Abstract
Several mechanisms enable immunological self-tolerance. Regulatory T cells (Tregs) are a specialized T cell subset that prevents autoimmunity and excessive immune responses, but can also mediate detrimental tolerance to tumors and pathogens in a Foxp3-dependent manner. Genetic tools exploiting the foxp3 locus including bacterial artificial chromosome (BAC)-transgenic DEREG mice have provided essential information on Treg biology and the potential therapeutic modulation of tolerance. In DEREG mice, Foxp3+ Tregs selectively express eGFP and diphtheria toxin (DT) receptor, allowing for the specific depletion of Tregs through DT administration. We here provide a detailed overview about important considerations such as DT toxicity, which affects any mouse strain treated with DT, and Treg rebound after depletion. Additionally, we point out the specific advantages of BAC-transgenic DEREG mice including their suitability to study organ-specific autoimmunity such as type I diabetes. Moreover, we discuss recent insights into the role of Tregs in viral infections. In summary, DEREG mice are an important tool to study Treg-mediated tolerance and its therapeutic circumvention.
Keywords: Autoimmunity, DEREG, diphtheria toxin (DT), regulatory T cells, tolerance, Treg
Introduction
Regulatory T cells (Tregs) play a non-redundant role in the control of immune responses. The importance of Foxp3 for Treg lineage specification and function in both mice and humans has led several laboratories to the development of genetic tools for the diphtheria toxin (DT)-based depletion of Foxp3+ Tregs [1–3]. These mouse models, including bacterial artificial chromosome (BAC)-transgenic DEREG mice [1], have been instrumental in unraveling Foxp3+ Treg biology [1–6]. T cell-restricted Foxp3 expression could be clearly demonstrated previously as a requirement for maintaining tolerance [4,7–9]. Despite the resolution of these controversies, there is recent confusion about putative disadvantages of DEREG mice. We here provide a detailed technical discussion about DEREG mice and other frequently used and important Foxp3DTR strains.
Results and Discussion
DT Toxicity
DEREG mice express a Foxp3 BAC-driven primate DT-receptor (DTR) fused to eGFP, enabling specific Foxp3+ Treg depletion following DT administration [1]. Although the sensitivity of rodent cells to DT is several orders of magnitude lower compared to that of cells expressing the primate/human DTR, it is well known that DT has side effects in mice. For example, DT administration induces weight loss and transient proteinuria [10,11], and different DT batches can vary in this unspecific toxicity [12]. Additionally, combining DT treatment with adjuvants including pertussis toxin, CFA, or CpG-ODN enhance DT toxicity [6,10]. It is thus not surprising that viral infection has similar effects [13]. Indeed, we found that DT treatment of WT mice during the peak of sub-lethal influenza A virus infection intensifies lethal inflammation and body weight loss (Fig. 1). Similarly, Schmitz et al. [14] already reported that WT C57BL/6 mice chronically infected with LCMV are much more susceptible to DT toxicity and titrated the DT dose accordingly. Importantly, all these observations are independent of DEREG mice. Unspecific DT effects seen in WT mice should not be confused with effects induced by the depletion of specific cell populations in transgenic mice. Examples for the latter are DC depletion models where the targeting of radioresistant non-hematopoietic cells can cause death, or where neutrophilia can occur following DC depletion. We conclude that DT has known unspecific effects that are manageable by careful dose titration and by the use of DT-treated WT controls.
Figure 1.
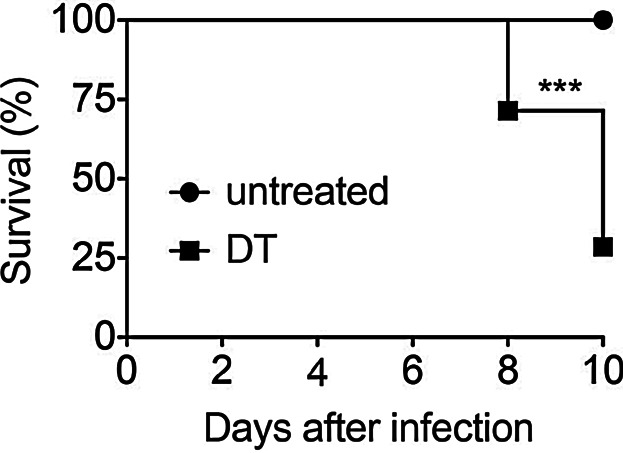
DT induces lethal toxicity in WT mice after influenza infection. WT mice were intranasally infected with a sub-lethal dose of influenza A virus. One group of mice received DT on d4 and d5 after infection, while the other group was left untreated. The graph shows survival of mice in both groups. Data were pooled from two independent experiments (n = 14; ***p < 0.001, Log-rank and Gehan–Breslow–Wilcoxon tests).
Treg Rebound
A rebound of Tregs following the withdrawal of DT treatment is described for several Foxp3DTR strains [1–3]. Thus, the re-appearance of Tregs in short-term depletion protocols [13] is fully expected and has certain advantages (see section “Advantages of Foxp3 BAC Transgenes”). Daily DT treatment of DEREG mice for 5–6 consecutive days reduces Treg outgrowth on d6-7 [1]. However, transient Treg depletion on two consecutive days is often sufficient to demonstrate strong biological effects in comparison to DT-treated controls [12]. Potential anti-DT antibody formation should be considered in long-term depletion regimen depending on the genetic background. Reduced dosing is also favored regarding DT side effects (see section “DT Toxicity”). Of note, Treg rebound can also be influenced by infections (see sections “Advantages of Foxp3 BAC transgenes” and “Tregs in Viral Infections”) and presumably by the commensal microflora.
Advantages of Foxp3 BAC Transgenes
We have previously described that eGFP−Foxp3+ Tregs can be selected by long-term DT treatment or by certain infections combined with DT treatment of DEREG mice [12,15,16]. Similar observations were made with Foxp3.LuciDTR mice undergoing prolonged DT treatment [3]. The existence of DT-resistant Tregs has provided important insights into Treg homeostasis and self-tolerance [3,17]. Additionally, adult DEREG mice are protected from lethal autoimmunity as opposed to Foxp3DTR knock-in mice due to DT-resistant Tregs [1,2,17]. This important advantage allows studying the specific effects of transient Treg depletion. For example, the DEREG transgene is particularly suited to study organ-specific autoimmunity in mice on genetically susceptible backgrounds. We have crossed DEREG mice on the NOD background as a model of human type I diabetes. Interestingly, 70% of non-diabetic NOD.DEREG mice develop overt diabetes after Treg depletion, whereas DT-treated NOD controls remain non-diabetic (Fig. 2). Thus, Foxp3+ Tregs maintain self-tolerance to insulin-producing beta cells in the absence of TCR transgenes. Independent of Treg depletion, the Foxp3 BAC-encoded eGFP in DEREG mice has been instrumental in Treg isolation and functional analyses [18]. This is particularly important to mention because Foxp3 modifications in knock-in strains can alter Treg functions [17]. In contrast, Foxp3 maintains its native state in DEREG mice.
Figure 2.
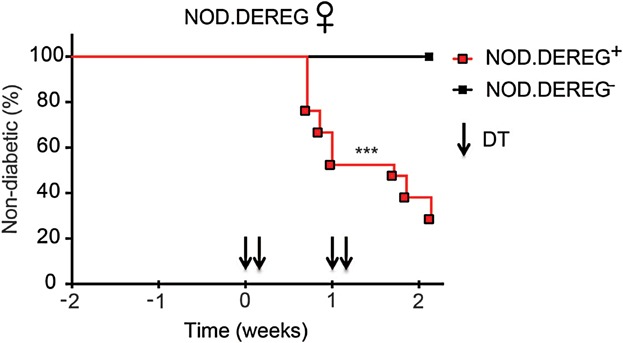
Rapid diabetes onset upon acute Foxp3+ Treg cell ablation in NOD.DEREG mice. Normoglycemic NOD.DEREG+ females (n = 21; N = 3) were injected with DT (arrowheads). Age-matched NOD.DEREG− females were included as controls (n = 6; N = 1). Pooled percentages of normoglycemic mice are shown over time (***p < 0.001, Log-rank and Gehan–Breslow–Wilcoxon tests).
Tregs in Viral Infections
DT toxicity must be especially controlled in viral infections (see section “DT toxicity”). After careful DT dose titration, Schmitz et al. [14] demonstrated enhanced pathology after Treg depletion during LCMV infection. Treatment of DEREG mice with low dose DT effectively depleted all transgenic GFP+Foxp3+ cells but spared a considerable proportion of expanded GFPnegFoxp3+ T cells. An overall reduction of 50–60% Foxp3+ T cells in DEREG compared to C57BL/6 mice was sufficient to reduce T cell exhaustion by increasing anti-viral CD8+ T cell effector function. Inhibition of T cell exhaustion is known to drive immunopathology. Consequently, partial depletion of Foxp3+ T cells in DEREG mice resulted in 100% mortality [14]. In contrast to LCMV clone 13, Friend Virus (FV) induces a life-long chronic infection and does not induce severe immunopathology since this retrovirus mainly replicates and induces effector T cell responses in lymphatic organs. Hence, Treg depletion is associated with improved control of FV infection rather than enhanced pathology [19–21]. Thus, if immunopathology is not a critical factor in an infection model, Treg depletion has no detrimental effects given that the right source and dose of DT is administered. This underlines the power of the method despite the circumstance that Foxp3+ T cells are depleted only partially in some settings [13]. Our data suggest that Treg manipulation might be an interesting new therapeutic approach in certain persistent viral infections. In patients, such a treatment could only be performed for a short period of time to avoid the onset of severe autoimmune diseases [16,17]. In this regard, the DEREG mouse is an ideal model to study the effect of a transient Treg depletion on a chronic viral infection. Interestingly, such a transient Treg depletion resulted in a sustained reduction in chronic retroviral set points in the FV model [21,22].
In conclusion, Foxp3 BAC transgenic mice have known limitations, as every model, while DEREG mice also have several important advantages that underline their broad use.
Material and Methods
Mice
WT (C57BL/6JRj) mice and DEREG mice [1] were maintained at the animal facilities of Twincore (Hanover, Germany) and the Helmholtz-Center for Infection Research (Braunschweig, Germany) under SPF conditions. DEREG mice were crossed to the NOD background for 12 generations and maintained at the DFG-Center for Regenerative Therapies (Dresden, Germany) under SPF conditions. All animal experiments were in accordance with institutional and state guidelines.
Influenza infection
10–14 weeks old female WT mice were intranasally infected with a sub-lethal dose of influenza A virus (mouse-adapted H1N1 PR8, 0.1 LD50). One group of mice received an intraperitoneal injection with 1 µg DT (Calbiochem) diluted in 100 µl PBS on days 4 and 5 after infection, while the other group was left untreated. Health status was followed every second day until day 10. Mice showing very restricted activity for two consecutive days or losing more than 20% of body weight within two days were euthanized, with infection being considered lethal.
Diabetes
15–18 week-old normoglycemic NOD.DEREG+ or control NOD.DEREG− females were injected intraperitoneally with 0.5 µg DT (Calbiochem) on days 0, 1, 7 and 8. Blood glucose levels were determined every other day. Mice were considered diabetic at blood glucose levels above 150 mg/dl. 71.4% (15 out of 21) of initially normoglycemic NOD.DEREG+ mice developed hyperglycemia within 2 weeks after the first DT injection.
Statistical analysis
Survival curves were assessed by Log-rank and Gehan-Breslow-Wilcoxon tests using Prism. p values <0.05 are considered significant.
Acknowledgments
We thank Drs. Peter Openshaw and Mark J. Smyth for helpful discussions. C. T. M. was supported by the German National Academic Foundation.
Conflict of Interest
None declared.
References
- 1.Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med. 2007;204(1):57–63. doi: 10.1084/jem.20061852. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JM, Rasmussen JP. Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007;8(2):191–197. doi: 10.1038/ni1428. and. [DOI] [PubMed] [Google Scholar]
- 3.Suffner J, Hochweller K, Kuhnle MC, Li X, Kroczek RA, Garbi N. Dendritic cells support homeostatic expansion of Foxp3+ regulatory T cells in Foxp3.LuciDTR mice. J. Immunol. 2010;184(4):1810–1820. doi: 10.4049/jimmunol.0902420. et al. [DOI] [PubMed] [Google Scholar]
- 4.Kim J, Lahl K, Hori S, Loddenkemper C, Chaudhry A, deRoos P. Cutting edge: depletion of Foxp3+ cells leads to induction of autoimmunity by specific ablation of regulatory T cells in genetically targeted mice. J. Immunol. 2009;183(12):7631–7634. doi: 10.4049/jimmunol.0804308. et al. [DOI] [PubMed] [Google Scholar]
- 5.Berod L, Puttur F, Huehn J. Sparwasser T. Tregs in infection and vaccinology: heroes or traitors. Microb. Biotechnol. 2012;5(2):260–269. doi: 10.1111/j.1751-7915.2011.00299.x. and. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klages K, Mayer CT, Lahl K, Loddenkemper C, Teng MW, Ngiow SF. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 2010;70(20):7788–7799. doi: 10.1158/0008-5472.CAN-10-1736. et al. [DOI] [PubMed] [Google Scholar]
- 7.Mayer CT, Floess S, Baru AM, Lahl K, Huehn J. Sparwasser T. CD8+ Foxp3+ T cells share developmental and phenotypic features with classical CD4+ Foxp3+ regulatory T cells but lack potent suppressive activity. Eur. J. Immunol. 2011;41(3):716–725. doi: 10.1002/eji.201040913. and. [DOI] [PubMed] [Google Scholar]
- 8.Mayer CT, Kuhl AA, Loddenkemper C. Sparwasser T. Lack of Foxp3+ macrophages in both untreated and B16 melanoma-bearing mice. Blood. 2012;119(5):1314–1315. doi: 10.1182/blood-2011-11-392266. and. [DOI] [PubMed] [Google Scholar]
- 9.Liston A, Farr AG, Chen Z, Benoist C, Mathis D, Manley NR. Lack of Foxp3 function and expression in the thymic epithelium. J. Exp. Med. 2007;204(3):475–480. doi: 10.1084/jem.20062465. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer Zu Horste G, Zozulya AL, El-Haddad H, Lehmann HC, Hartung HP, Wiendl H. Active immunization induces toxicity of diphtheria toxin in diphtheria resistant mice--implications for neuroinflammatory models. J. Immunol. methods. 2010;354(1–2):80–84. doi: 10.1016/j.jim.2010.01.012. et al. [DOI] [PubMed] [Google Scholar]
- 11.Goldwich A, Steinkasserer A, Gessner A. Amann K. Impairment of podocyte function by diphtheria toxin—a new reversible proteinuria model in mice. Lab. Invest. 2012;92(12):1674–1685. doi: 10.1038/labinvest.2012.133. and. [DOI] [PubMed] [Google Scholar]
- 12.Lahl K. Sparwasser T. In vivo depletion of FoxP3+ Tregs using the DEREG mouse model. Methods Mol. Biol. 2011;707:157–172. doi: 10.1007/978-1-61737-979-6_10. and. [DOI] [PubMed] [Google Scholar]
- 13.Christiaansen AF, Boggiatto PM. Varga SM. Limitations of Foxp3 Treg depletion following viral infection in DEREG mice. J. Immunol. Methods. 2014 doi: 10.1016/j.jim.2014.03.005. and PubMed PMID: 24642426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitz I, Schneider C, Frohlich A, Frebel H, Christ D, Leonard WJ. IL-21 restricts virus-driven Treg cell expansion in chronic LCMV infection. PLoS Pathogens. 2013;9(5):e1003362. doi: 10.1371/journal.ppat.1003362. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rausch S, Huehn J, Loddenkemper C, Hepworth MR, Klotz C, Sparwasser T. Establishment of nematode infection despite increased Th2 responses and immunopathology after selective depletion of Foxp3+ cells. Eur. J. Immunol. 2009;39(11):3066–3077. doi: 10.1002/eji.200939644. et al. [DOI] [PubMed] [Google Scholar]
- 16.Berod L, Stueve P, Varela F, Behrends F, Swallow M, Kruse F. Rapid rebound of the Treg compartment in DEREG mice limits the impact of Treg depletion on mycobacterial burden, but prevents autoimmunity. PLoS ONE. 2014;9(7):e102804. doi: 10.1371/journal.pone.0102804. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer CT, Ghorbani P, Kuhl AA, Stuve P, Hegemann M, Berod L. Few Foxp3 regulatory T cells are sufficient to protect adult mice from lethal autoimmunity. Eur. J. Immunol. 2014 doi: 10.1002/eji.201344315. et al. doi: 10.1002/eji.201344315. [DOI] [PubMed] [Google Scholar]
- 18.Mayer CT. Sparwasser T. Assessing the suppressive activity of foxp3(+) regulatory T cells. Methods Mol. Biol. 2014;193:85–96. doi: 10.1007/978-1-4939-1212-4_9. and. [DOI] [PubMed] [Google Scholar]
- 19.Zelinskyy G, Dietze K, Sparwasser T. Dittmer U. Regulatory T cells suppress antiviral immune responses and increase viral loads during acute infection with a lymphotropic retrovirus. PLoS Pathogens. 2009;5(8):e1000406. doi: 10.1371/journal.ppat.1000406. and. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zelinskyy G, Dietze KK, Husecken YP, Schimmer S, Nair S, Werner T. The regulatory T-cell response during acute retroviral infection is locally defined and controls the magnitude and duration of the virus-specific cytotoxic T-cell response. Blood. 2009;114(15):3199–207. doi: 10.1182/blood-2009-03-208736. et al. [DOI] [PubMed] [Google Scholar]
- 21.Dietze KK, Zelinskyy G, Gibbert K, Schimmer S, Francois S, Myers L. Transient depletion of regulatory T cells in transgenic mice reactivates virus-specific CD8+ T cells and reduces chronic retroviral set points. Proc. Natl. Acad. Sci. U.S.A. 2011;108(6):2420–2425. doi: 10.1073/pnas.1015148108. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dietze KK, Zelinskyy G, Liu J, Kretzmer F, Schimmer S. Dittmer U. Combining regulatory T cell depletion and inhibitory receptor blockade improves reactivation of exhausted virus-specific CD8+ T cells and efficiently reduces chronic retroviral loads. PLoS Pathogens. 2013;9(12):e1003798. doi: 10.1371/journal.ppat.1003798. and. [DOI] [PMC free article] [PubMed] [Google Scholar]


