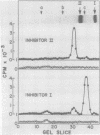
Abstract
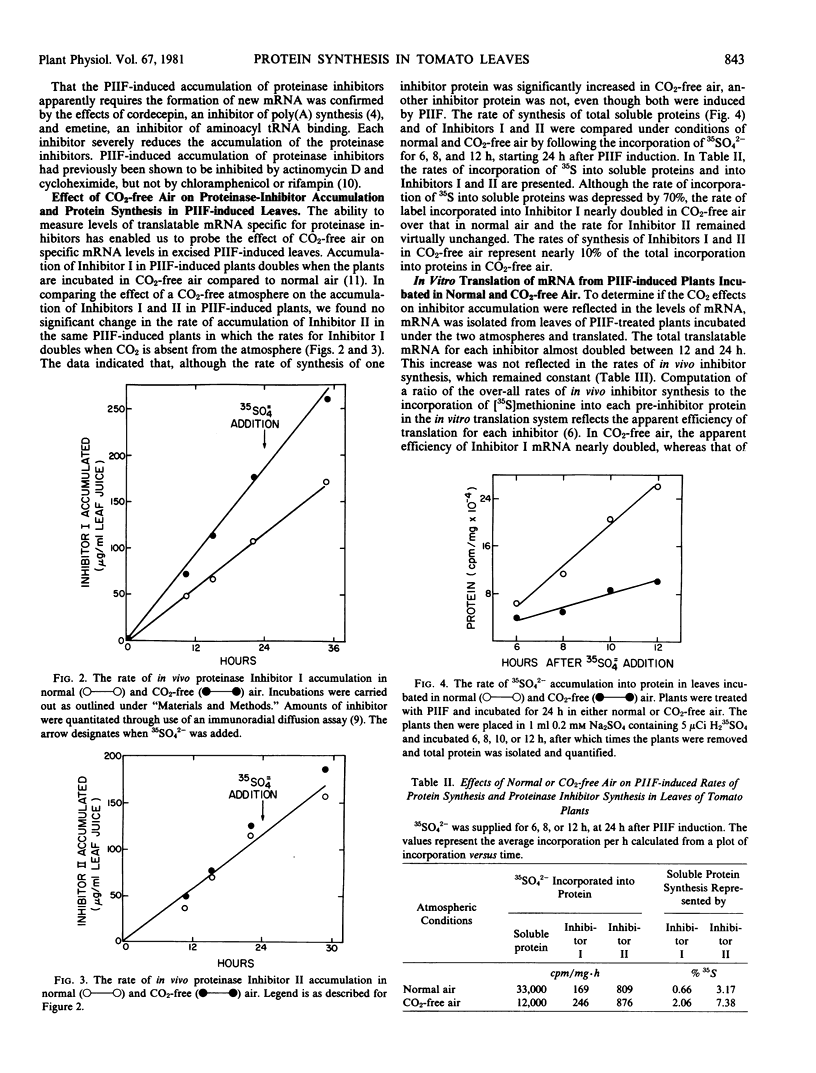
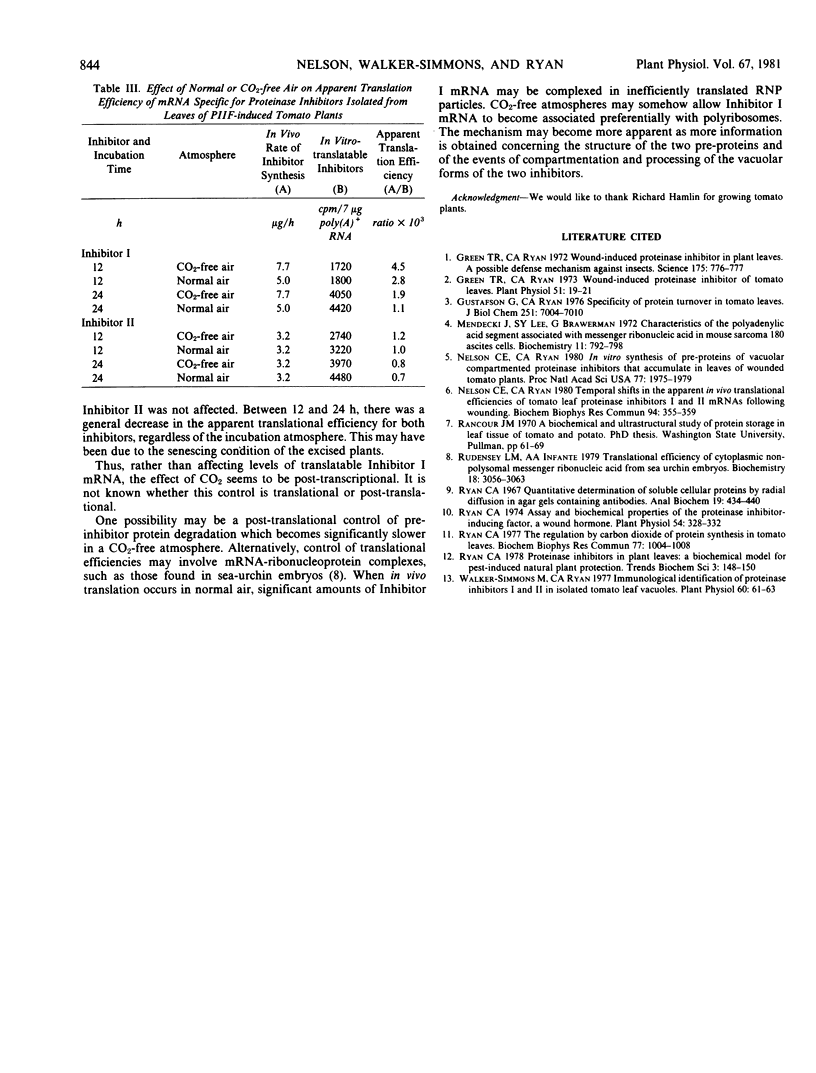
Messenger RNA was isolated from young excised tomato leaves, induced to accumulate proteinase Inhibitors I and II with the proteinase inhibitor inducing factor (PIIF), and translated in vitro in a rabbit reticulocyte lysate system. Translatable messenger RNAs specific for Inhibitors I and II were present in PIIF-induced leaves but were not present without PIIF induction. The nascent in vitro-synthesized inhibitors migrated with an apparent molecular weight 2,000 to 3,000 daltons larger than that of the two inhibitors isolated from leaves. The molecular weights of the preinhibitors were identical whether translated from mRNA from PIIF-induced leaves or translated from mRNA isolated from wounded leaves. Incubation of excised PIIF-induced plants in CO2-free air doubled the rate of in vivo synthesis of Inhibitor I over that in normal air (Ryan CA 1977 Biochem Biophys Res Commun 77: 1004-1008) but did not affect the rate of in vivo Inhibitor II accumulation. The rate of incorporation of 35SO42− into soluble proteins was 70% less when leaves were incubated in CO2-free air rather than normal air. Messenger RNA isolated from PIIF-induced plants incubated in the presence or absence of CO2 was translated in vitro. The amount of in vitro-translatable mRNA present for each inhibitor (per microgram total mRNA) was the same in leaves incubated in either atmosphere. Therefore, the increased rate of synthesis and accumulation of Inhibitor I in a CO2-free atmosphere does not appear to result from an increased level of mRNA but appears to be controlled at a posttranscriptional level.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Green T. R., Ryan C. A. Wound-Induced Proteinase Inhibitor in Plant Leaves: A Possible Defense Mechanism against Insects. Science. 1972 Feb 18;175(4023):776–777. doi: 10.1126/science.175.4023.776. [DOI] [PubMed] [Google Scholar]
- Green T. R., Ryan C. A. Wound-induced Proteinase Inhibitor in Tomato Leaves: Some Effects of Light and Temperature on the Wound Response. Plant Physiol. 1973 Jan;51(1):19–21. doi: 10.1104/pp.51.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson G., Ryan C. A. Specificity of protein turnover in tomato leaves. Accumulation of proteinase inhibitors, induced with the wound hormone, PIIF. J Biol Chem. 1976 Nov 25;251(22):7004–7010. [PubMed] [Google Scholar]
- Mendecki J., Lee S. Y., Brawerman G. Characteristics of the polyadenylic acid segment associated with messenger ribonucleic acid in mouse sarcoma 180 ascites cells. Biochemistry. 1972 Feb 29;11(5):792–798. doi: 10.1021/bi00755a018. [DOI] [PubMed] [Google Scholar]
- Nelson C. E., Ryan C. A. In vitro synthesis of pre-proteins of vacuolar compartmented proteinase inhibitors that accumulate in leaves of wounded tomato plants. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1975–1979. doi: 10.1073/pnas.77.4.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C. E., Ryan C. A. Temporal shifts in the apparent in vivo translational efficiencies of tomato leaf proteinase inhibitors I and II mRNAs following wounding. Biochem Biophys Res Commun. 1980 May 14;94(1):355–359. doi: 10.1016/s0006-291x(80)80228-2. [DOI] [PubMed] [Google Scholar]
- Rudensey L. M., Infante A. A. Translational efficiency of cytoplasmic nonpolysomal messenger ribonucleic acid from sea urchin embryos. Biochemistry. 1979 Jul 10;18(14):3056–3063. doi: 10.1021/bi00581a023. [DOI] [PubMed] [Google Scholar]
- Ryan C. A. Assay and Biochemical Properties of the Proteinase Inhibitor-inducing Factor, a Wound Hormone. Plant Physiol. 1974 Sep;54(3):328–332. doi: 10.1104/pp.54.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C. A. Quantitative determination of soluble cellular proteins by radial diffusion in agar gels containing antibodies. Anal Biochem. 1967 Jun;19(3):434–440. doi: 10.1016/0003-2697(67)90233-3. [DOI] [PubMed] [Google Scholar]
- Ryan C. A. The regulation by carbon dioxide of protein synthesis in tomato leaves. Biochem Biophys Res Commun. 1977 Aug 8;77(3):1004–1008. doi: 10.1016/s0006-291x(77)80077-6. [DOI] [PubMed] [Google Scholar]
- Walker-Simmons M., Ryan C. A. Immunological Identification of Proteinase Inhibitors I and II in Isolated Tomato Leaf Vacuoles. Plant Physiol. 1977 Jul;60(1):61–63. doi: 10.1104/pp.60.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]