The aim of this study was to explore whether geriatric assessment (GA) also predicts 1- and 5-year survival after colorectal cancer surgery in older patients and to compare the predictive power of GA with that of established prognostic factors such as tumor stage and age. In localized and regional disease, the impact of frailty upon 5-year survival was comparable with that of TNM stage.
Keywords: Geriatric assessment, Colorectal neoplasms, Frail elderly, Mortality
Abstract
Background.
Colorectal cancer (CRC) is prevalent in the older population. Geriatric assessment (GA) has previously been found to predict treatment tolerance and postoperative complications in older cancer patients. The aim of this study was to explore whether GA also predicts 1-year and 5-year survival after CRC surgery in older patients and to compare the predictive power of GA with that of established prognostic factors such as TNM classification of malignant tumors (TNM) stage and age.
Materials and Methods.
A cohort of 178 CRC patients aged 70 and older were followed prospectively. All patients went through elective surgery, and GA was performed presurgery. The GA resulted in patients being divided into two groups: frail or nonfrail. All patients were followed for 5 years or until death. Data were analyzed by Kaplan-Meier plots and the Cox proportional hazards model.
Results.
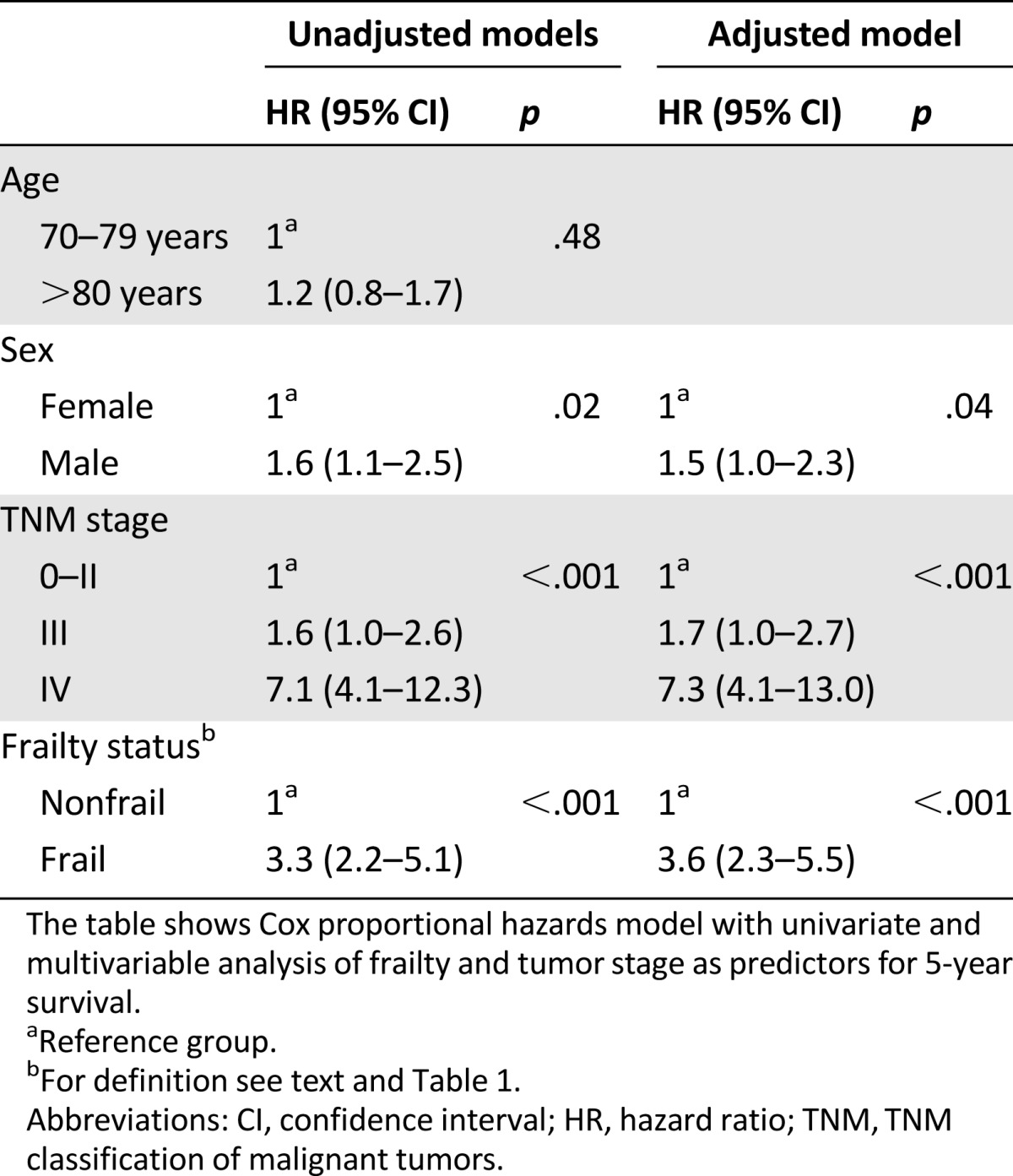
Seventy-six patients (43%) were frail, and one hundred and two (57%) were nonfrail. Twenty-three patients (13%) died during the first year after surgery. One-year survival was 80% in the frail group and 92% in the nonfrail group. Five-year survival was significantly lower in frail (24%) than nonfrail patients (66%), and this difference was apparent both within the stratums of TNM stages 0–II and TNM stage III. In multivariable analysis adjusting for TNM stage, age, and sex, frailty was an independent prognostic factor for survival.
Conclusion.
A GA-based frailty assessment predicts 1-year and 5-year survival in older patients after surgery for CRC. In localized and regional disease, the impact of frailty upon 5-year survival is comparable with that of TNM stage.
Implications for Practice:
Geriatric assessment has previously been found to predict several outcomes when treating older cancer patients, such as postoperative complications and treatment tolerance. The result of a geriatric assessment can be expressed as the patient's degree of frailty. In this study, we find that frailty is a strong predictor for 5-year survival after surgery for colorectal cancer in a group of patients aged 75 and older. Performing a geriatric assessment took 20-60 minutes, and is recommended as part of the decision making process in older cancer patients.
Introduction
Cancer is a disease of aging, with approximately 60% of all cancers and 70% of all cancer deaths occurring in people aged 65 and older [1]. Colorectal cancer (CRC) is no exception, with sharply increasing incidence rates with age in all industrialized countries [2]. In Norway, colorectal cancer is now the most frequent cancer in women aged 70 and older, and in men it is second only to prostate cancer [3]. CRC is also the second leading cause of cancer death in Europe [3, 4]. Over the last decades, mortality rates from CRC have declined [4, 5], but this improvement in survival has been less evident in the older age groups [4, 6, 7].
Tumor stage at diagnosis is critical for prognosis and choice of treatment strategies [8]. In the older cancer patient, prognosis and treatment decisions are also influenced by patient-related factors such as comorbidity and functional status. Therefore, the International Society for Geriatric Oncology (SIOG) recommends that a geriatric assessment (GA) should be performed in all cancer patients more than 70 years of age. The most frail patients seem to benefit most from a GA-based approach, and a two-step model including some form of screening may be used [9]. Geriatric assessment is widely used in clinical, nononcology geriatric practice, and its value for in-hospital patients in this setting has been demonstrated through randomized controlled trials [10]. It is a systematic assessment of the patient encompassing a multitude of domains such as functional status, medication, comorbidities, nutrition, cognitive abilities, and signs of depression. The aim of the assessment in an older cancer patient is threefold: (a) to estimate the patient’s physiological reserves and capacity, (b) to serve as decision-making support, and (c) to detect other and potentially reversible conditions such as depression, malnourishment, and harmful polypharmacy [11]. The result of the GA may be expressed as an estimation of the patient’s frailty: is this a fit patient who will tolerate standard treatment or a frail patient who might profit from a more individualized approach [12]?
In cancer patients, GA has been found to predict treatment tolerance [13] and postoperative complications [14], to identify previously unrecognized and potentially reversible conditions [15], and to predict mortality [16]. However, two recent reviews conclude that the evidence is still conflicting and that further research is needed to establish the role of GA in predicting outcomes and affecting treatment decisions [16, 17].
In a previous prospective study, we showed that frailty can predict short-term outcomes such as postoperative complications in a cohort of older colorectal cancer patients [14]. The aim of the current study was to explore whether a GA-based frailty assessment and individual components of GA were prognostic for 1-year and 5-year survival after surgery and to compare their prognostic impact to that of established prognostic factors such as tumor stage and chronological age.
Material and Methods
Study Group
Patients were recruited from three university hospitals in the Oslo area: Oslo University Hospital, Ullevaal; Oslo University Hospital, Aker; and Akershus University Hospital. The inclusion period was from November 2006 to June 2008. All patients scheduled for elective surgery for confirmed or suspected colorectal cancer and aged 70 and older were eligible for inclusion. Patients had to be able to provide written consent. There were no other exclusion criteria. The study was approved by the Regional Committee for Medical and Health Research Ethics in South East Norway.
Geriatric Assessment and Frailty Definition
All patients were examined in hospital 0–14 days before surgery by a medical doctor trained in geriatrics (S.R.). The doctor performed a geriatric assessment consisting of six domains: functional status, use of medication, comorbidities, nutritional status, cognitive abilities, and signs of depression. Validated scoring systems with predefined and established cutoff values for frailty were used for each domain (Table 1). We used a modified version of the criteria for frailty first proposed by Balducci and Extermann [12], described in detail previously [14]. If the patient scored outside the cutoff value within one or more of the domains, he was considered frail.
Table 1.
Components of the geriatric assessment with cutoff values for frailty
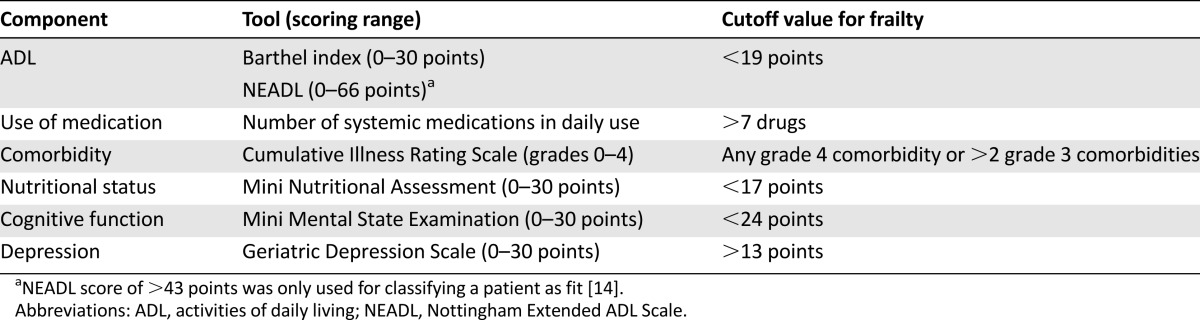
For functional status, the Barthel index [18] and Nottingham Extended Activities for Daily Living Scale (NEADL) [19] were used. The Barthel index measures dependence in basic personal activities of daily living (ADL) such as showering, feeding, and walking. The index yields a score of 0–20 points, where 20 points signifies full independence. The NEADL measures dependence in the more complex daily activities, often referred to as instrumental ADL (IADL) [19]. Examples of such activities are domestic work, leisure activities, kitchen work, and mobility. The sum score ranges from 0 to 66 points, and a score above 43 points signifies independence in IADL.
Data on comorbidity and number of systemic medications in daily use were gathered from the interview with the patient and from the patient records. Comorbidity was scored according to the revised Cumulative Illness Rating Scale (CIRS) [20, 21]. This scale assesses 14 organ systems and rates comorbidities in each organ system according to degree of severity: from grade 0 (no comorbidity) to grade 4 (extremely severe comorbidity).
Nutritional status was assessed with the 18-item Mini Nutritional Assessment [22], giving a score of 0–30 points, where <17 points signifies malnourishment.
For cognitive assessment, we used the Mini Mental State Examination [23], a widely used 20-item screening tool for cognitive impairment. The score ranges from 0 to 30 points, and a score below 24 is considered indicative of reduced cognitive abilities.
Finally, for assessment of depression, we used the Geriatric Depression Scale, a 30-item yes/no questionnaire. A score of 14 or higher has been found to indicate depression with a sensitivity of 80% and specificity of 100% [24, 25].
The oncologists and surgeons responsible for the treatment were blinded to the results of the GA.
Outcome Variables and Explanatory Variables
Follow-up was for 5 years or until death, when all-cause mortality data were gathered. Five-year survival after surgery was used as primary outcome variable. Survival data were collected from the National Registry of Norway.
As the main explanatory variable, we used frailty status based on the GA. In a second multivariable analysis, the individual components of the GA were used as explanatory variables.
Statistical Analyses
Analyses were performed using SPSS Statistics version 18. Time from surgery to death was estimated by Kaplan-Meier analysis and compared with log-rank tests. The Cox proportional hazards regression model was applied to estimate independent effects of the different explanatory variables. The assumption of proportional hazards was checked by visual inspection of log minus log plots. To detect potential multicollinearity, we estimated pairwise correlation between explanatory variables and compared standard errors of the estimates from unadjusted and adjusted models. In addition, variance inflation factors were informally inspected. All variables that were statistically significant in unadjusted analyses with a p value < 0.05 were entered into the multivariable model. Subsequently variables were selected by backward elimination to reduce the model to include statistically significant variables only. Two Cox models for 5-year survival were made. The first model included frailty as the main explanatory variable, correcting for tumor stage, age, and sex. The second model included the individual frailty indicators as explanatory variables, correcting for tumor stage and sex. Age was not included in the second model, because it was not found to be significant in unadjusted analysis. Hazard ratios with a 95% confidence interval are reported for all variables.
Results
Patient Characteristics
One hundred ninety-five patients were assessed for eligibility. Ten were not able to give consent, refused participation, or were deemed unfit for surgery and thus excluded from the study presurgery. Seven patients were excluded postsurgery because complete GA data had not been collected, surgical resection had not been performed, or reoperation had been performed for other reasons. Details on inclusion into the study have been published previously [14].
The final study cohort consisted of 178 patients. As seen in Table 2, the median age was 80 years (range, 70–94 years), and 57% were women. Almost all patients were living at home before surgery, and the majority (83%) were independent in IADL. However, comorbidities were common, with 74% of patients having moderate or serious comorbidity according to CIRS. Cognitive impairment was found in 7%, and malnutrition was found in 10%.
Table 2.
Patient characteristics
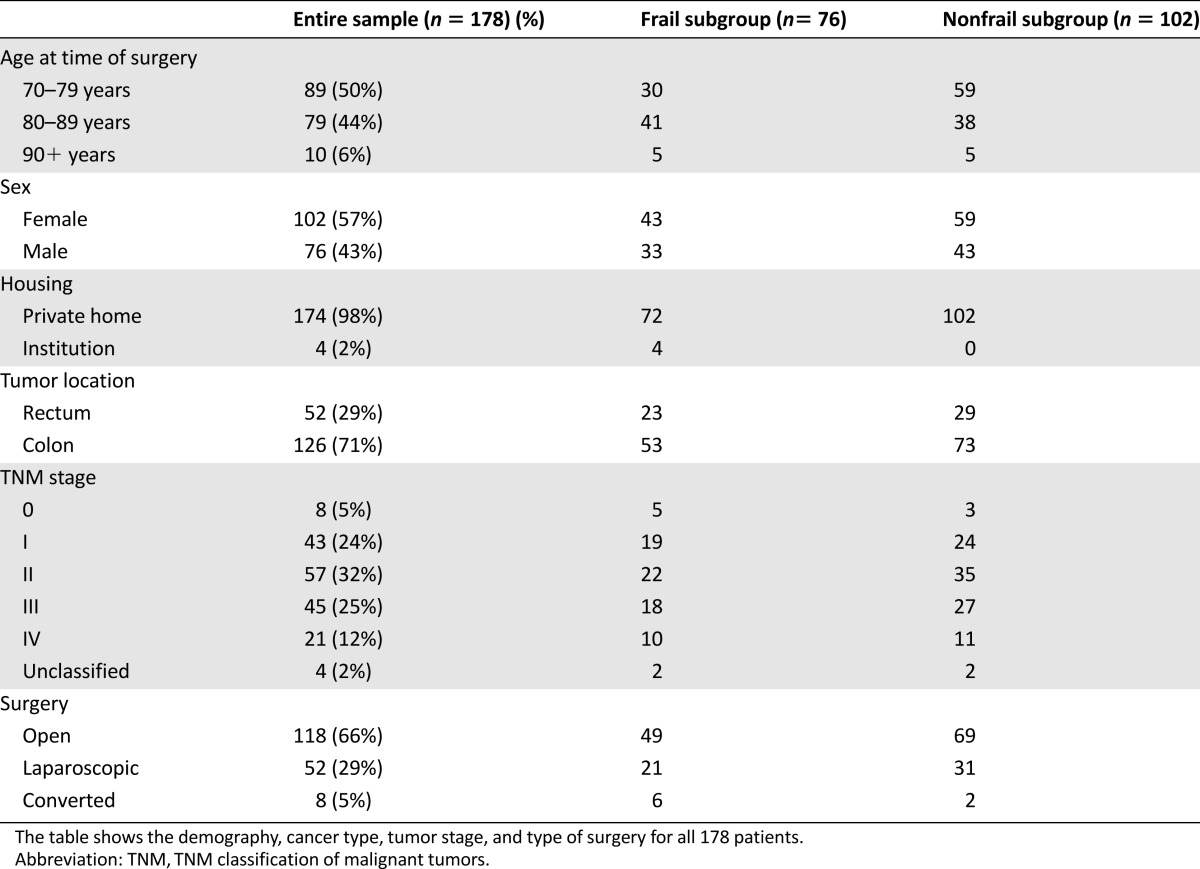
Based on the GA data and the predefined cutoff values for each domain of the geriatric assessment, 76 patients (43%) were frail, and 102 (57%) were nonfrail. Fifty-two patients (29%) had rectal cancer. Eight patients had carcinoma in situ and were classified as TNM stage 0. A large majority had potentially curable disease with 61% with TNM stages 0–II (localized disease), 25% with TNM stage III (regional disease), and only 12% with metastatic disease (TNM stage IV).
During the first year after surgery, 23 patients (13%) died, and 93 (52%) died within 5 years after surgery. Median follow-up was 57.5 months (range, 1–60). There was no association between frailty and tumor stage: 43% of patients with localized disease, 40% of patients with regional disease and 48% of patients with distant metastases were frail.
Association Between Frailty and Survival
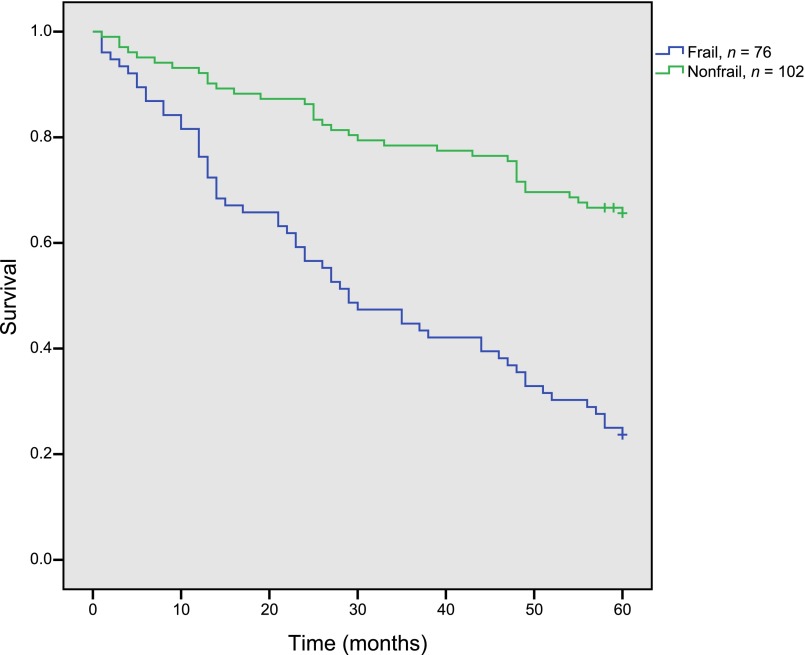
In frail and nonfrail patients, 1-year survival was 80% and 92%, respectively. The difference in survival increased further during the study period. In frail patients, 5-year survival was 24%, and median survival was 24 months. In nonfrail patients, 5-year survival was 66% (log rank p < .001) (Fig. 1).
Figure 1.
Five-year overall survival by frailty status. Kaplan-Meier plot of 5-year survival in frail (n = 76) versus nonfrail (n = 102) patients.
In univariate analysis, frailty, tumor stage, and sex were all prognostic factors for 5-year survival (Table 3). Age above 80 years as compared with age of 70–79 years was not a prognostic factor in the univariate analysis (Table 3) and was therefore not a part of the multivariable model. In multivariable Cox regression analysis, frailty status was a significant prognostic factor for survival when adjusting for TNM stage and sex, with a hazard ratio (HR) of 3.6 (95% confidence interval [CI] 2.3–5.5).
Table 3.
Predictive models for survival (Cox proportional hazards) with frailty and tumor stage
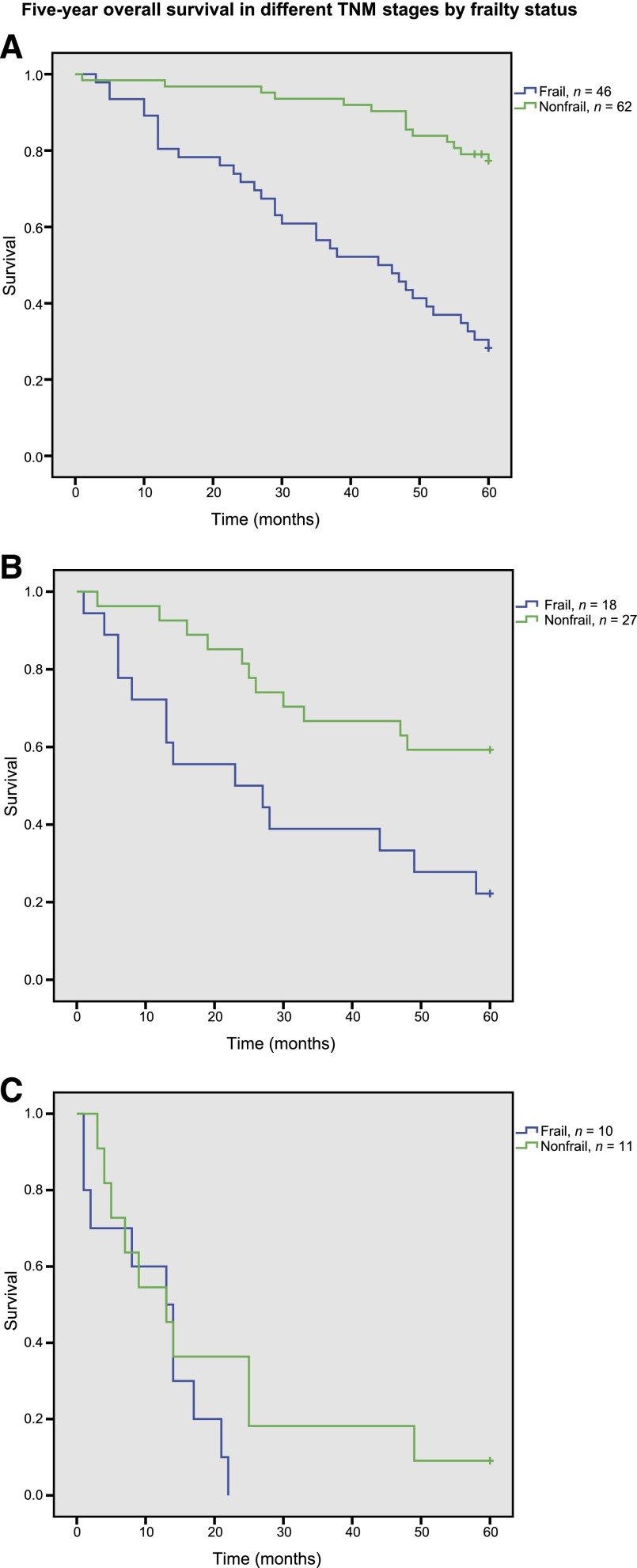
Within the strata of localized and regional disease, 5-year survival was significantly higher in nonfrail than in frail patients (Fig. 2), with log-rank p value of <0.001 in localized disease and of p = .007 in regional disease. In metastatic disease, mortality was high in both frail and nonfrail patients (log-rank p = .2).
Figure 2.
Five-year survival in different tumor stages by frailty status. Kaplan-Meier plots of 5-year survival in frail versus nonfrail patients are stratified according to TNM classification of malignant tumors (TNM) stage. (A): TNM stages 0–II (localized disease; n = 108; log rank p < .001). (B): TNM stage III (regional disease; n = 45; log rank p = .007). (C): TNM stage IV (distant mestastases; n = 21; log rank p = .2).
Association Between Individual Frailty Indicators and 5-Year Survival
We further analyzed the impact of the single components of the GA upon survival. In univariate analysis, comorbidity, nutritional status, cognitive function, polypharmacy, and IADL dependency were all prognostic factors for survival. Severe comorbidity and IADL dependency had the strongest associations with decreased survival, with a HR of 2.8 (1.8–4.3) and 2.8 (1.8–4.4), respectively. The only individual GA element that was not a significant prognostic factor for 5-year survival was depression (Table 4).
Table 4.
Predictive models for survival (Cox proportional hazards) with the individual frailty indicators
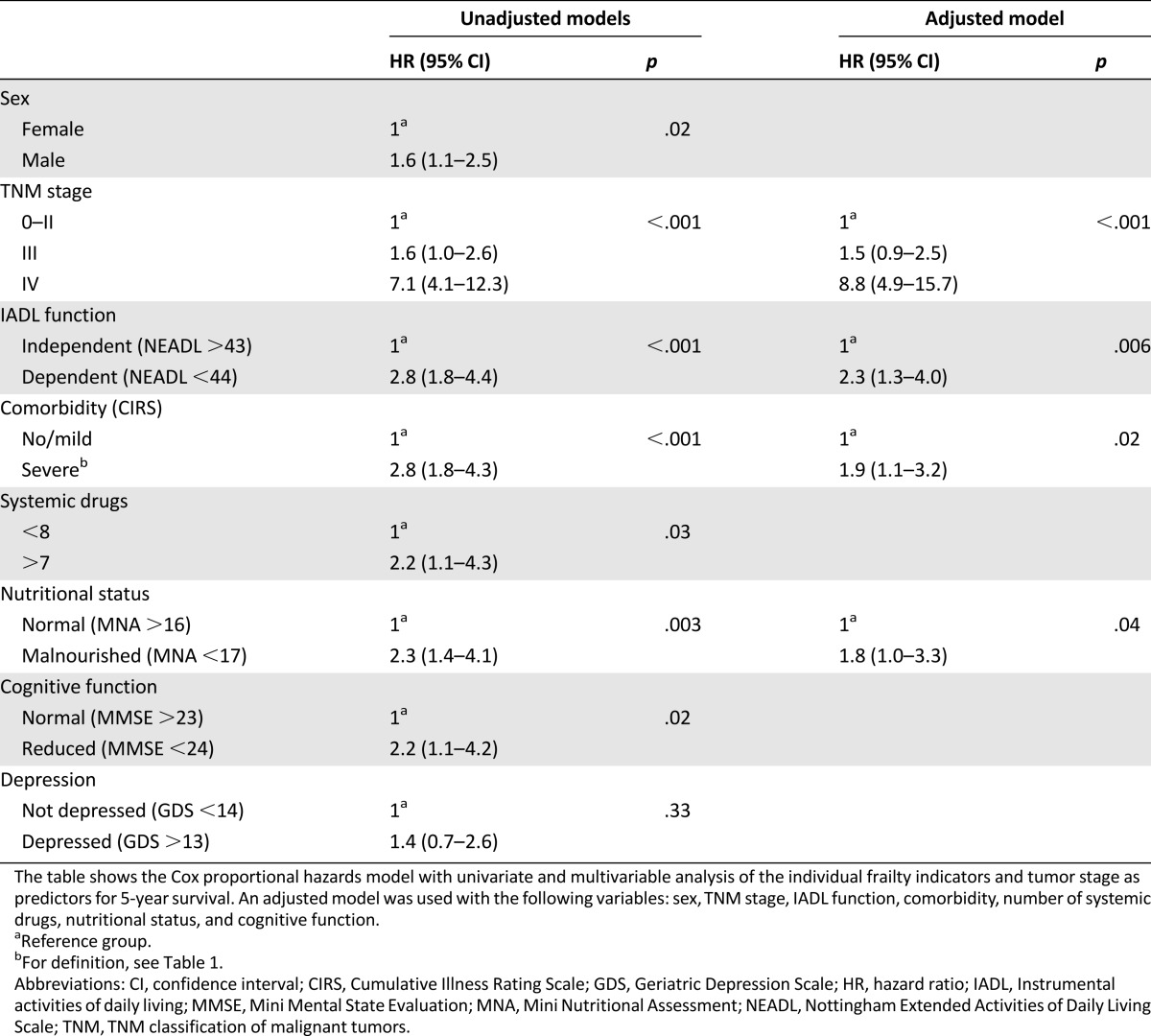
In multivariable analysis correcting for TNM stage and sex, IADL function, comorbidity, and nutritional status remained individual prognostic factors for 5-year survival (Table 4). Because age had already been found to be without statistical significance in univariate analysis, we did not correct for age in this multivariable analysis.
Discussion
Our main finding was that frailty predicts both short-term (1-year) and long-term (5-year) survival after elective surgery for colorectal cancer in older patients. The individual components of GA that were independently prognostic for survival were functional status (IADL), comorbidity, and nutritional status. As expected, TNM stage had a strong prognostic impact on survival, but the association between frailty and mortality remained pronounced within TNM stages 0–II, as well as within stage III. Of note, in tumor stages 0–II, only 28% of frail patients were alive after 5 years compared with 77% of nonfrail patients. In multivariable analysis, being frail (as opposed to nonfrail) implied a higher HR for mortality than having TNM stage III as opposed to stages 0–II. Thus, whereas tumor stage is essential for the prognosis and treatment of older patients with colorectal cancer, assessing frailty seems to be comparable in importance for evaluating overall prognosis in localized and regional disease.
Two recent reviews [16, 17] have summarized the prognostic properties of GA in older cancer patients. Puts et al. [17] found 13 prospective studies addressing the association between GA domains and mortality in different cancer groups. Although their conclusion is that the evidence so far is insufficient, most of the identified studies reported mental health, comorbidity, polypharmacy, malnutrition, and ADL impairments to be indicators of importance. Hamaker et al. [16] reviewed 25 studies and found comorbidity and malnutrition to be the most consistently reported risk factors for mortality. Our findings accord well with these reviews, but whereas they assessed the impact of GA domains in a variety of cancer patients and thus found that the primary reports diverged in their conclusions, we provide data on the prognostic validity of GA in a well-defined homogeneous group of CRC patients. In a recent large study restricted to CRC patients, Gooiker et al. [26] likewise found comorbidity to be a profound risk factor for mortality during the first postoperative year, in addition to age, tumor stage, emergency surgery, and postoperative adverse events. Other GA components such as functional status were, however, not included in that study.
Major strengths of our study are a prospective design with a long follow-up period, a standardized GA performed in the same preoperative setting of a truly aged population, and the fact that the same doctor performed all GAs. However, there are also weaknesses limiting the external validity of the results. The number of participants was limited, and they represent a selected group of patients who were referred to and accepted for elective surgery. Furthermore, the frailty assessment in these analyses did not include any physical performance measures such as gait speed. Recent studies have shown that clinical frailty scores that contain one or two physical performance measures have better predictive ability than those without [27]. In addition, cause of death is not reported. Further studies are warranted to explore whether frailty is of prognostic value for cancer-specific mortality in this population.
Undertreatment and overtreatment are well-known pitfalls in geriatric oncology. Many of the patients are frail with limited remaining life expectancy. Understanding the heterogeneity of the aging process, in which some patients become frail while others remain fit and independent despite advanced chronological age, is of critical importance in prognostics and decision making. To avoid overtreatment, an essential question is whether the patient is going to live long enough to experience complications from his cancer disease [28]. In order to be able to answer this question, the treating physician needs knowledge of both the life expectancy of the patient and the expected progression of the cancer if left untreated. These are difficult tasks, and elements of uncertainty are almost always present. In our cohort, 93% of frail patients survived the first 3 months after surgery, and 80% of frail patients survived the first year. This may imply that overtreatment was not a major concern in our population. However, many older patients consider preserved physical and cognitive function after treatment to be more important than prolonged survival per se [29]. We have previously looked at physical function in a subsample of 84 patients from the same cohort. In that study, we found a significant reduction in ADL and IADL scores from the preoperative state until 16–28 months after surgery [30]. Approximately one-third of the patients had lost ADL function, while two-thirds had lost IADL function.
Undertreatment of older cancer patients has been reported in several studies [31, 32], although there may have been some improvement in this field in recent years [33]. Undertreatment of colorectal cancer may give rise to serious and painful complications such as bleeding, bowel obstruction, and perforation. Emergency surgery carries a far higher risk than elective surgery [26], especially in older patients. Elective surgery is the preferred treatment, either for cure or for palliation, and after careful selection of patients [34, 35].
Despite recommendations, GA is still not fully incorporated into everyday clinical practice. One reason may be feasibility and time needed for the assessment. In our study, the assessment took 20–60 minutes per patient. To reduce the time needed for the assessment, one might wish to exclude some of its domains and assess only those with the strongest predictive power. In our study, these were comorbidity, functional status, and nutritional status. However, the evidence regarding the predictive value of individual domains of the GA is still inconsistent [16]. In addition, some domains have obvious clinical value irrespective of predictive power. For example, it is essential to discover cognitive dysfunction in a patient prior to discussing consent and when providing treatment that requires the patient to be alert of possible complications. Depression in older cancer patients is prevalent, causes considerable morbidity and suffering, and can be treated [36]. Polypharmacy is a large and increasing problem in the older population, causing considerable morbidity and mortality [37]. Therefore, a full GA is still recommended.
Conclusion
Our study provides evidence on the prognostic value of GA-based frailty in older cancer patients. Geriatric assessment adds information about patient’s short-term and long-term overall survival, which, along with information of tumor stage at diagnosis, is of critical importance for decision making in older cancer patients. Performing a GA in older cancer patients is recommended by the SIOG but is not yet fully incorporated into everyday clinical practice. For future research, emphasis should be on patient-centered outcomes such as cognitive and physical function and self-reported health. In addition, because evidence from randomized controlled trials on the effects of GA is lacking, intervention studies are needed.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgment
Funding for this study was provided through a grant from the Norwegian Cancer Society.
Footnotes
For Further Reading:Schroder Sattar, Shabbir M.H. Alibhai, Hans Wildiers et al. How to Implement a Geriatric Assessment in Your Clinical Practice. The Oncologist 2014;19:1056–1068.
Implications for Practice:This article explains how to conduct a geriatric assessment, how to implement it into clinical practice, and how to use the results of the assessment in clinical oncology practice. Furthermore, information is provided about available resources on geriatric assessment as well as geriatric assessment and screening tools, and important challenges of implementing geriatric assessment in practice are highlighted.
Author Contributions
Conception/Design: Nina Ommundsen, Torgeir B. Wyller, Arild Nesbakken, Marit S. Jordhøy, Arne Bakka, Eva Skovlund, Siri Rostoft
Provision of study material or patients: Arild Nesbakken, Arne Bakka
Collection and/or assembly of data: Nina Ommundsen, Siri Rostoft
Data analysis and interpretation: Nina Ommundsen, Torgeir B. Wyller, Arild Nesbakken, Marit S. Jordhøy, Eva Skovlund, Siri Rostoft
Manuscript writing: Nina Ommundsen, Torgeir B. Wyller, Arild Nesbakken, Marit S. Jordhøy, Arne Bakka, Eva Skovlund, Siri Rostoft
Final approval of manuscript: Nina Ommundsen, Torgeir B. Wyller, Arild Nesbakken, Marit S. Jordhøy, Arne Bakka, Eva Skovlund, Siri Rostoft
Disclosures
The authors indicated no financial relationships.
References
- 1.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: A feasibility study. Cancer. 2005;104:1998–2005. doi: 10.1002/cncr.21422. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Registry of Norway . Cancer in Norway 2010: Cancer Incidence, Mortality, Survival and Prevalence in Norway. Oslo, Norway: Cancer Registry of Norway; 2012. [Google Scholar]
- 4.Brenner H, Bouvier AM, Foschi R, et al. Progress in colorectal cancer survival in Europe from the late 1980s to the early 21st century: The EUROCARE study. Int J Cancer. 2012;131:1649–1658. doi: 10.1002/ijc.26192. [DOI] [PubMed] [Google Scholar]
- 5.Angell-Andersen E, Tretli S, Coleman MP, et al. Colorectal cancer survival trends in Norway 1958–1997. Eur J Cancer. 2004;40:734–742. doi: 10.1016/j.ejca.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 6.Morris EJA, Sandin F, Lambert PC, et al. A population-based comparison of the survival of patients with colorectal cancer in England, Norway and Sweden between 1996 and 2004. Gut. 2011;60:1087–1093. doi: 10.1136/gut.2010.229575. [DOI] [PubMed] [Google Scholar]
- 7.Nedrebø BS, Søreide K, Eriksen MT, et al. Survival effect of implementing national treatment strategies for curatively resected colonic and rectal cancer. Br J Surg. 2011;98:716–723. doi: 10.1002/bjs.7426. [DOI] [PubMed] [Google Scholar]
- 8.Hari DM, Leung AM, Lee J-H, et al. AJCC cancer staging manual 7th edition criteria for colon cancer: Do the complex modifications improve prognostic assessment? J Am Coll Surg. 2013;217:181–190. doi: 10.1016/j.jamcollsurg.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wildiers H, Heeren P, Puts M, et al. International society of geriatric oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32:2595–2603. doi: 10.1200/JCO.2013.54.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis G, Whitehead MA, O’Neill D, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2011:CD006211. doi: 10.1002/14651858.CD006211.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alibhai SMH. Comprehensive geriatric assessment in older patients with cancer: Two steps forward? J Geriatr Oncol. 2013;4:205–207. doi: 10.1016/j.jgo.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Balducci L, Extermann M. Management of cancer in the older person: A practical approach. The Oncologist. 2000;5:224–237. doi: 10.1634/theoncologist.5-3-224. [DOI] [PubMed] [Google Scholar]
- 13.Clough-Gorr KM, Stuck AE, Thwin SS, et al. Older breast cancer survivors: Geriatric assessment domains are associated with poor tolerance of treatment adverse effects and predict mortality over 7 years of follow-up. J Clin Oncol. 2010;28:380–386. doi: 10.1200/JCO.2009.23.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kristjansson SR, Nesbakken A, Jordhøy MS, et al. Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: A prospective observational cohort study. Crit Rev Oncol Hematol. 2010;76:208–217. doi: 10.1016/j.critrevonc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Extermann M, Meyer J, McGinnis M, et al. A comprehensive geriatric intervention detects multiple problems in older breast cancer patients. Crit Rev Oncol Hematol. 2004;49:69–75. doi: 10.1016/s1040-8428(03)00099-4. [DOI] [PubMed] [Google Scholar]
- 16.Hamaker ME, Vos AG, Smorenburg CH, et al. The value of geriatric assessments in predicting treatment tolerance and all-cause mortality in older patients with cancer. The Oncologist. 2012;17:1439–1449. doi: 10.1634/theoncologist.2012-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puts MTE, Hardt J, Monette J, et al. Use of geriatric assessment for older adults in the oncology setting: A systematic review. J Natl Cancer Inst. 2012;104:1133–1163. doi: 10.1093/jnci/djs285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahoney FI, Barthel DW. Functional evaluation: The Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 19.Lincoln NB, Gladman JRF. The Extended Activities of Daily Living Scale: A further validation. Disabil Rehabil. 1992;14:41–43. doi: 10.3109/09638289209166426. [DOI] [PubMed] [Google Scholar]
- 20.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: Application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 21.Salvi F, Miller MD, Grilli A, et al. A manual of guidelines to score the modified cumulative illness rating scale and its validation in acute hospitalized elderly patients. J Am Geriatr Soc. 2008;56:1926–1931. doi: 10.1111/j.1532-5415.2008.01935.x. [DOI] [PubMed] [Google Scholar]
- 22.Guigoz Y. The mini nutritional assessment (MNA) review of the literature--what does it tell us? J Nutr Health Aging. 2006;10:466–485. [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982–1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 25.Brink TL, Yesavage JA, Lum O, et al. Screening tests for geriatric depression. Clin Gerontol. 1982;1:37–43. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 26.Gooiker GA, Dekker JW, Bastiaannet E, et al. Risk factors for excess mortality in the first year after curative surgery for colorectal cancer. Ann Surg Oncol. 2012;19:2428–2434. doi: 10.1245/s10434-012-2294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woo J, Leung J, Morley JE. Comparison of frailty indicators based on clinical phenotype and the multiple deficit approach in predicting mortality and physical limitation. J Am Geriatr Soc. 2012;60:1478–1486. doi: 10.1111/j.1532-5415.2012.04074.x. [DOI] [PubMed] [Google Scholar]
- 28.Balducci L, Colloca G, Cesari M, et al. Assessment and treatment of elderly patients with cancer. Surg Oncol. 2010;19:117–123. doi: 10.1016/j.suronc.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Fried TR, Bradley EH, Towle VR, et al. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346:1061–1066. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 30.Rønning B, Wyller TB, Jordhøy MS, et al. Frailty indicators and functional status in older patients after colorectal cancer surgery. J Geriatr Oncol. 2014;5:26–32. doi: 10.1016/j.jgo.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Van Leeuwen BL, Rosenkranz KM, Feng LL, et al. The effect of under-treatment of breast cancer in women 80 years of age and older. Crit Rev Oncol Hematol. 2011;79:315–320. doi: 10.1016/j.critrevonc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Vulto AJ, Lemmens VE, Louwman MW, et al. The influence of age and comorbidity on receiving radiotherapy as part of primary treatment for cancer in South Netherlands, 1995 to 2002. Cancer. 2006;106:2734–2742. doi: 10.1002/cncr.21934. [DOI] [PubMed] [Google Scholar]
- 33.Bernardi D, Errante D, Tirelli U, et al. Insight into the treatment of cancer in older patients: Developments in the last decade. Cancer Treat Rev. 2006;32:277–288. doi: 10.1016/j.ctrv.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Simmonds PD, Best L, George S, et al. Surgery for colorectal cancer in elderly patients: A systematic review. Lancet. 2000;356:968–974. [PubMed] [Google Scholar]
- 35.Kunitake H, Zingmond DS, Ryoo J, et al. Caring for octogenarian and nonagenarian patients with colorectal cancer: What should our standards and expectations be? Dis Colon Rectum. 2010;53:735–743. doi: 10.1007/DCR.0b013e3181cdd658. [DOI] [PubMed] [Google Scholar]
- 36.Mottram P, Wilson K, Strobl J. Antidepressants for depressed elderly. Cochrane Database Syst Rev. 2006:CD003491. doi: 10.1002/14651858.CD003491.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajjar ER, Cafiero AC, Hanlon JT. Polypharmacy in elderly patients. Am J Geriatr Pharmacother. 2007;5:345–351. doi: 10.1016/j.amjopharm.2007.12.002. [DOI] [PubMed] [Google Scholar]