Abstract
The effects of cyanide, anoxia, and temperatures varying from 2 to 42 C on the cell membrane electropotential difference (PD) of washed and freshly excised corn roots have been determined. Respiration rates of freshly excised root segments in response to cyanide and to varying temperatures were also measured. The cell membrane PD of roots which had been washed for 12 to 15 hours was almost insensitive to cyanide and anoxia but sensitive to low temperature. In contrast, the cell membrane PD of freshly excised roots was reversibly depolarized by all three treatments, cyanide depolarized from −117 to −86 millivolts and the sequential imposition of anoxia further lowered the PD to −69 millivolts. Anoxia applied first depolarized maximally and the PD was not further lowered by sequential cyanide treatment. Arrhenius plot analysis of the temperature response of respiration showed an apparent transition at 13 C with an activation energy of 20.0 kilocalories per mole below and 8.8 kilocalories per mole above the transition temperatures. The energy of activation for repolarization of PD is much higher; 53.4 kilocalories per mole below 7 to 8 C and 25.4 kilocalories per mole above this apparent transition. The energy requirement for polarization of the cell membrane PD was calculated based on the temperature responses of the cell membrane PD and respiration. It was estimated that 3.5% of the energy output from respiration at 22 C is required for cell polarization. It is unlikely that ion transport is limited by energy availability below the 8 C transition in this chill sensitive species.
Full text
PDF
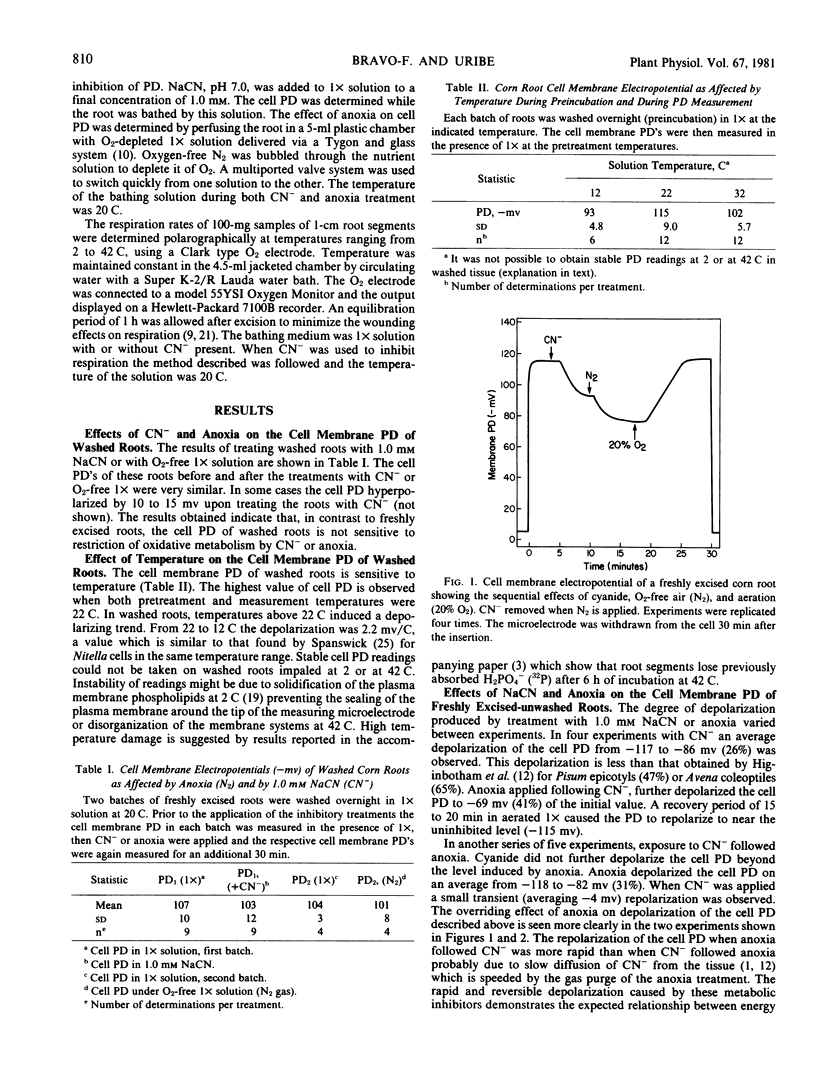
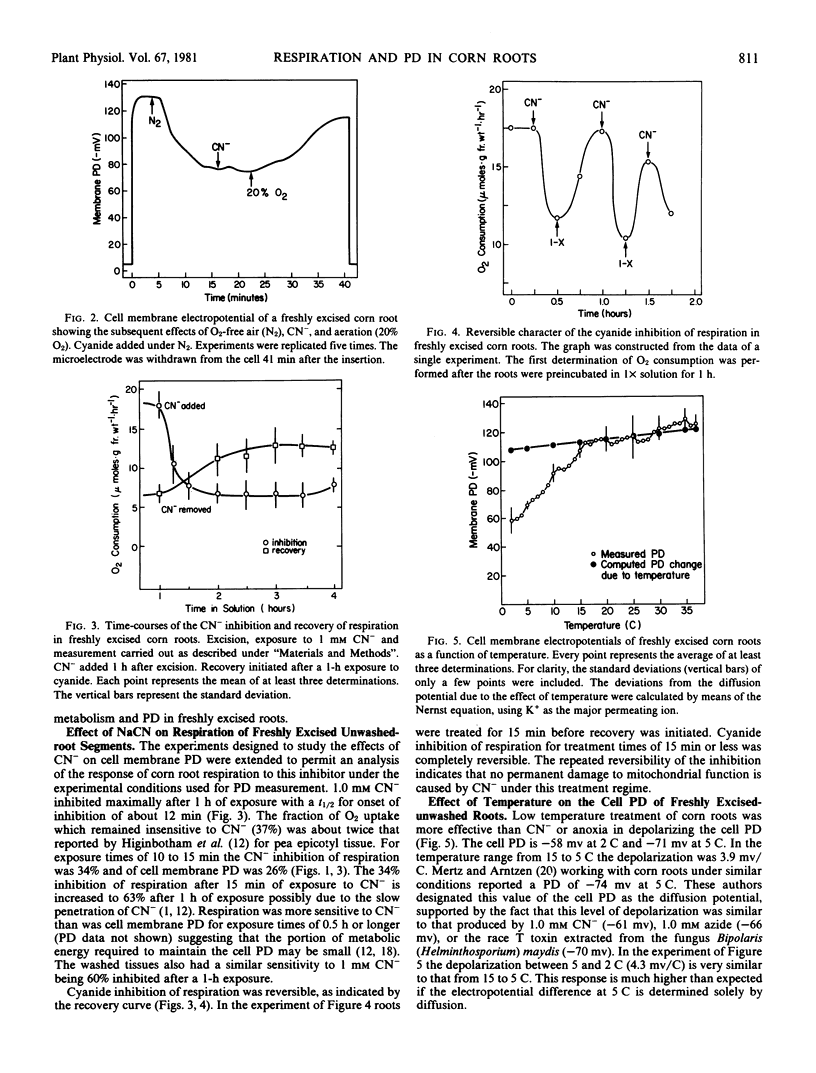



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. P., Hendrix D. L., Higinbotham N. The effect of cyanide and carbon monoxide on the electrical potential and resistance of cell membranes. Plant Physiol. 1974 Nov;54(5):712–716. doi: 10.1104/pp.54.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo-F P., Uribe E. G. Temperature dependence of the concentration kinetics of absorption of phosphate and potassium in corn roots. Plant Physiol. 1981 Apr;67(4):815–819. doi: 10.1104/pp.67.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidenbach R. W., Waring A. J. Response to chilling of tomato seedlings and cells in suspension cultures. Plant Physiol. 1977 Aug;60(2):190–192. doi: 10.1104/pp.60.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey R. W., Berry J. A. Effects of low temperature on respiration and uptake of rubidium ions by excised barley and corn roots. Plant Physiol. 1978 May;61(5):858–860. doi: 10.1104/pp.61.5.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman J. M., Hanson J. B. Energy-linked Potassium Influx as Related to Cell Potential in Corn Roots. Plant Physiol. 1979 Nov;64(5):842–845. doi: 10.1104/pp.64.5.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. F., Higinbotham N. Effects of external cations and respiratory inhibitors on electrical potential of the xylem exudate of excised corn roots. Plant Physiol. 1969 Oct;44(10):1383–1392. doi: 10.1104/pp.44.10.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. F., Jaworski A. Z. Effects of ouabain and low temperature on the sodium efflux pump in excised corn roots. Plant Physiol. 1979 May;63(5):940–946. doi: 10.1104/pp.63.5.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronewald J. W., Cheeseman J. M., Hanson J. B. Comparison of the responses of corn root tissue to fusicoccin and washing. Plant Physiol. 1979 Feb;63(2):255–259. doi: 10.1104/pp.63.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higinbotham N., Etherton B., Foster R. J. Mineral ion contents and cell transmembrane electropotentials of pea and oat seedling tissue. Plant Physiol. 1967 Jan;42(1):37–46. doi: 10.1104/pp.42.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Potassium and Phosphate Uptake in Corn Roots: Further Evidence for an Electrogenic H/K Exchanger and an OH/Pi Antiporter. Plant Physiol. 1979 May;63(5):952–955. doi: 10.1104/pp.63.5.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons J. M., Raison J. K. Oxidative activity of mitochondria isolated from plant tissues sensitive and resistant to chilling injury. Plant Physiol. 1970 Apr;45(4):386–389. doi: 10.1104/pp.45.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz S. M., Arntzen C. J. Depolarization of the Electrogenic Transmembrane Electropotential of Zea mays L. by Bipolaris (Helminthosporium) maydis Race T Toxin, Azide, Cyanide, Dodecyl Succinic Acid, or Cold Temperature. Plant Physiol. 1978 Nov;62(5):781–783. doi: 10.1104/pp.62.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz S. M., Higinbotham N. Transmembrane electropotential in barley roots as related to cell type, cell location, and cutting and aging effects. Plant Physiol. 1976 Feb;57(2):123–128. doi: 10.1104/pp.57.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanswick R. M. Evidence for an electrogenic ion pump in Nitella translucens. I. The effects of pH, K + , Na + , light and temperature on the membrane potential and resistance. Biochim Biophys Acta. 1972 Oct 23;288(1):73–89. doi: 10.1016/0005-2736(72)90224-6. [DOI] [PubMed] [Google Scholar]


