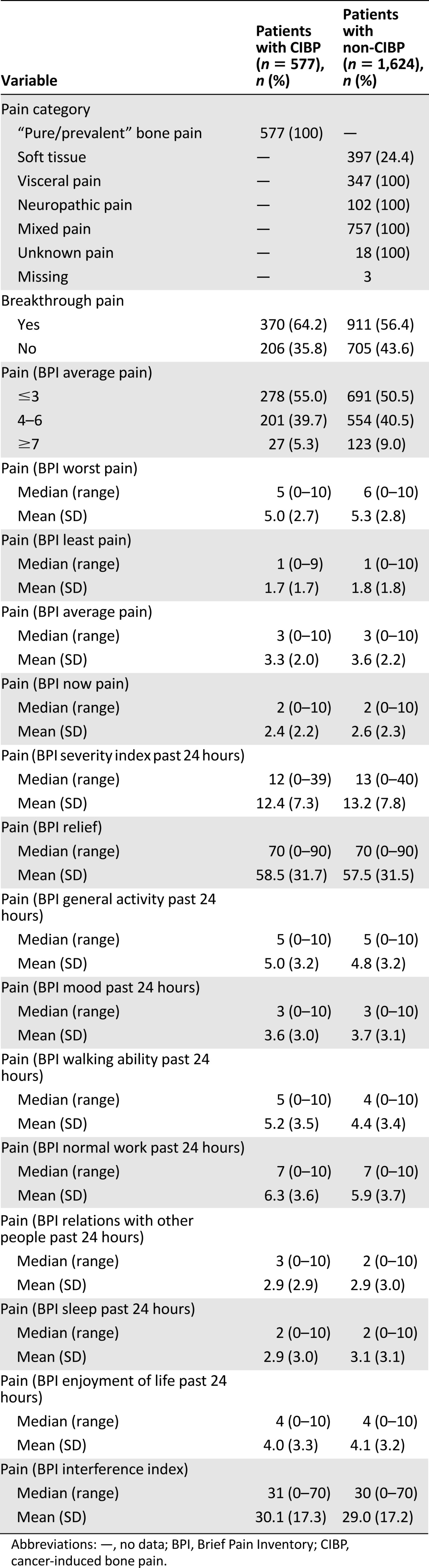
Clinical and demographic characteristics of the patients with CIBP (n = 577, 26.2%) and patients with non-CIBP (n = 1,624, 73.8%) were similar except for cancer diagnoses and presence of bone metastasis (Table 1). Prostate and breast cancer were more frequent in those with CIBP, whereas gastrointestinal and cancer in reproductive organs were more frequent in those with non-CIPB. Patients with CIBP had by definition 100% presence of bone metastasis, whereas 26% of the patients with non-CIBP had bone metastases. The reports related to pain were generally similar (Table 2). However, episodes of breakthrough cancer pain were present in 370 patients (64.2%) with CIBP and 911 patients (56.4%) with non-CIBP (p = .001). Patients with bone pain had a significantly higher pain interference in “walking ability past 24 hours” (p < .0001).
Table 1.
Characteristics of study population
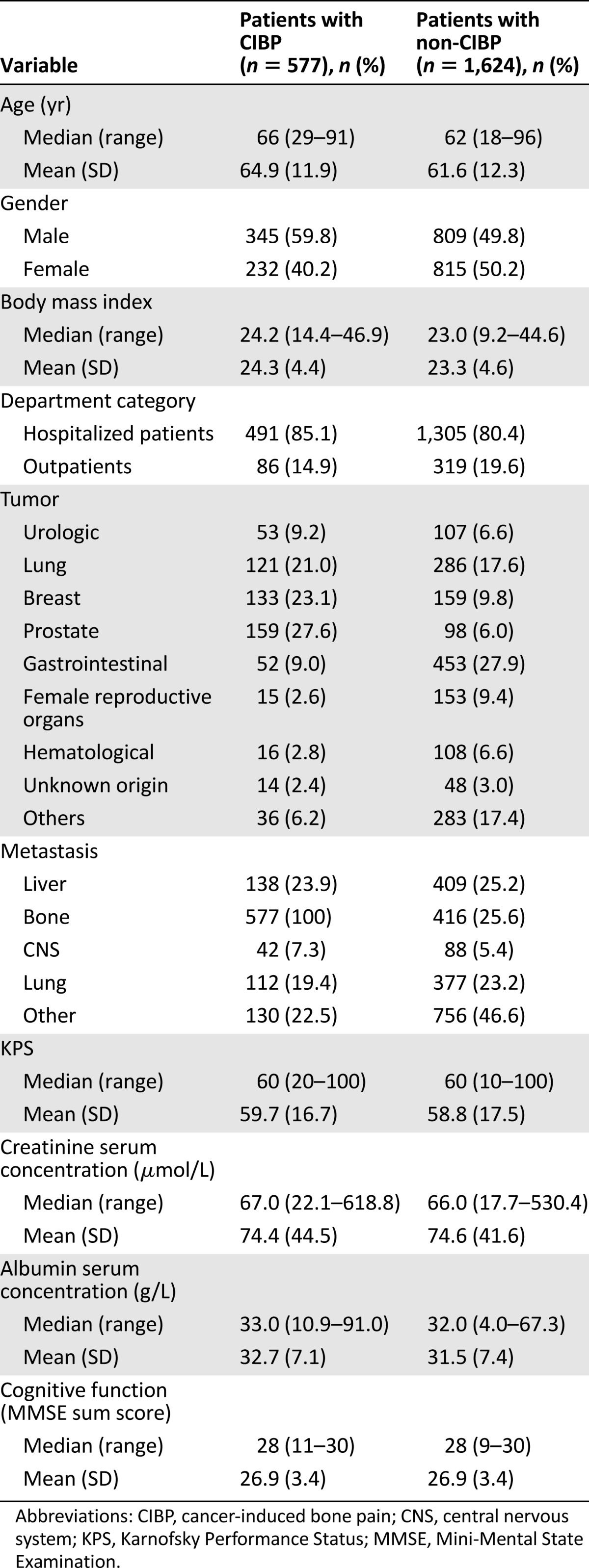
Table 2.
Characteristics of study population related to cancer pain
Analysis of quality of life reveals that physical and role function scales were associated with patients with CIBP or non-CIBP. Mean (SD) scores of these scales of EORTC QLQ-C30 were higher in those with non-CIBP than in those with CIBP (42.1 [26.2] vs. 34.8 [24.0], p < .0001 for physical function and 28.9 [31.1] vs. 23.1 [26.7], p = .0002 for role function). In regard to symptoms, patients with CIBP had a mean constipation score of 50.2 (37.2) compared with 43.2 (36.8) for patients with non-CIBP (p = .0003) and a mean pain score of 63.8 (27.7) compared with 61.4 (28.5), respectively (p = .096).
The median opioid dose expressed as the oral equivalent daily morphine use was similar in the two groups of patients: CIBP group, median value 160 mg/24 hours (quartiles 80–380); non-CIBP group, median value 180 mg/24 hours (quartiles 80–400) (p = .10). More details on the use of analgesics are shown in Table 3. Patients with CIBP more often used paracetamol or corticosteroids, whereas nonsteroidal anti-inflammatory drugs and gabapentins were equally applied (Table 3). Globally, patients with CIBP received adjuvant drugs more frequently than patients with non-CIBP (84.1% vs. 78.3%; p = .003).
Table 3.
Use of analgesics (as the primary opioid)
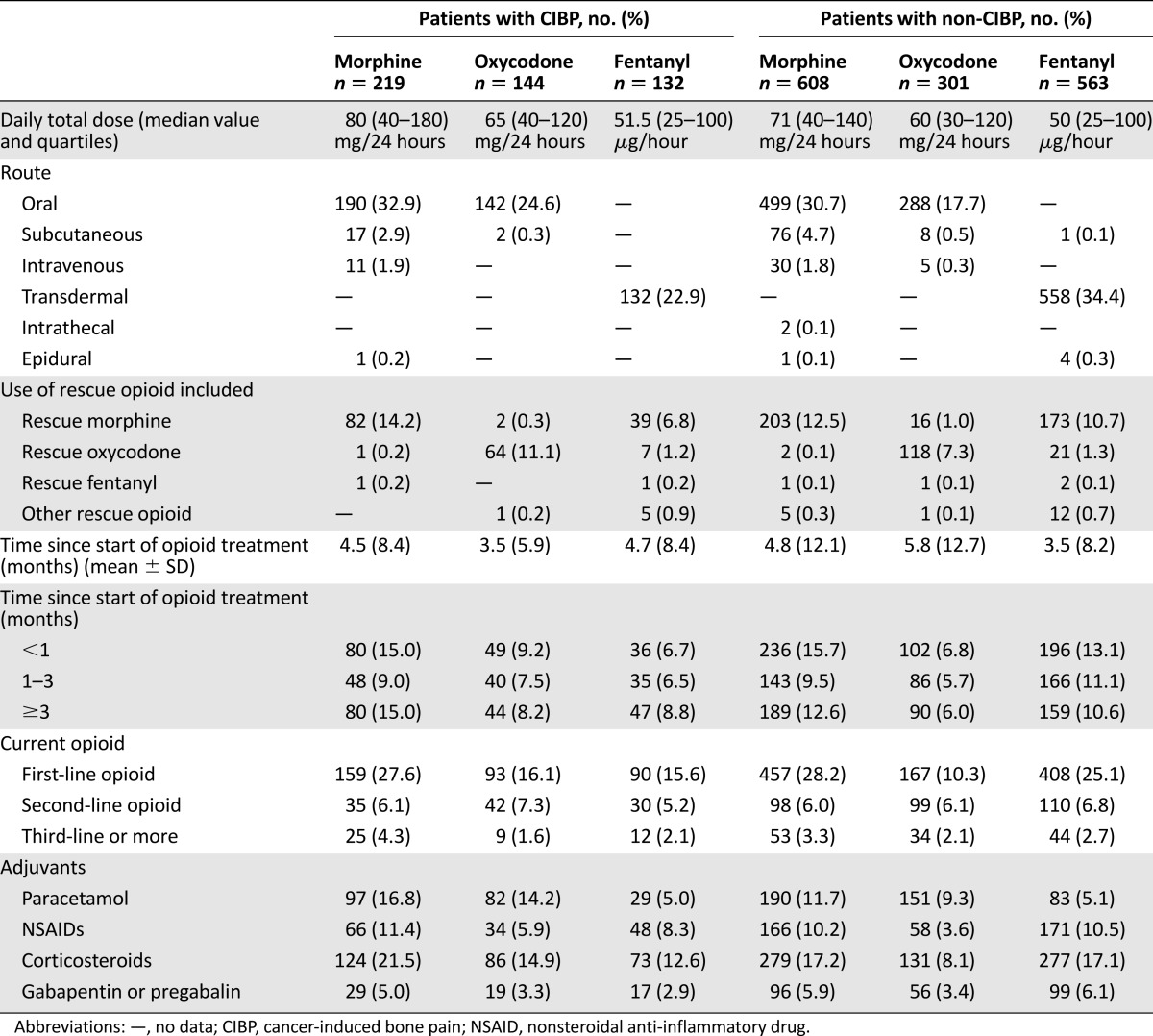
The efficacy of opiates to relieve the pain was similar in the CIBP and non-CIBP groups. Median opioid doses of morphine, oxycodone, and fentanyl were 80 mg/24 hours, 60 mg/24 hours, and 75 μg/hour, respectively, for patients with CIBP compared with 60 mg/24 hours, 55 mg/24 hours, and 50 μg/hour, respectively, for patients with non-CIBP (p = .088, p = .075, and p = .090, respectively).
In regard to life expectancy, patients with CIBP had a higher, albeit nonsignificant, median OS (3.8 months; 95% CI, 3.2%–4.2%) than patients with non-CIBP (2.9 months; 95% CI, 2.6%–3.3%) (p = .716). The median OS of patients with CIBP who received high doses of opiates and experienced relief of pain was higher than in patients who received high doses of opiates and did not experience relief of pain: morphine, 7.2 (2.9–10.2) months versus 4.5 (1.7–5.5) months (p = .573); oxycodone, 6.6 (3.2–9.0) months versus 2.7 (0.5–4.1) months (p = .004); and fentanyl, 3.4 (1.8–4.6) months versus 2.4 (0.6–13.4) months (p = .476).
Haplotypes Analyses and Pain
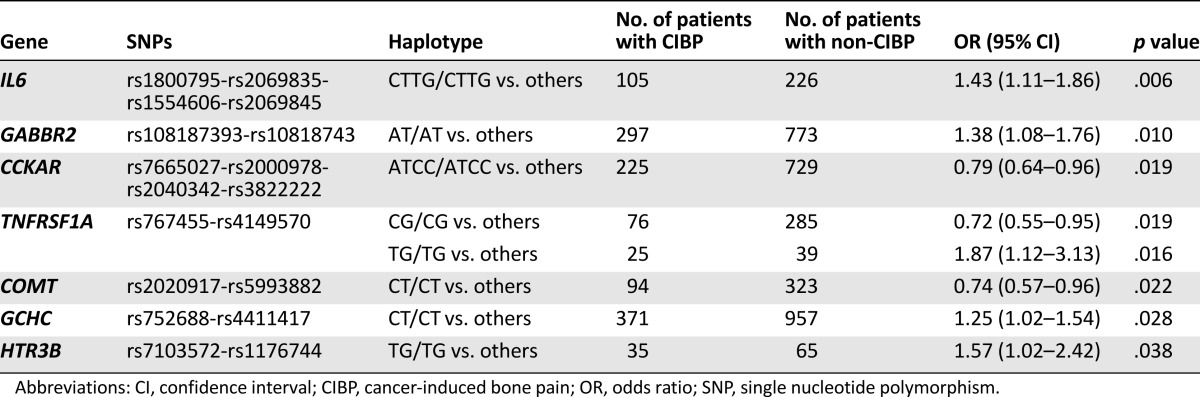
Of 125 SNPs analyzed, 13 were excluded from the study: 3 SNPs (rs1202181 in ABCB1, rs7175823 in CHRM5, and rs33940208 in HTR3A) because no genotypes were recorded and 10 SNPs (rs7815824 in OPRK1, rs16954146 in ARRB2, rs4878 and rs11558046 in HINT1, rs1805009 in MC1R, rs1800496 in DRD2, rs1799920 in HTR1, rs34327364 in HTR3A, rs3831455 in HTR3B, and rs34826744 in HTR4) because allele frequency was less than 5%. All remaining 112 SNPs were in Hardy-Weinberg equilibrium and were retained for analyses. A linkage disequilibrium analysis was performed for all SNPs according to gene position. We identified 43 different haplotype blocks that were evaluated according to bone pain and pain relief. Among these, 7 haplotypes, in 6 different genes, were associated (uncorrected p < .05) with the presence of CIBP, but none exceeded the Benjamini-Hochberg criterion (Table 4).
Table 4.
Haplotypes with an initial significant association with the presence of CIBP
Patients with CIBP who experienced good pain relief and poor pain relief were analyzed with respect to haplotype variability. Two haplotypes were associated with a good pain relief response (uncorrected p < .05) (Table 5). However, none of the associations passed the Benjamini-Hochberg criterion in this case.
Table 5.
Haplotype with an initial significant association with pain relief in patients with CIBP

Discussion
In this study comparing patients with CIBP and patients with non-CIBP, we observed that except for more frequent episodes of breakthrough pain, reduced ability to walk, and more use of corticosteroids, the two pain groups were rather similar. In a selection of pain-relevant genes, we did not find any genetic variability, which after correction for multiple testing was significantly associated with pain relief from opioid analgesic therapy.
Not all patients with bone metastases exhibit symptoms. Still, as many as two thirds of patients with metastatic bone disease experience severe pain, particularly those who are in advanced stages of cancer disease [39]. Bone involvement increases the risk of serious complications, such as pathologic fractures, hypercalcemia, and bone marrow compression, and significantly reduces quality of life. In the absence of metastatic disease at other sites, the presence of bone metastases is associated with a relatively long life expectancy [40–43]. Therefore, optimizing treatment is important, but CIBP can be a therapeutic challenge because of a combination of an ongoing baseline component, neuropathic pains, and episodes of breakthrough pain.
This study represents an expansion of previously published analyses in an unselected population with cancer pain. In this study, all patients with cancer regardless of pain etiology were included [27]. Cancer pain may be a result of several pain mechanisms, each with the potential for a unique pain pathophysiology. Thus, the negative findings from this study may be caused by a variable phenotype, for instance, pain caused by bone pain versus pain caused by nerve destruction. Therefore, we wanted to analyze the special characteristics and potential genetic influence in CIBP. In our study, patients with bone pain, as expected, more frequently had cancer diagnoses that are associated with a higher risk of bone metastases (e.g., breast and prostate cancer). In a study by Berger et al. [44], patients with bone metastasis required more frequent treatment with opioids. However, in our study in which patient inclusion was based on the use of opioids, we did not observe any differences in opioid dose for those with bone pain. Also, for other characteristics, the similarities between those with and without CIBP were more striking than the differences. In addition to more frequent breakthrough pain, reduced ability to walk and more use of corticosteroids in the two populations were similar. This observation implies that in patients with moderate or severe pain indicating the use of opioids, similar clinical observations can be expected regardless of the presence of bone pain.
In our previously published study including unselected patients with cancer pain, the candidate genes were primarily selected on the basis of their known or putative involvement in opioid analgesia. The results suggested that none of 112 SNPs in 25 candidate genes showed significant associations with opioid dose, and these findings did not support the use of pharmacogenetic analyses for the assessed SNPs to guide opioid treatment. Later reviews on the role of COMT and OPRM1 genes demonstrated negative results [45, 46]. Still, the individual variability of opioid pharmacology suggests that patients’ genetic characteristics influence the response to opioids. Thus, the lack of genetic association may be caused by an imprecise phenotype and/or the study has not analyzed the relevant genes. In this study, we have made an effort to address these two issues: first, by selecting a cohort with bone cancer pain, and second, by adding genes important for inflammation. Still, we did not observe any genetic variations that were convincingly predictive of pain relief.
Generally, in studies evaluating the association of polymorphisms with palliative therapies, each single SNP has been correlated separately with pain perception and/or analgesic efficacy, and few data are available on the association and clustering of SNPs in specific haplotypes. By evaluating the nonrandom association of single alleles, we identified a number of haplotype blocks. Several of these showed an initial association with bone pain and pain relief, but none could be concluded to represent a significant association because they did not exceed the Benjamini-Hochberg criterion. The lack of a statistical association of molecular markers with bone pain and pain relief could be due to some limitations of the study. First, the low number of cases for some haplotypes may limit statistical power and result in false-negative findings. Second, the categorization of bone pain was based on the clinical decision by the treating physician, and the presence of bone metastasis was based on available clinical evidence. It is evident that clinicians may err in the categorization, and patients with advanced cancer may have bone metastasis not detected by examination. The presence of bone metastasis and pain is not equal to the presence of bone pain. Finally, in this study a candidate gene approach was selected primarily on the basis of inflammation and opioid pharmacogenetics. Other genes involved in other aspects of bone pain physiology or in opioid pharmacology could certainly be involved, and other genetic analytical approaches could be applied to detect effects from unknown, important genetic variability.
Conclusion
This secondary analysis showed that patients with CIBP had more breakthrough pain, had less walking ability, used more corticosteroids, and had a slightly higher overall survival than patients with other pain causes. Other clinical and treatment characteristics, in general, were similar. We did not observe any genetic variability that predicted pain intensity or pain relief in patients with CIBP. Further research is needed to elucidate the role, if any, of genetic predispositions in the management of CIBP.
Acknowledgments
We thank all principal investigators of each center contributing to the EPOS: G. Jakobsen and T. Nilsen (Trondheim, Norway), J. Havard Loge (Oslo, Ulleval, Norway), K. Bjordal (Oslo, Radiumhospitalet, Norway), P. Sjøgren (Copenhagen, Denmark), E. Salminen (Turku, Finland), M. Kloke (Essen, Germany), L. Radbruch (Aachen, Germany), R. Sabatowski (Dresden, Germany), E. Argyra (Athens, Greece), V. Sigurdardottir and S. Gunnarsdottir (Reykjavik, Iceland), D. Miotti (Pavia, Italy), A. Pigni and A. Caraceni (Milan, Italy), C. Modonesi, S. Derni, L. Fabbri, F. Martini, M. Dall’Agata (Forli, Italy), I. Poviloniene (Vilnius, Lithuania), S. Lundström (Stockholm, Sweden), F. Strasser (St. Gallen, Switzerland), and A. Davies (Sutton/Chelsea, United Kingdom). The Norwegian Research Council and the European Union’s 6th framework (Contract 037777) financially supported the EPOS.
Footnotes
For Further Reading:Charles J. Ryan, Philip J. Saylor, Jason J. Everly et al. Bone-Targeting Radiopharmaceuticals for the Treatment of Bone-Metastatic Castration-Resistant Prostate Cancer: Exploring the Implications of New Data. The Oncologist 2014;19:1012–1018.
Implications for Practice:Bone metastases remain a clinical challenge in prostate cancer and lead to substantial morbidity and impairment of quality of life. A number of bone-targeted agents have been studied and approved in this setting for their abilities to palliate bone pain or to prevent skeletal events, but radium-223 is the first agent to produce an improvement in overall survival. Further study is required to optimize clinical use of radium-223 in sequence or in combination with other available agents.
Author Contributions
Conception/Design: Emanuela Scarpi, Pål Klepstad, Frank Skorpen, Oriana Nanni, Marco Maltoni
Provision of study material or patients: Pål Klepstad, Stein Kaasa, Frank Skorpen
Collection and/or assembly of data: Emanuela Scarpi, Daniele Calistri, Pål Klepstad, Stein Kaasa, Frank Skorpen, Marco Maltoni
Data analysis and interpretation: Emanuela Scarpi, Daniele Calistri, Pål Klepstad, Stein Kaasa, Ragnhild Habberstad, Oriana Nanni, Dino Amadori, Marco Maltoni
Manuscript writing: Emanuela Scarpi, Daniele Calistri, Pål Klepstad, Stein Kaasa, Frank Skorpen, Ragnhild Habberstad, Oriana Nanni, Dino Amadori, Marco Maltoni
Final approval of manuscript: Emanuela Scarpi, Daniele Calistri, Pål Klepstad, Stein Kaasa, Frank Skorpen, Ragnhild Habberstad, Oriana Nanni, Dino Amadori, Marco Maltoni
Disclosures
The authors indicated no financial relationships.
References
- 1.van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: A systematic review of the past 40 years. Ann Oncol. 2007;18:1437–1449. doi: 10.1093/annonc/mdm056. [DOI] [PubMed] [Google Scholar]
- 2.Banning A, Sjøgren P, Henriksen H. Pain causes in 200 patients referred to a multidisciplinary cancer pain clinic. Pain. 1991;45:45–48. doi: 10.1016/0304-3959(91)90163-R. [DOI] [PubMed] [Google Scholar]
- 3.Mercadante S. Prevalence, causes and mechanisms of pain in home care patients with advanced cancer. Pain Clinic. 1994;7:131–136. [Google Scholar]
- 4.Grond S, Zech D, Diefenbach C, et al. Assessment of cancer pain: A prospective evaluation in 2266 cancer patients referred to a pain service. Pain. 1996;64:107–114. doi: 10.1016/0304-3959(95)00076-3. [DOI] [PubMed] [Google Scholar]
- 5.Pecherstorfer M, Vesely M. Diagnosis and monitoring of bone metastases. In: Body JJ, editor. Bone and Cancer. New York, NY: Marcel Dekker; 2000. pp. 97–129. [Google Scholar]
- 6.Fidler IJ. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 7.Middlemiss T, Laird BJ, Fallon MT. Mechanisms of cancer-induced bone pain. Clin Oncol (R Coll Radiol) 2011;23:387–392. doi: 10.1016/j.clon.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Lozano-Ondoua AN, Symons-Liguori AM, Vanderah TW. Cancer-induced bone pain: Mechanisms and models. Neurosci Lett. 2013;557:52–59. doi: 10.1016/j.neulet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Portenoy RK, Hagen NA. Breakthrough pain: Definition, prevalence and characteristics. Pain. 1990;41:273–281. doi: 10.1016/0304-3959(90)90004-W. [DOI] [PubMed] [Google Scholar]
- 10.Deandrea S, Corli O, Consonni D, et al. Prevalence of breakthrough cancer pain: A systematic review and a pooled analysis of published literature. J Pain Symptom Manage. 2014;47:57–76. doi: 10.1016/j.jpainsymman.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Knudsen AK, Brunelli C, Kaasa S, et al. Which variables are associated with pain intensity and treatment response in advanced cancer patients?—Implications for a future classification system for cancer pain. Eur J Pain. 2011;15:320–327. doi: 10.1016/j.ejpain.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Vasudev NS, Brown JE. Medical management of metastatic bone disease. Curr Opin Support Palliat Care. 2010;4:189–194. doi: 10.1097/SPC.0b013e32833d3024. [DOI] [PubMed] [Google Scholar]
- 13.Levy MH. Pharmacologic treatment of cancer pain. N Engl J Med. 1996;335:1124–1132. doi: 10.1056/NEJM199610103351507. [DOI] [PubMed] [Google Scholar]
- 14.Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999;353:1695–1700. doi: 10.1016/S0140-6736(99)01310-0. [DOI] [PubMed] [Google Scholar]
- 15.Luger NM, Sabino MA, Schwei MJ, et al. Efficacy of systemic morphine suggests a fundamental difference in the mechanisms that generate bone cancer vs inflammatory pain. Pain. 2002;99:397–406. doi: 10.1016/S0304-3959(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 16.Martucci C, Panerai AE, Sacerdote P. Chronic fentanyl or buprenorphine infusion in the mouse: Similar analgesic profile but different effects on immune responses. Pain. 2004;110:385–392. doi: 10.1016/j.pain.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Sacerdote P, Bianchi M, Gaspani L, et al. The effects of tramadol and morphine on immune responses and pain after surgery in cancer patients. Anesth Analg. 2000;90:1411–1414. doi: 10.1097/00000539-200006000-00028. [DOI] [PubMed] [Google Scholar]
- 18.Sacerdote P, Limiroli E, Gaspani L. Experimental evidence for immunomodulatory effects of opioids. Adv Exp Med Biol. 2003;521:106–116. [PubMed] [Google Scholar]
- 19.Bell RF, Wisløff T, Eccleston C, et al. Controlled clinical trials in cancer pain. How controlled should they be? A qualitative systematic review. Br J Cancer. 2006;94:1559–1567. doi: 10.1038/sj.bjc.6603162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muralidharan A, Smith MT. Pain, analgesia and genetics. J Pharm Pharmacol. 2011;63:1387–1400. doi: 10.1111/j.2042-7158.2011.01340.x. [DOI] [PubMed] [Google Scholar]
- 21.Kleine-Brueggeney M, Musshoff F, Stuber F, et al. Pharmacogenetics in palliative care. Forensic Sci Int. 2010;203:63–70. doi: 10.1016/j.forsciint.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Lacroix-Fralish ML, Mogil JS. Progress in genetic studies of pain and analgesia. Annu Rev Pharmacol Toxicol. 2009;49:97–121. doi: 10.1146/annurev-pharmtox-061008-103222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lötsch J, Geisslinger G, Tegeder I. Genetic modulation of the pharmacological treatment of pain. Pharmacol Ther. 2009;124:168–184. doi: 10.1016/j.pharmthera.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Branford R, Droney J, Ross JR. Opioid genetics: The key to personalized pain control? Clin Genet. 2012;82:301–310. doi: 10.1111/j.1399-0004.2012.01923.x. [DOI] [PubMed] [Google Scholar]
- 25.Arendt-Nielsen L, Olesen AE, Staahl C, et al. Analgesic efficacy of peripheral kappa-opioid receptor agonist CR665 compared to oxycodone in a multi-modal, multi-tissue experimental human pain model: Selective effect on visceral pain. Anesthesiology. 2009;111:616–624. doi: 10.1097/ALN.0b013e3181af6356. [DOI] [PubMed] [Google Scholar]
- 26.Kim H, Clark D, Dionne RA. Genetic contributions to clinical pain and analgesia: Avoiding pitfalls in genetic research. J Pain. 2009;10:663–693. doi: 10.1016/j.jpain.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klepstad P, Fladvad T, Skorpen F, et al. Influence from genetic variability on opioid use for cancer pain: A European genetic association study of 2294 cancer pain patients. Pain. 2011;152:1139–1145. doi: 10.1016/j.pain.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 28.Fladvad T, Fayers P, Skorpen F, et al. Lack of association between genetic variability and multiple pain-related outcomes in a large cohort of patients with advanced cancer: The European Pharmacogenetic Opioid Study (EPOS) BMJ Support Palliat Care. 2012;2:351–355. doi: 10.1136/bmjspcare-2012-000212. [DOI] [PubMed] [Google Scholar]
- 29.American College of Physicians. 2004: Dosing and conversion chart of opioid analgesics. Available at: http://www.acponline.org/clinical_information/journals_publications/acp_internist/dec04/pain/dosing_conv.pdf. Accessed July 1, 2014.
- 30.Fainsinger RL, Nekolaichuk CL, Lawlor PG, et al. A multicenter study of the revised Edmonton Staging System for classifying cancer pain in advanced cancer patients. J Pain Symptom Manage. 2005;29:224–237. doi: 10.1016/j.jpainsymman.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 32.Tombaugh TN, McIntyre NJ. The mini-mental state examination: A comprehensive review. J Am Geriatr Soc. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 33.Anthony JC, LeResche L, Niaz U, et al. Limits of the ‘Mini-Mental State’ as a screening test for dementia and delirium among hospital patients. Psychol Med. 1982;12:397–408. doi: 10.1017/s0033291700046730. [DOI] [PubMed] [Google Scholar]
- 34.Karnofsky DA, Abelmann WH, Craver LF, et al. The use of the nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- 35.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 36.Hardy GH. Mendelian proportions in a mixed population. Science. 1908;28:49–50. doi: 10.1126/science.28.706.49. [DOI] [PubMed] [Google Scholar]
- 37.Barrett JC, Fry B, Maller J, et al. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 38.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 39.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 40.Chow E, Yee A. Bone secondaries. In: Glare P, Christakis NA, editors. Prognosis in Advanced Cancer. Oxford: Oxford University Press; 2008. pp. 269–283. [Google Scholar]
- 41.Angelo K, Dalhaug A, Pawinski A, et al. Survival prediction score: A simple but age-dependent method predicting prognosis in patients undergoing palliative radiotherapy. ISRN Oncol. 2014;2014:912865. doi: 10.1155/2014/912865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ouyang WW, Su SF, Ma Z, et al. Prognosis of non-small cell lung cancer patients with bone oligometastases treated concurrently with thoracic three-dimensional radiotherapy and chemotherapy. Radiat Oncol. 2014;9:147. doi: 10.1186/1748-717X-9-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harries M, Taylor A, Holmberg L, et al. Incidence of bone metastases and survival after a diagnosis of bone metastases in breast cancer patients. Cancer Epidemiol. 2014;38:427–434. doi: 10.1016/j.canep.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Berger A, Dukes E, Smith M, et al. Use of oral and transdermal opioids among patients with metastatic cancer during the last year of life. J Pain Symptom Manage. 2003;26:723–730. doi: 10.1016/s0885-3924(03)00255-0. [DOI] [PubMed] [Google Scholar]
- 45.Andersen S, Skorpen F. Variation in the COMT gene: Implications for pain perception and pain treatment. Pharmacogenomics. 2009;10:669–684. doi: 10.2217/pgs.09.13. [DOI] [PubMed] [Google Scholar]
- 46.Walter C, Lötsch J. Meta-analysis of the relevance of the OPRM1 118A>G genetic variant for pain treatment. Pain. 2009;146:270–275. doi: 10.1016/j.pain.2009.07.013. [DOI] [PubMed] [Google Scholar]


