Abstract
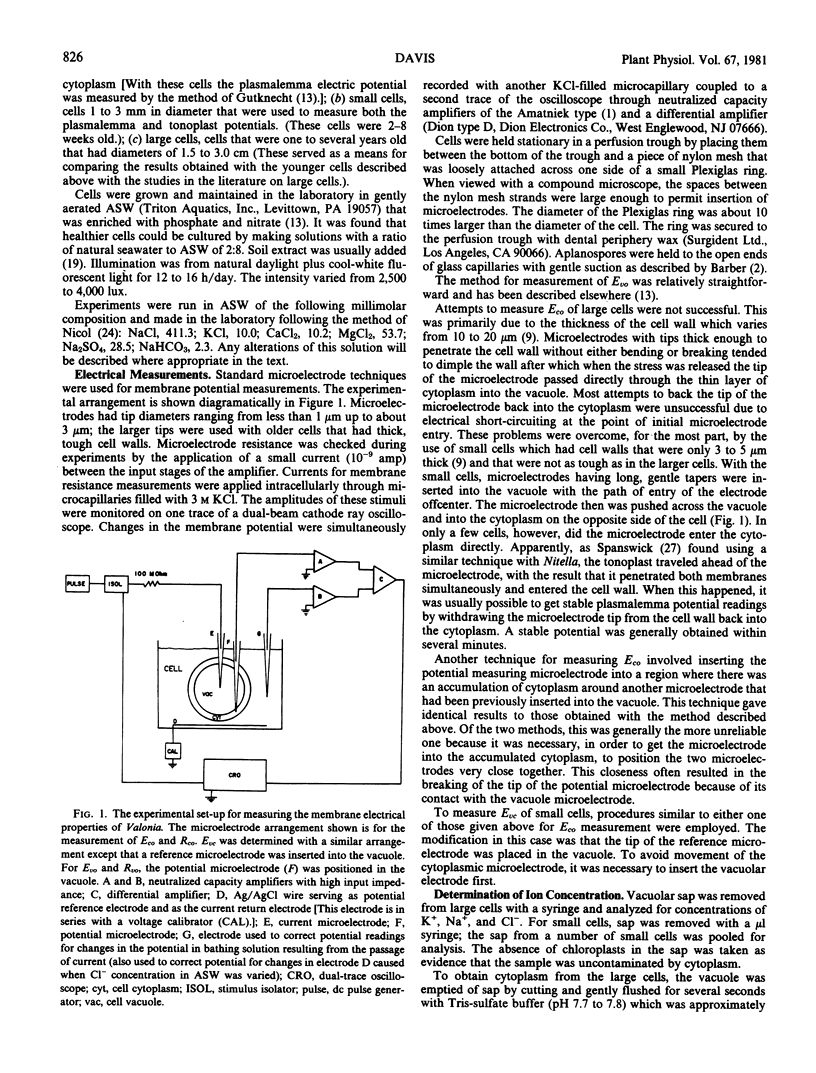
Studies were made on the electric potentials of the plasmalemma (Eco) and tonoplast (Evc) in small cells (1-3 mm diameter) of Valonia ventricosa. To measure Eco, microelectrodes with long tapers were inserted into the vacuole with the path of electrode entry off-center. The microelectrode then was pushed across the vacuole and into the cytoplasm on the opposite side of the cell. A reference electrode was placed in the artificial seawater bathing the cell. A similar method was used to measure Evc except that the reference electrode was placed in the vacuole.
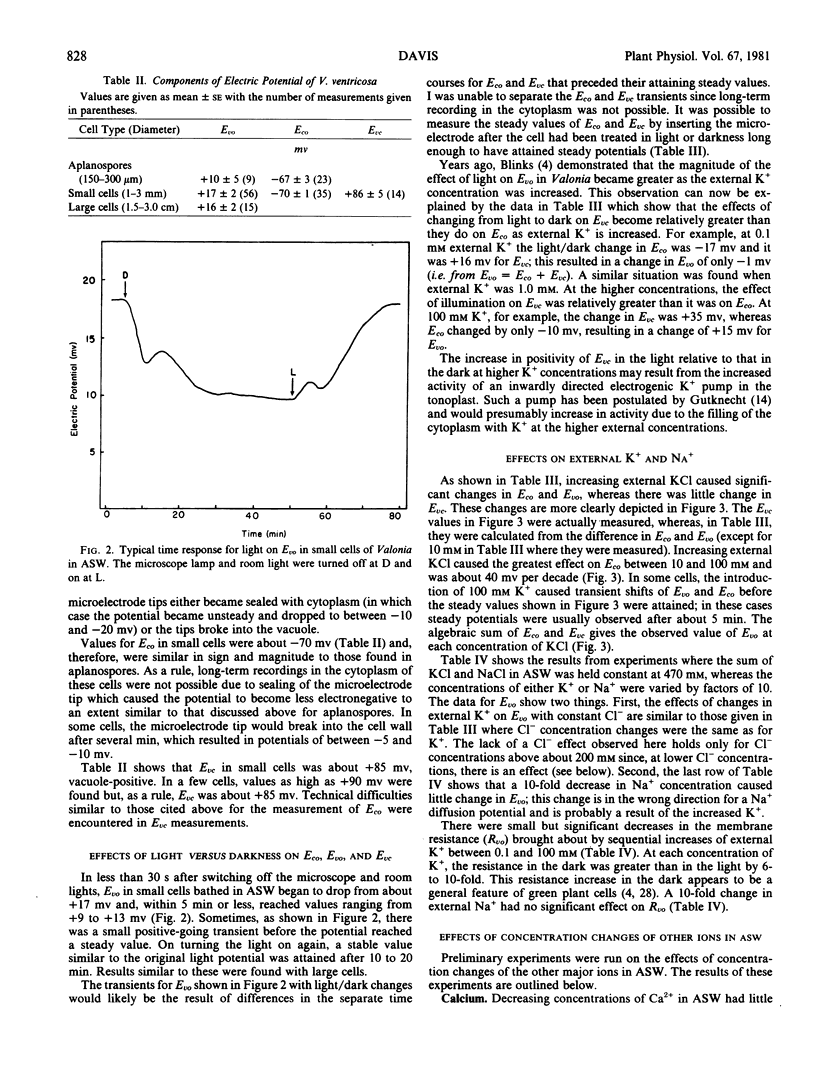
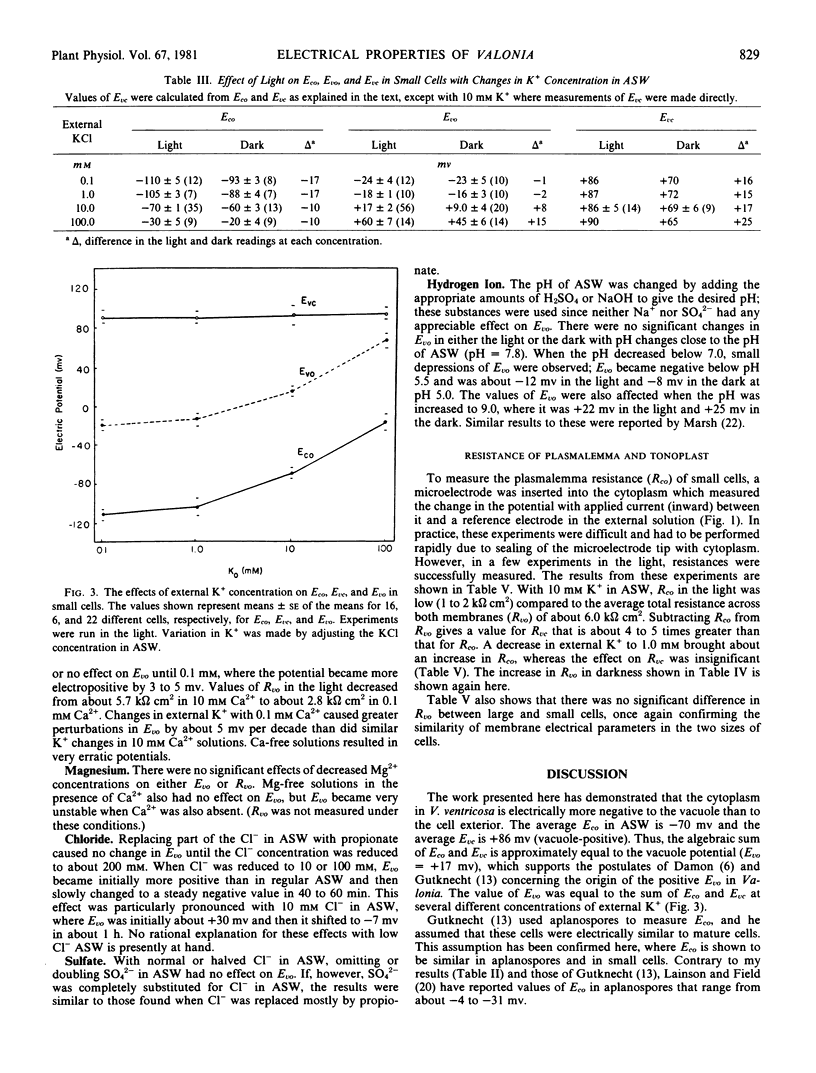
Both Eco and Evc were influenced by light. In the light, Eco was −70 millivolts and it changed to −60 millivolts in the dark (cytoplasm-negative to outside). For Evc, the potentials were +86 millivolts in the light and +69 millivolts in the dark (vacuole-positive to cytoplasm). The vacuole potential (Evo) was demonstrated to be the algebraic sum of Eco and Evc. For example, in the light, the sum of the means (±se) for Eco (= −70 ± 1) and Evc (= +86 ± 5) is +16 millivolts, which is comparable to the measured Evo of +17 ± 2 millivolts. In the dark, the sum of Eco (= −60 ± 3) and Evc (+69 ± 6) is +9 millivolts and the measured value of Evo is +9 ± 4 millivolts.
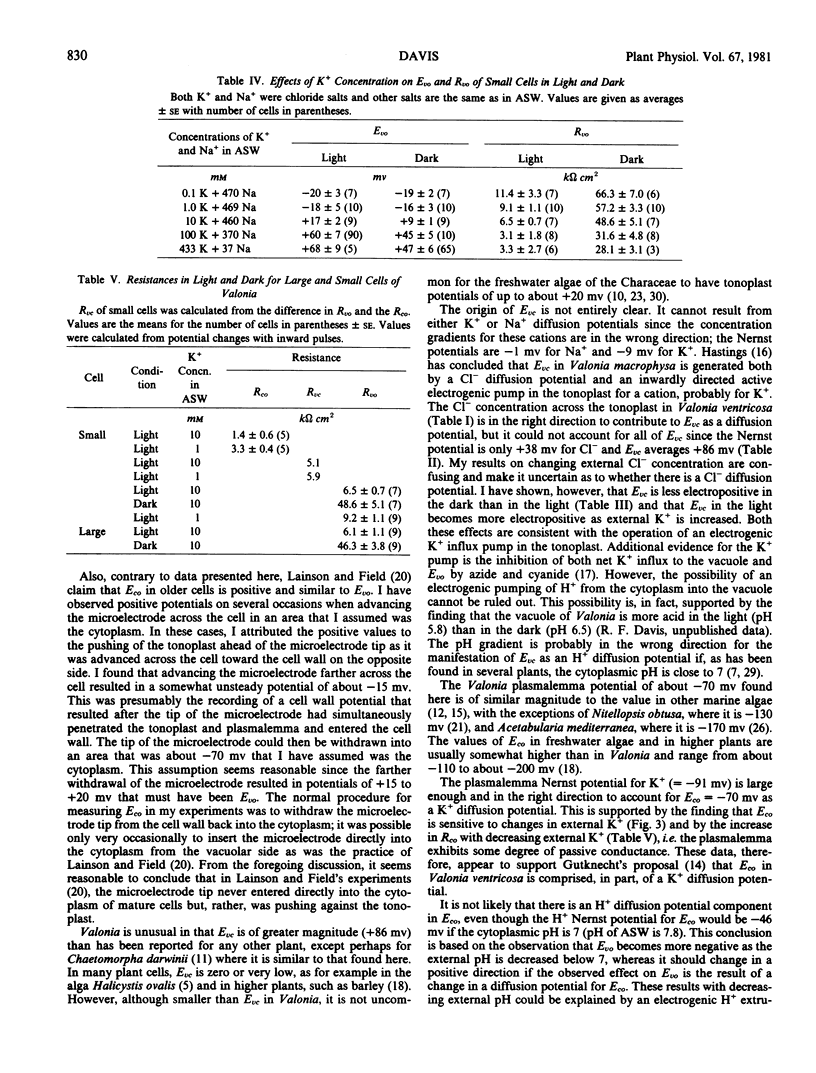
The external K+ concentration had a controlling effect on both Eco and the direct current resistance of the plasmalemma, which suggests that Eco is largely a K+ diffusion potential. The tonoplast electrical properties were affected only slightly by external K+.
The data presented are indicative of a K+ electrogenic influx pump in the tonoplast. It is also considered possible that H+ might be electrogenically pumped from the cytoplasm both into the vacuole and to the cell exterior.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLINKS L. R. The source of the bioelectric potentials in large plant cells. Proc Natl Acad Sci U S A. 1949 Oct;35(10):566–575. doi: 10.1073/pnas.35.10.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOUNT R. W., LEVEDAHL B. H. Active sodium and chloride transport in the single celled marine alga Halicystis ovalis. Acta Physiol Scand. 1960 May 25;49:1–9. doi: 10.1111/j.1748-1716.1960.tb01922.x. [DOI] [PubMed] [Google Scholar]
- Barber J. Measurement of the membrane potential and evidence for active transport of ions in Chlorella pyrenoidosa. Biochim Biophys Acta. 1968 Jun 11;150(4):618–625. doi: 10.1016/0005-2736(68)90051-5. [DOI] [PubMed] [Google Scholar]
- Damon E. B. DISSIMILARITY OF INNER AND OUTER PROTOPLASMIC SURFACES IN VALONIA. II. J Gen Physiol. 1929 Nov 20;13(2):207–221. doi: 10.1085/jgp.13.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves J. S., Gutknecht J. Ion transport studies and determination of the cell wall elastic modulus in the marine alga Halicystis parvula. J Gen Physiol. 1976 May;67(5):579–597. doi: 10.1085/jgp.67.5.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht J. Ion fluxes and short-circuit current in internally perfused cells of Valonia ventricosa. J Gen Physiol. 1967 Aug;50(7):1821–1834. doi: 10.1085/jgp.50.7.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainson R., Field C. D. Electrical properties of Valonia ventricosa. J Membr Biol. 1976 Oct 20;29(1-2):81–94. doi: 10.1007/BF01868953. [DOI] [PubMed] [Google Scholar]
- MACROBBIE E. A., DAINTY J. Ion transport in Nitellopsis obtusa. J Gen Physiol. 1958 Nov 20;42(2):335–353. doi: 10.1085/jgp.42.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhout W. J. ON THE IMPORTANCE OF MAINTAINING CERTAIN DIFFERENCES BETWEEN CELL SAP AND EXTERNAL MEDIUM. J Gen Physiol. 1925 Mar 20;7(4):561–564. doi: 10.1085/jgp.7.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddler H. D. The membrane potential of Acetabularia mediterranea. J Gen Physiol. 1970 Jun;55(6):802–821. doi: 10.1085/jgp.55.6.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanswick R. M. Evidence for an electrogenic ion pump in Nitella translucens. I. The effects of pH, K + , Na + , light and temperature on the membrane potential and resistance. Biochim Biophys Acta. 1972 Oct 23;288(1):73–89. doi: 10.1016/0005-2736(72)90224-6. [DOI] [PubMed] [Google Scholar]
- Spanswick R. M., Miller A. G. Measurement of the Cytoplasmic pH in Nitella translucens: Comparison of Values Obtained by Microelectrode and Weak Acid Methods. Plant Physiol. 1977 Apr;59(4):664–666. doi: 10.1104/pp.59.4.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobiev L. N. Potassium selective microelectrodes with precipitate in the tip. Nature. 1968 Feb 3;217(5127):450–451. doi: 10.1038/217450a0. [DOI] [PubMed] [Google Scholar]


