Abstract
Introduction
Patients undergoing radical prostatectomy (RP) suffer from erectile dysfunction (ED) refractory to PDE5 inhibitors, which act downstream of CN-mediated release of nitric oxide (NO). Direct delivery of NO to the penis could potentially circumvent this limitation.
Aim
To determine if topically applied NO-releasing nanoparticles (NO-np) can elicit erections in a rat model of RP and demonstrate that the mechanism is through increased blood flow.
Methods
26 Sprague–Dawley rats underwent bilateral transection of the CN. One week later NO-np were applied topically to the penile shaft in DMSO-gel (10 animals) or coconut oil (6 animals). Control animals were treated with empty-np. Erectile function was determined through the intracorporal pressure/blood pressure ratio (ICP/BP). The effect of the NO-np on blood flow was determined using a hamster dorsal window chamber.
Main Outcome Measures
Animals were investigated for spontaneous erections, onset and duration of erectile response and basal ICP/BP ratio. Microcirculatory blood-flow was determined through arteriolar and venular diameter and blood flow.
Results
Eight of ten animals treated with NO-np suspended in DMSO-gel had significant increases in basal ICP/BP, and six out of these ten animals demonstrated spontaneous erections of approximately one minute duration. Onset of spontaneous erections ranged from 5–37 minutes and occurred for at least 45 minutes. Similar results were observed with NO-np applied in coconut oil. No erectile response was observed in control animal models treated with empty-np. The hamster dorsal window chamber demonstrated NO-np applied as a suspension in coconut oil caused a significant increase in the microcirculatory blood flow, sustained over 90 minutes.
Conclusions
Topically applied NO-np induced spontaneous erections and increased basal ICP in an animal model of RP. These effects are most likely due to increased microcirculatory blood flow. These characteristics suggest that the NO-np would be useful in penile rehabilitation of patients following RP.
Keywords: Nanoparticle, Nitric oxide, erectile dysfunction, radical prostatectomy, cavernous nerve, microcirculation
Introduction
Data from the National Cancer Institute shows that prostate cancer is the second most common cancer in men. The number of new cases of prostate cancer in 2013 was 152.0 per 100,000 men per year. Approximately 15.3 percent of men will be diagnosed with prostate cancer at some point during their lifetime. Radical prostatectomy (RP) is the most commonly used treatment option for localized prostate cancer, particularly in younger sexually active men. Virtually all men experience erectile dysfunction (ED) after surgery, with a profound loss of nocturnal erections [1]. The etiology of the immediate loss of nocturnal activity is considered to be the result of intraoperative neuropraxia (temporary loss of cavernous nerve (CN) function). As the neuropraxia resolves there is a slow recovery of erectile function taking between 18 to 24 months. However, 30–90% of patients do not recover erectile function in this time frame [2]. A recent meta-analysis found that new robotic surgical techniques did not significantly improve erectile function after RP. Compounding the issue of impotence, many men observe that their penises are smaller, with a reduction in penile circumference and length occurring in the first 3 months after surgery. These observations are supported in animal models of penile denervation [3–7]. These changes in penile architecture due to lack of erectile function represent an obstacle to subsequent recovery or treatment of ED following RP.
The precise cause of neuropraxia is unclear, but has been hypothesized to include direct trauma during surgery (for example retraction injury), damage from tissue electrocautery, disruption of the neural vasculature and generalized local inflammation associated with the procedure [8]. As a consequence of neurological damage, mechanisms that facilitate cavernosal oxygenation fail. Fibrosis ensues and is marked by the presence of transforming growth factor β, a marker of chronic inflammation and fibrosis. Additionally, anti-fibrotic mediators such as prostaglandin E1 (PGE1) and cyclic adenosine monophosphate are under-expressed during this process. This destructive cycle leads to cavernosal smooth muscle apoptosis. Whereas neuropraxia may be reversible, the penile fibrosis resulting from poor oxygenation permanently damages cavernosal function and produces chronic ED [7]. An additional factor that could contribute to impotence following the immediate effects of neuropraxia could be related to lowered testosterone levels that have been reported to occur following RP in both patients and animal models [9, 10].
These sequence of events, and the hypothesis that hypoxia results in irreparable cavernosal tissue damage, has led to a clinical strategy of “penile rehabilitation” where cavernosal tissue oxygenation through increased corporal blood flow is attempted to reduce the prevalence of chronic inflammation and cavernosal fibrosis until a time when functionality of the CN is recovered. Several approaches have been used in penile rehabilitation, all of which have drawbacks and are controversial in terms of success in restoring erectile function. The primary methods are; 1) Regular dosing with phosphodiesterase-5 (PDE5) inhibitors [11]. PDE5 inhibition tends not to result in an erection in RP patients but may result in sufficient increased blood flow and oxygenation of the corporal tissue to provide higher free oxygen levels needed for nitric oxide production and prevention of collagen formation; 2) Intracaversonal injection of PGE1 (allprostadil®). Injection results in an erection in most patients [12], however, the mechanism of application (ie. penile injection) both limits patient compliance, and may only cause intermittent rises in penile oxygen levels; 3) Vacuum Erection Devices (VED). Although commonly recommended in penile rehabilitation, these devices do cause a transient increase in arterial blood flow and oxygen supply. However, application of a constriction band (to facilitate an erection) drops oxygen saturation and therefore the VED is applied without the constriction band in penile rehabilitation [13].
A recent review of all studies related to penile rehabilitation by Fode, M et al. [11] came to the conclusion that although theoretical considerations warrant early implementation of penile rehabilitation to ensure cavernous oxygenation, there is little clinical evidence to support the use of current protocols. They instead recommend that in accordance with patient wishes, treatments should be prescribed in doses and combinations that actually induce erections and allow sexual intercourse if possible and should be offered early after RP to minimize the possible detrimental psychological effects. However, no easily administered effective treatment of ED following RP has yet to emerge.
We recently published a “proof-of-principle” paper demonstrating that topical application of NO-releasing hydromer nanoparticles (sialorphin-np, NO-np) can elicit an erectile response in an aging rat model of ED [14] without stimulation of the CN. The principle of the investigation was that the NO-np could topically deliver sufficient NO to relax corporal smooth muscle tissue and elicit an erection without the need for stimulation of the CN. In the present studies, we futher tested this hypothesis to determine if topically applied NO-np would elicit an erectile response in an animal model of ED resulting from CN transection. We hypothesiszed that the prolonged release of NO from the NO-np would lead to an overall increase in intracorporal blood pressure because of increased blood flow into the penis. The potential of topically applied NO-releasing nanoparticles to both induce erections and increase blood flow into the penis would make them useful therapeutics in penile rehabilitation following RP.
Materials and Methods
Animal model of radical prostatectomy
A total of 26 male Sprague–Dawley rats (4–5 months old, weighing ≈275 g) were obtained from Charles River Laboratories (Boston, MA). The model of radical prostatectomy was bi-lateral transection of the cavernous nerve (CN) as previously described [15–19]. Briefly the procedure involves performing a vertical low abdominal midline incision, identifying the major pelvic ganglion (MPG) on either side of the dorsolateral lobes of the prostate (which has two inputs, the hypogastric nerve and the pelvic nerve, and one of its outputs is the CN). CN transection was achieved by directly cutting the nerve 2–5 mm distal to the MPG under dissecting microscope visualization. After one week, erectile function before and after the application np was determined visually and through measurement of the intracorporal pressure/blood pressure ratio (ICP/BP).
Determination of erectile function
We have previously described ICP/BP determination [20]. Briefly rats are first anesthetized via intraperitoneal injection of sodium pentobarbital (35 mg/kg) and a cannula will be inserted into the carotid artery for systemic BP. Next an incision is made in the perineum, the ischiocavernosus muscle removed to expose the corpus cavernosum crus, and a 23-gauge needle inserted to measure ICP. The changes in ICP and systemic BP are monitored continuously throughout the experiments as described in [20]. After establishing baseline ICP/BP for at least 30 min, 200 μl NO-np was applied, either using DMSO-gel (10 animals) or coconut oil (6 animals) as carrier. In a similar set of experiments animal were treated with 200 μl empty-np as a negative control either using DMSO-gel (6 animals) or coconut oil (4 animals) as carrier. Animals were studied for demonstration of spontaneous erections (evidenced by peaks in ICP/BP ratio >0.6), time for onset of initial erections, duration of the erectile response and if there is increased ICP/BP ratio insufficient to cause an erection, but indicative of increased blood flow to the penis.
Hamster dorsal window chamber to monitor microcirculatory blood flow: Hamster dorsal window preparation
55–65 g male Golden Syrian Hamsters (Charles River Laboratories, Boston, MA) were fitted with a dorsal window chamber. Animal handling and care followed the NIH Guide for the Care and Use of Laboratory Animals. The experimental protocol was approved by the local animal care committee. The hamster window chamber model is widely used for microvascular studies and the complete surgical technique is described in detail elsewhere [21, 22]. Briefly, the animal was prepared for chamber implantation with a 50 mg/kg ip injection of pentobarbital sodium anesthesia. After hair removal, sutures were used to lift the dorsal skin away from the animal, and one frame of the chamber was positioned on the animal's back. A chamber consisted of two identical titanium frames with a 15-mm circular window. With the aid of backlighting and a stereomicroscope, one side of the skin fold was removed following the outline of the window until only a thin layer of retractor muscle and the intact subcutaneous skin of the opposing side remained. The intact skin of the other side was exposed to the ambient environment. Animals were allowed 2 days for recovery before experiment. Hamsters were suitable for the experiments if microscopic examination of the tissue in the chamber observed under ×650 magnification did not reveal signs of edema or bleeding.
Microvascular experimental setup
The hamsters instrumented with the dorsal window were restrained in a well-ventilated acrylic tube. The protruding window chamber was fixed to the microscopic stage for transillumination with a customized intravital microscope (BX51WI, Olympus, New Hyde Park, NY). The tissue image was projected onto a charge-coupled device camera (COHU 4815) connected to a videocassette recorder and viewed on a monitor. Measurements were carried out using a 40X (LUMPFL-WIR, numerical aperture 0.8, Olympus) water immersion objective. Animals were given 20 min to adjust to the tube environment before any measurement. Detailed mappings were made of the chamber vasculature to study the same vessels at baseline (before treatment) and throughout the experiment (after treatment). Blood vessels were chosen by a distinctive anatomic landmark to easily and quickly reestablish the same fields and vessels at each observation time point. Eight to ten arterioles and venules were selected in each preparation.
Microhemodynamics
A video image-shearing method was used to measure vessel diameter (D) [23]. Changes in arteriolar and venular diameter from baseline were used as indicators of a change in vascular tone. Arteriolar and venular centerline velocities were measured on-line by using the photodiode cross-correlation method (Photo Diode/Velocity Tracker Model 102B, Vista Electronics, San Diego, CA). The measured centerline velocity (V) was corrected according to vessel size to obtain the mean RBC velocity [24]. Blood flow (Q) was calculated from the measured values as Q = π × V (D/2)2. This calculation assumes a parabolic velocity profile and has been ftound to be applicable to tubes of 15 – 80 μm internal diameters and for Hcts in the range of 6 – 60% [24].
Topical NO-np application on the hamster window chamber
Eight hamsters were randomly divided into two experimental groups. One group (n=4) received NO-np (2 mg/cm2) in coconut oil as carrier, and the other group (n=4), NO-np (2 mg/cm2) in hyaluronic acid as a carrier. NO-np were applied on 50% of the intact skin of the opposing side of the window chamber (Figure 3). The other 50% of the intact skin received the same amount of the carrier. Microhemodynamics (diameter and blood flow) were studied over 90 min after NO-np application.
Figure 3.
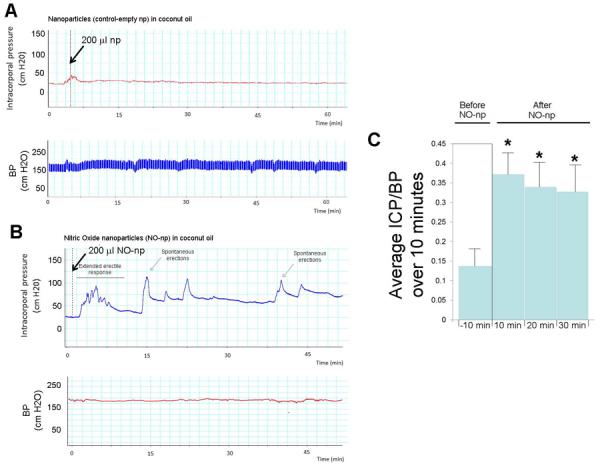
3A. Example of a continuous trace of intracorporal pressure (ICP) (upper panel) and systemic blood pressure (BP) (lower panel) over the course of an experiment following administration of 200 μl empty-nanoparticles in coconut oil performed topically to a rat that had cavernous nerve transection one week prior to the study. The time line is the time after application of the empty-nanoparticles. 3B. The experiment was repeated using nanoparticles releasing NO (NO-np). 3C. ICP/BP average over 10 minutes for 6 animals prior to the addition of NO-np, and at 10 minute intervals following the administration of the NO-np in the coconut oil carrier. *= significant increase in ICP/BP ratio over 10 minutes compared to pretreatment value
Preparation and characterization of the NO releasing nanoparticles (NO-np)
The NO-np used in this study are based on our previously reported protocol[25] but with minor modifications designed to make the resulting np more uniform with respect to size distribution and more compact with respect to the internal polymeric network.
Briefly, the NO-np are prepared using the following sequence of steps: 1) Hydrolyzing Tetramethylorthosilicate (TMOS): Stock of 5 ml of TMOS, 600 μl of deioinized water, and 560 μl of 2 mM hydrochloric acid are added to a small vial. The contents of the vial are sonicated for approximately 20–30 minutes yielding a clear solution that is then placed on ice. 2) Mixing the sol-gel components: 1.49 g of sodium nitrite are dissolved in 4 ml of PBS buffer at pH 7.5 followed by sequential addition and mixing of 0.5 ml of PEG-200, 500μl of chitosan (1mg/ml), and 34 ml of methanol. The resulting mixture is then vortexed thoroughly. Then, 2 ml of previously hydrolyzed TMOS is added to the solution along with approximately 50–75μl of 3-aminopropyltrimethoxysilane followed by vigorous vortexing until complete gelation. 3) Lyophilizing the sol-gel: The resulting gelled material is then lyophilized for 24–48 hrs which removes all volatile components including the methanol. 4) Ball-Milling the lyophilized solgel: Following lyophilization the dry material is ball milled at 150 rpm for 8 hours. The resulting material is a very fine white powder.
This above next generation protocol includes alcohol to reduce the number of water molecules integrated into the hydrogen bonding network (thus decreasing the internal water content) of the nanoparticles. Additionally, amine groups are incorporated into the polymeric network through the addition of aminopropyltrimethoxysilane which accelerates the polymerization process and also contributes to what appears to be a tighter internal hydrogen bonding network.
Electron Microscopy
Nanoparticles were plated on poly-L-lysine-coated coverslips, critical point dried using liquid CO2 in a Samdri-795 Critical Point Dryer (Tousimis, Rockville, MD), and sputter coated with chromium in a Q150T ES Sputter Coater (Quorum Technologies Ltd, United Kingdom). Samples were examined under a Supra Field Emission Scanning Electron Microscope (Carl Zeiss Microscopy, LLC North America) with accelerating voltage of 3 kV.
Dynamic Light Scattering
A suspension of NO-np (1 mg/ml) was sonicated in distilled water, and particle size was measured using DynaPro NanoStar (Wyatt Technology, Santa Barbara, CA). Experiments were conducted in triplicate, and 40 acquisition attempts at an acquisition length of 5 seconds were obtained per sample. Average particle hydrodynamic diameter and polydispersity index were calculated from the results.
Measurement of Gaseous NO release
Gaseous NO levels were measured using a chemiluminescent NO analyzer (Sievers NO analyzer, Model 280i, Boulder, CO): 1 mg NO-np was added to Di water (5 ml). pH was balanced using Trizma Base and Trizma HCI (Sigma-Aldrich, St. Louis, MO). Solutions were continuously bubbled with pure nitrogen (0.2 L/min). The gas phase was collected into the NO analyzer for a minute every set period of time, and the excess flow was evacuated through an underwater seal (1 cm H2O).
Statistical Analysis
We compared the observed difference (i.e., an erectile response) between NO-np treated and empty np (control) groups using Fisher's exact test. For comparing development of ICP/BP response between NO-np and empty-np, and for differences in microhemodynamics we used Student's t-test.
Results
NO-np characterization
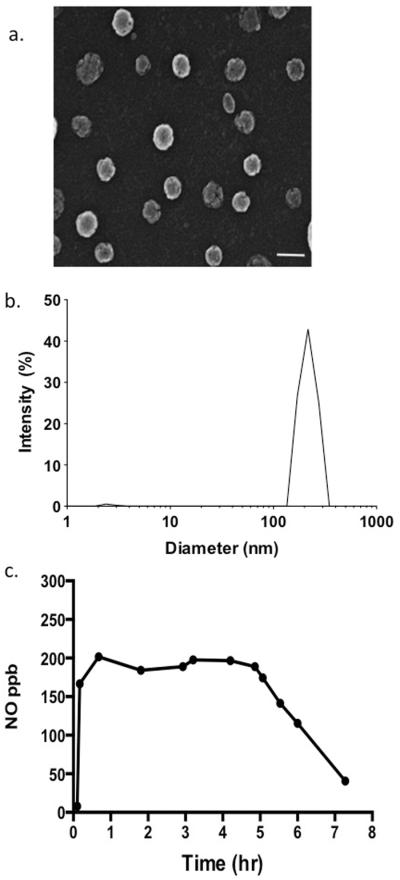
The resulting NO-np is a very fine white powder with no visible granularities. With scanning EM, results showed nanoparticles with a mean diameter of 55.6 ± 14.8 nm (Figure 1a). DLS analysis demonstrated a relatively narrow distribution of sizes for the NO-np, that is centered at 226.5 nm based on 40 acquisition attempts. The standard deviation is 8.9, proving that NO-np are homogenous in size. Since NO-np swell with moisture, the average diameter is likely an overestimate (Figure 1b).
Figure 1.
Characterization of the NO-np. The structure was analyzed using (a) scanning electron microscopy (SEM; bar 100 nm) and (b) analytical sizing performed using dynamic light scattering (DLS). (c)The graph shows the release of NO from the NO-np once placed in an aqueous environment over the course of 8 hours.
NO release from NO-np is depicted in Figure 1c. A peak release concentration was reached at 40.2 minutes, after which a steady state release ranging between 184–196 ppb NO was achieved, with subsequent decline of release rate extending to the end of the investigation at 7.2 hours. Measurements at lower pH levels showed small changes in release profiles, suggesting that limited amounts of nitrite remain in the nanoparticles.
Topically applied NO-np can elicit an erection in a rat model of radical prostatectomy
We investigated the ability of topically applied hydromer-nanoparticle releasing NO (NO-np) to elicit an erectile response in a rat model of RP (animals underwent CN transection one week prior to the experimental procedure). Two carriers were used, either DMSO gel or coconut oil. With the DMSO gel carrier six control animals were treated by topical application with empty-np (control) and ten experimental animals were topically treated with NO-np. An example is shown in Figure 2A for the effect of the empty-np on erectile response. None of the animals treated with the empty-np (control) had any erectile response (ie, a change ICP/BP ratio). An example of the effect of the NO-np on erectile response is shown in Figure 2B. Following treatment with the NO-np there was no significant effect on BP. There were two types of erectile responses; 1) a prolonged increase in the ICP/BP ratio or 2) spontaneous erections, where the ICP/BP ratio was >0.6 for approximately one minute. In eight of the ten animals there was a statistically significant increase in ICP/BP from an average of 0.182 +/− 0.03 prior to the addition of NO-np to 0.317 +/− 0.12 over 30 minutes following the application of the NO-np. This ICP/BP ratio equates to increased blood flow into the penis but not a visible erection. The average ICP/BP at 10 minute intervals following the application of the NO-np is shown in Figure 2C. Six out of the eight animals treated with the NO-np which had increased ICP/BP ratios of long duration also showed spontaneous erections, represented by peaks in the ICP/BP ratio of >0.6 of duration of approximately one minute (labeled in Figure 2B), representing a significant increase in occurrence of spontaneous erections (P=0.02). There was a range from the onset of spontaneous erections (from 5–37 minutes) with an average of 15 +/− 11 minutes. The spontaneous erections were of approximately 1 minute duration and were noted to occur after one single application for at least 45 minutes. None of the animals treated with the NO-np had a significant change in BP.
Figure 2.
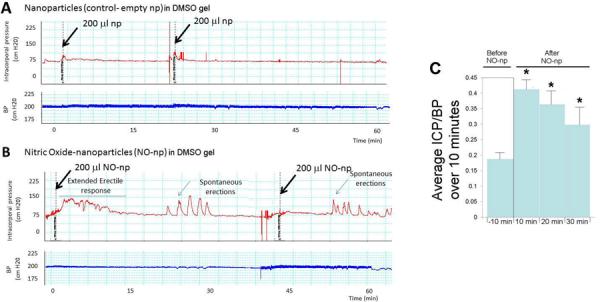
2A. Example of a continuous trace of intracorporal pressure (ICP) (upper panel) and systemic blood pressure (BP) (lower panel) over the course of an experiment following administration of 200 μl empty-nanoparticles in a DMSO-gel carrier performed topically to a rat that had cavernous nerve transection one week prior to the study. The time line is the time after application of the empty-nanoparticles. 2B. The experiment was repeated using nanoparticles releasing NO (NO-np). 2C. ICP/BP average over 10 minutes for 10 animals prior to the addition of NO-np, and at 10 minute intervals following the administration of the NO-np in the DMSO-gel carrier. *= significant increase in ICP/BP ratio over 10 minutes compared to pretreatment value.
Similar results were obtained using coconut oil as the carrier. The four control animals treated by topical application with empty-np (control) did not display any erectile response (ie, a change ICP/BP ratio or spontaneous erections). An example is shown in Figure 3A. As with the NO-np in DMSO-gel, when coconut oil was used as the carrier there was no significant effect on BP and there were two types of erectile responses (increased basal ICP/BP and spontaneous erections, Figure 3C). In all six animals tested there was a statistically significant increase in ICP/BP from an average of 0.157 +/− 0.04 prior to the addition of NO-np to 0.233 +/−0.16 over 30 minutes following the application of the NO-np. The average ICP/BP at 10 minute intervals following the application of the NO-np is shown in Figure 3C. In four of the six animals treated with the NO-np there were spontaneous erections, represented by peaks in the ICP/BP ratio of >0.6 of approximately one minute duration (an example is shown in Figure 3C) representing a significant increase in occurrence of spontaneous erections (P=0.02). The time for onset of these spontaneous erections was also similar to that observed with the DMSO gel carrier ranging from 7 to 20 minutes (with an average of 12 +/− 6 minutes) and had a duration of approximately 1 minute.
Topically applied NO-np increased blood flow
In light of the data presented above, it was hypothesized that the increased ICP was due to increased blood flow into the penis. Direct determination of blood flow into corporal tissue is not feasible with conventional techniques therefore to confirm the ability of the NO-np to increase blood flow when applied topically a hamster window model was utilized. As depicted in Figure 4A the window chamber is excised from the hamster, skin is removed from one side protected with a glass cover slip as described in the methods section (Figure 4B) and the back side is intact skin (Figure 4C) [26]. NO-np were applied at 2 mg/cm2 to half of the intact side area of the window chamber. Blood flows were compared between the untreated area (control) and the area treated with NO-np. Two carriers were compared, coconut oil (Figure 4D) and hyaluronic acid (Figure 4E) and with both carriers the NO-np caused a significant increase in the microcirculatory blood flow, sustained over the duration of the experiment (90 minutes). HA has been investigated as a drug delivery agent for various routes of administration, including ophthalmic, nasal, pulmonary, parenteral and topical. In fact, regulatory approval in the USA, Canada and Europe was granted for various drugs in 2.5% HA gel.
Figure 4.
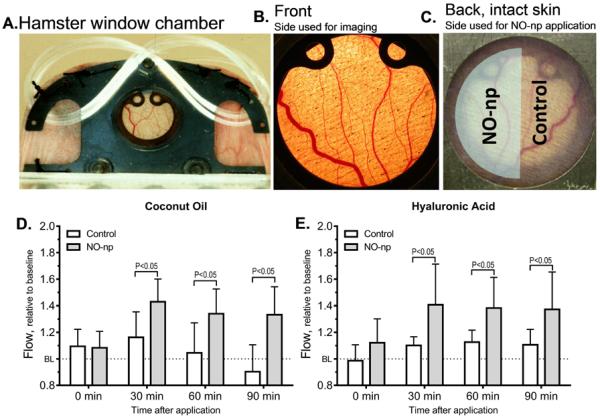
4A, Hamster window chamber model, the front side (4B) was observed using intra vital microscopy and the back side (4C) or intact skin received the NO-np. On one half of the intact skin exposed area the NO-np were applied (shaded) and blood flow compared with the untreated area (control). The control area received the carrier: The difference in microcirculatory blood flow caused by NO-np applied using coconut oil (4D) or hyaluronic acid (4E) as carrier from time of application is shown.
Discussion
We demonstrate for the first time that topical application of hydromer nanoparticles releasing NO (NO-np) to the penis of a rat model of RP will elicit spontaneous erections of approximately one minute duration and develop increased ICP/BP for a prolonged period of time, at least 30 minutes. It should be noted a normal erection in the rat has a duration of approximately 1 minute [27] and therefore the spontaneous erections resulting from the application of NO-np would equate to a the duration of a normal physiologic erection in the rat. Although it might be expected that the NO-np would exert a similar effect on blood flow and erectile function in normal animals (ie, without CN transection) this has not been determined. A potential side effect of administering NO-np could be a rise in BP if sufficient NO enters the systemic circulation. However with the animal numbers used in the present study there was no significant increase in BP following administration of the NO-np.
We hypothesized that the increased ICP was a result of increased blood flow into the penis, and in an established in vivo perfusion model (the hamster windows chamber flap) we demonstrated that the topically applied NO-np can generate an increase in blood flow over at least 90 minutes. The hamster window model includes muscle and connective tissue; therefore predominant vascular changes in blood flow will translate to other tissues independent of species exposed. Although the duration of physiologic experiments are restricted to several hours for the well-being of animals, we demonstrate that the NO-np can release NO over at least 7 hours, suggesting the physiologic effects of the NO-np could be of at least this duration. In our previous study using the same NO-np [20] we described that in an aging model of ED at the conclusion of the experiment there were no morphological changes. We do not expect given the duration of the present experiment that morphological changes would be apparent. In previous studies on the same animal model chronic administration of tadalafil, which was hypothesized to increase the basal level of blood-flow and thereby cavernous oxygenation, preserved penile smooth muscle content and function [28]. Further studies, where animals are given repeated treatment by the NO-np and monitored over a longer time course would likely to be necessary to determine a restorative effect on penile architecture.
NO is known to play a critical role in erectile function coordinating activity between the nervous system, the vascular system, and cavernosal smooth muscle tissues. Decreased tone (relaxation) of the cavernosal smooth muscle tissue results in penile erection (tumescence) [29]. At the biochemical level, normal smooth muscle tone is achieved through a balance of biochemical pathways that together regulate contraction or relaxation of the smooth muscle via myosin-driven actin filament sliding. To initiate the process of cavernosal smooth muscle relaxation, agonists bind to their membrane receptors and activate the synthesis of secondary messengers, such as NO and cGMP. The secondary messengers co-ordinate several biochemical pathways, leading to a decrease in [Ca2+]i and smooth muscle tone. One mechanism involves the activation of protein kinases A, C, and G (PKA, PKC, and PKG), which in turn activates the Maxi-K potassium channels, causing membrane hyperpolarization, which inhibits L-type calcium channels, lowering [Ca2+]i. It has been suggested that NO generation from nNOS plays two roles in erection: a rapid, brief, calcium-dependent activation of nNOS initiates the erectile process, with subsequent cAMP-dependent phosphorylation of nNOS sustaining NO production and erection [30]. Similarly PI3-kinase/Akt-dependent phosphorylation of eNOS results in sustained NO production to maintain an erection [31, 32]. It is also possible that increased blood flow into the penis further stimulates NO production from nitrite [33]. Although NO has been recognized for several years as playing a central role in eliciting an erection, prior to the development of the NO-np technology described here, topical delivery of NO has not been feasible.
It has been reported that lowered testosterone levels occur following RP in both patients and animal models [9, 10]. Since it is believed that the primary biochemical mediator of the effect of testosterone on erectile function is through the regulation of NOS activity, the use of NO-np would also be effective in treating ED resulting from low testosterone resulting from RP, In addition the erectogenic target of PDE5 inhibitors is downstream of NO. In patients undergoing RP, where the ability of CN to generate NO is perturbed, treatment with PDE5 inhibitors does not result in an erection. However, the efficacy of PDE5 inhibitors could be enhanced if local levels of NO in the penis were increased. Similarly it is also possible that the efficacy of the NO-np could also be enhanced synergistically if there was greater expression of down-stream targets, such as Maxi-K potassium channel. Gene transfer of vectors expressing Maxi-K are presently being evaluated in Phase I clinical trials for the treatment of ED [34, 35]. Therefore, in future experiments the efficacy of treatments will be determined in which treatments targeting downstream of NO (such as PDE5i or over-expression of MaxiK) will be combined with NO-np.
Several technologies have been proposed for cutaneous delivery of NO, including but not limited to, compressed gas cylinders that deliver NO directly to wounds [36], acidified nitrite cream that release NO [37] and diazeniumdiolates [38] that give off NO spontaneously from a donor compound. Unlike these and other NO delivery platforms, the NO-np do not rely on enzymatic conversion nor donor compounds for NO delivery. In the np delivery platform NO is spontaneously generated through the reduction of nitrite to NO, facilitated by the rich hydrogen bonding network provided by this unique nanoparticle technology [25, 39, 40]. As a result neither depletion of host thiols nor toxicity as a result of residual parent compound, both of which have been previously reported, are of concern here[41].
The present study demonstrates the hemodynamic effects of topically applied NO-np in various carriers. The NO-np carriers formulation released measurable quantities of NO over several hours. Mixing the NO-np into a hydrophobic carrier, like coconut oil or DMSO, or hydrophilic carrier, like hyaluronic acid gel, produced an increase of microcirculation blood flow. Although, coconut oil slows the transport of water to the NO-np and potentially reduces the rate at which NO is released, there were not differences in blood flow between the two carriers. From a translational standpoint, coconut oil facilitates the application of the nanoparticles on the skin due to its low temperature melting point. In addition, coconut oil can allows for several reactions that result in fatty acid nitration by the reactions of NO and oxygen. Recently, nitrated fatty acids, linoleic acid and oleic acid were found to be endogenous molecules with several attractive signaling properties [42]. Nitro-fatty acid derivatives are electrophilic lipid signaling mediators generated endogenously by NO and NO derived reactive species. Endogenous nitro-fatty acids have been associated with inflammation as they serve as ligands for peroxisome proliferator-activated receptors [43]. However, nitrated oleic can be a pluripotent signaling molecule by serving as a hydrophobically stabilized reserve for ·NO [42]. The strong vessel relaxation and increased blood flow produced by topical NO-np suspended in coconut oil or hyaluronic acid does not necessarily require that the NO released from the NO-np be stabilized and transported to the vascular smooth muscle by molecules in the carrier.
The current study demonstrates a new strategy to increase microvascular blood flow by topical application of NO-np in combination with coconut oil or hyaluronic acid. The comprehensive examination performed in this study defines that the combination strategy produced effective therapeutic synergism to increase blood flow in an experimental model. Our data show that treatment with either NO-np carried in coconut oil or hyaluronic acid was able to significantly increase blood flow of blood vessel 200 microns away from the site of NO-np application to intact skin. Although further studies will be required to determine a dose response curve for the NO-np in producing the effects on erectile physiology and if the application of the NO-np are capable of protecting or producing a more rapid recovery of penile architecture following RP (which is the primary goal of penile rehabilitation) our results demonstrate that topical application of NO-np to induce penile erections regardless of cavernous function.
Conclusion
We have demonstrated in an animal model of RP that the NO-np used in these studies have characteristics that would make them potentially useful agents in penile rehabilitation. They can both elicit a full erection and provide a persistent increase in the blood flow into the penis. Our in vitro data suggests that the release of NO can persist over at least 7 hours suggesting topical application to the penis would provide extended periods of increased blood-flow into corporal tissue. It remains to be determined if application of the NO-np protects corporal tissue from the pathological changes in penile architecture that accompany of hypoxia or can shorten the time for recovery of CN function (and thereby neurogenic erectile function). However, in other animal models the same hydromer gel NO-np have shown promise in accelerating cutaneous wound healing [44–46], suggesting that the NO-np may have several desirable characteristics to improve erectile function following RP.
Acknowledgments
This work was partly supported by grant by grant R01DK087872 from the NIH/NIDDK to Kelvin P. Davies. AJF acknowledges the Dermatology Foundation Career Development Award Program.
Authors Roles All authors were involved in designing of the study, data collection and analysis, interpretation of results and manuscript preparation. All authors read and approved the final manuscript.
Abbreviations
- CN
cavernous nerve
- DMSO
dimethyl sulfoxide
- ED
erectile dysfunction
- ICP/BP
intracorporal pressure/ blood pressure ratio
- MPG
major pelvic ganglion
- NO
nitric oxide; np nanoparticle
- PDE5
Phosphodiesterase-5
- RP
radical prostatectomy
Footnotes
Conflict of Interest The authors declare they have no conflict of interest.
References
- 1.McCullough AR. Sexual dysfunction after radical prostatectomy. Rev Urol. 2005;7(Suppl 2):S3–S10. [PMC free article] [PubMed] [Google Scholar]
- 2.Alemozaffar M, et al. Prediction of erectile function following treatment for prostate cancer. Jama. 2011;306(11):1205–14. doi: 10.1001/jama.2011.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canguven O, Burnett A. Cavernous nerve injury using rodent animal models. J Sex Med. 2008;5(8):1776–85. doi: 10.1111/j.1743-6109.2008.00955.x. [DOI] [PubMed] [Google Scholar]
- 4.User HM, et al. Penile weight and cell subtype specific changes in a post-radical prostatectomy model of erectile dysfunction. J Urol. 2003;169(3):1175–9. doi: 10.1097/01.ju.0000048974.47461.50. [DOI] [PubMed] [Google Scholar]
- 5.Leungwattanakij S, et al. Cavernous neurotomy causes hypoxia and fibrosis in rat corpus cavernosum. J Androl. 2003;24(2):239–45. doi: 10.1002/j.1939-4640.2003.tb02668.x. [DOI] [PubMed] [Google Scholar]
- 6.Klein LT, et al. Apoptosis in the rat penis after penile denervation. J Urol. 1997;158(2):626–30. [PubMed] [Google Scholar]
- 7.Ferrini MG, et al. Fibrosis and loss of smooth muscle in the corpora cavernosa precede corporal veno-occlusive dysfunction (CVOD) induced by experimental cavernosal nerve damage in the rat. J Sex Med. 2009;6(2):415–28. doi: 10.1111/j.1743-6109.2008.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magheli A, Burnett AL. Erectile dysfunction following prostatectomy: prevention and treatment. Nat Rev Urol. 2009;6(8):415–27. doi: 10.1038/nrurol.2009.126. [DOI] [PubMed] [Google Scholar]
- 9.Gacci M, et al. Changes in sex hormone levels after radical prostatectomy: Results of a longitudinal cohort study. Oncol Lett. 2013;6(2):529–533. doi: 10.3892/ol.2013.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vignozzi L, et al. Cavernous neurotomy in the rat is associated with the onset of an overt condition of hypogonadism. J Sex Med. 2009;6(5):1270–83. doi: 10.1111/j.1743-6109.2008.01208.x. [DOI] [PubMed] [Google Scholar]
- 11.Fode M, et al. Penile rehabilitation after radical prostatectomy: what the evidence really says. BJU Int. 2013;112(7):998–1008. doi: 10.1111/bju.12228. [DOI] [PubMed] [Google Scholar]
- 12.Montorsi F, et al. Recovery of spontaneous erectile function after nerve-sparing radical retropubic prostatectomy with and without early intracavernous injections of alprostadil: results of a prospective, randomized trial. J Urol. 1997;158(4):1408–10. [PubMed] [Google Scholar]
- 13.Hoyland K, Vasdev N, Adshead J. The use of vacuum erection devices in erectile dysfunction after radical prostatectomy. Rev Urol. 2013;15(2):67–71. [PMC free article] [PubMed] [Google Scholar]
- 14.Calenda G, et al. Reversal of Diabetic Vasculopathy in a Rat Model of Type 1 Diabetes by Opiorphin-Related Peptides. Am J Physiol Heart Circ Physiol. doi: 10.1152/ajpheart.00383.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinlan DM, et al. The rat as a model for the study of penile erection. J Urol. 1989;141(3):656–61. doi: 10.1016/s0022-5347(17)40926-8. [DOI] [PubMed] [Google Scholar]
- 16.Burnett AL, Becker RE. Immunophilin ligands promote penile neurogenesis and erection recovery after cavernous nerve injury. J Urol. 2004;171(1):495–500. doi: 10.1097/01.ju.0000089775.88825.ec. [DOI] [PubMed] [Google Scholar]
- 17.Ferrini MG, et al. Vardenafil prevents fibrosis and loss of corporal smooth muscle that occurs after bilateral cavernosal nerve resection in the rat. Urology. 2006;68(2):429–35. doi: 10.1016/j.urology.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Sezen SF, et al. Immunophilin ligand FK506 is neuroprotective for penile innervation. Nat Med. 2001;7(10):1073–4. doi: 10.1038/nm1001-1073. [DOI] [PubMed] [Google Scholar]
- 19.Zuzuarregui JL, et al. Parameters affecting the results in a program of artificial insemination with donor sperm. A 12-year retrospective review of more than 1800 cycles. J Assist Reprod Genet. 2004;21(4):109–18. doi: 10.1023/B:JARG.0000029494.55273.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han G, et al. Nanoparticles as a novel delivery vehicle for therapeutics targeting erectile dysfunction. J Sex Med. 2010;7(1 Pt 1):224–33. doi: 10.1111/j.1743-6109.2009.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colantuoni A, Bertuglia S, Intaglietta M. Quantitation of rhythmic diameter changes in arterial microcirculation. Am J Physiol. 1984;246:H508–H517. doi: 10.1152/ajpheart.1984.246.4.H508. [DOI] [PubMed] [Google Scholar]
- 22.Endrich B, et al. Technical report: A new chamber technique for microvascular studies in unanaesthetized hamsters. Res Exp Med. 1980;177:125–134. doi: 10.1007/BF01851841. [DOI] [PubMed] [Google Scholar]
- 23.Intaglietta M, Tompkins WR. Microvascular measurements by video image shearing and splitting. Microvasc Res. 1973;5(3):309–312. doi: 10.1016/0026-2862(73)90042-3. [DOI] [PubMed] [Google Scholar]
- 24.Lipowsky HH, Zweifach BW. Application of the “two-slit” photometric technique to the measurement of microvascular volumetric flow rates. Microvasc Res. 1978;15:93–101. doi: 10.1016/0026-2862(78)90009-2. [DOI] [PubMed] [Google Scholar]
- 25.Friedman AJ, et al. Sustained release nitric oxide releasing nanoparticles: Characterization of a novel delivery platform based on nitrite containing hydrogel/glass composites. Nitric Oxide-Biology and Chemistry. 2008;19(1):12–20. doi: 10.1016/j.niox.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Menger MD, Laschke MW, Vollmar B. Viewing the microcirculation through the window: some twenty years experience with the hamster dorsal skinfold chamber. Eur Surg Res. 2002;34(1–2):83–91. doi: 10.1159/000048893. [DOI] [PubMed] [Google Scholar]
- 27.Bernabe J, et al. Intracavernous pressure during erection in rats: an integrative approach based on telemetric recording. Am J Physiol. 1999;276(2 Pt 2):R441–9. doi: 10.1152/ajpregu.1999.276.2.R441. [DOI] [PubMed] [Google Scholar]
- 28.Vignozzi L, et al. Effect of chronic tadalafil administration on penile hypoxia induced by cavernous neurotomy in the rat. J Sex Med. 2006;3(3):419–31. doi: 10.1111/j.1743-6109.2006.00208.x. [DOI] [PubMed] [Google Scholar]
- 29.Andersson KE. Erectile physiological and pathophysiological pathways involved in erectile dysfunction. J Urol. 2003;170(2 Pt 2):S6–13. doi: 10.1097/01.ju.0000075362.08363.a4. discussion S13–4. [DOI] [PubMed] [Google Scholar]
- 30.Hurt KJ, et al. Cyclic AMP-dependent phosphorylation of neuronal nitric oxide synthase mediates penile erection. Proc Natl Acad Sci U S A. 2012;109(41):16624–9. doi: 10.1073/pnas.1213790109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurt KJ, et al. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc Natl Acad Sci U S A. 2002;99(6):4061–6. doi: 10.1073/pnas.052712499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burnett AL, et al. Noncholinergic penile erection in mice lacking the gene for endothelial nitric oxide synthase. J Androl. 2002;23(1):92–7. doi: 10.1002/j.1939-4640.2002.tb02601.x. [DOI] [PubMed] [Google Scholar]
- 33.Feelisch M, et al. Tissue processing of nitrite in hypoxia: an intricate interplay of nitric oxide-generating and -scavenging systems. J Biol Chem. 2008;283(49):33927–34. doi: 10.1074/jbc.M806654200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melman A, et al. Gene transfer with a vector expressing Maxi-K from a smooth muscle-specific promoter restores erectile function in the aging rat. Gene Ther. 2008;15(5):364–70. doi: 10.1038/sj.gt.3303093. [DOI] [PubMed] [Google Scholar]
- 35.Melman A, Davies KP. Gene therapy in the management of erectile dysfunction (ED): past, present, and future. ScientificWorldJournal. 2009;9:846–54. doi: 10.1100/tsw.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghaffari A, et al. Potential application of gaseous nitric oxide as a topical antimicrobial agent. Nitric Oxide. 2006;14(1):21–29. doi: 10.1016/j.niox.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Weller R, Finnen MJ. The effects of topical treatment with acidified nitrite on wound healing in normal and diabetic mice. Nitric Oxide. 2006;15(4):395–9. doi: 10.1016/j.niox.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Miranda KM, et al. Comparison of the NO and HNO donating properties of diazeniumdiolates: primary amine adducts release HNO in Vivo. J Med Chem. 2005;48(26):8220–8. doi: 10.1021/jm050151i. [DOI] [PubMed] [Google Scholar]
- 39.Han G, Friedman AJ, Friedman JM. Nitric oxide releasing nanoparticle synthesis and characterization. Methods Mol Biol. 2011;704:187–95. doi: 10.1007/978-1-61737-964-2_14. [DOI] [PubMed] [Google Scholar]
- 40.Cabrales P, et al. Sustained release nitric oxide from long-lived circulating nanoparticles. Free Radical Biology and Medicine. 2010;49(4):530–538. doi: 10.1016/j.freeradbiomed.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedman A, Friedman J. New biomaterials for the sustained release of nitric oxide: past, present and future. Expert Opinion on Drug Delivery. 2009;6(10):1113–1122. doi: 10.1517/17425240903196743. [DOI] [PubMed] [Google Scholar]
- 42.Schopfer FJ, et al. Fatty acid transduction of nitric oxide signaling. Nitrolinoleic acid is a hydrophobically stabilized nitric oxide donor. J Biol Chem. 2005;280(19):19289–97. doi: 10.1074/jbc.M414689200. [DOI] [PubMed] [Google Scholar]
- 43.Baker PR, et al. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem. 2005;280(51):42464–75. doi: 10.1074/jbc.M504212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blecher K, et al. Nitric oxide-releasing nanoparticles accelerate wound healing in NOD-SCID mice. Nanomedicine. 2012;8(8):1364–71. doi: 10.1016/j.nano.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez DA, Nosanchuk J, Friedman A. The purview of nitric oxide nanoparticle therapy in infection and wound healing. Nanomedicine (Lond) 2012;7(7):933–6. doi: 10.2217/nnm.12.67. [DOI] [PubMed] [Google Scholar]
- 46.Han G, et al. Nitric oxide-releasing nanoparticles accelerate wound healing by promoting fibroblast migration and collagen deposition. Am J Pathol. 2012;180(4):1465–73. doi: 10.1016/j.ajpath.2011.12.013. [DOI] [PubMed] [Google Scholar]