Abstract
The objective of the present study was to characterize the role of resveratrol (Res) and vitamin C (VC) in prevention of estrogen-induced breast cancer through regulation of CNC b-zip transcription factors. Human breast epithelial cell line MCF-10A was treated with 17β-estradiol (E2) and VC or Res with or without E2. mRNA and protein expression levels of CNC b-zip transcription factors: Nrf1, Nrf2, Nrf3 and Nrf2-regulated antioxidant enzymes SOD3 and NQO1 were quantified. Treatment with E2 suppressed while VC and Res prevented E2-mediated decrease in the expression levels of SOD3, NQO1, Nrf2 mRNA and protein in MCF-10A cells. Treatment with E2, Res or VC significantly increased mRNA and protein expression levels of Nrf1. 17β-estradiol treatment significantly increased but VC or Res decreased Nrf3 mRNA and protein expression levels. Our studies demonstrate that estrogen-induced breast cancer might be prevented through up-regulation of antioxidant enzymes via Nrf-dependent pathways.
Keywords: Estrogen, Breast cancer, Resveratrol, Vitamin C, CNC b-zip transcription factors
INTRODUCTION
Breast cancer is one of the leading causes of death among women in the US [1–3]. Most of the breast cancers are estrogen-dependent and estrogens are known to be carcinogenic in both humans and rodents [4, 5]. Prolonged exposure to estrogen is associated with development of breast cancer [6–11]. Estrogen metabolism-mediated oxidative stress plays important role in development of carcinogenesis [12–18]. According to epidemiologic observations, the incidence of breast cancer is lower in Asian women, who consume significantly higher amounts of phytoestrogens than those of Westerners [19]. Second- and third-generation descendants of Asian women who migrated to Western countries have breast cancer risks similar to Westerners, suggesting that lifestyle may explain the low breast cancer risk observed in Asian women [20]. However, the relationship between phytoestrogens and breast cancer remains controversial [21, 22]. Moreover, the possible mechanisms of phytoestrogen action in breast cancer have yet to be resolved.
Resveratrol (Res) is an extensively studied phytoestrogen found in grapes, nuts, red wine and various other plants, and is well known for its protective role against various diseases including cancer [23–26]. One important activity of Res is antioxidant activity, manifested as eliminating reactive oxygen species (ROS) [25, 26]. Excessive ROS can cause peroxidation of lipids, oxidative damage of DNA and proteins, and play important role in different diseases including cancer [16, 17, 27, 28]. Resveratrol has been shown to have anticancer activity in in vitro and in vivo studies [29, 30]. Vitamin C (VC) is very well known naturally occurring antioxidant found in a wide variety of fruits and vegetables. The use of VC in cancer chemoprevention and treatment has been reported extensively in the literature [11, 31–34]. The mechanism of breast cancer chemoprevention by antioxidants is not well understood and is the focus of our present studies. We have characterized in our current studies, the antioxidant mechanism of the naturally occurring antioxidants, Res and VC.
Transcription factors from Cap’n’collar (CNC) family of proteins are well known for mediating important developmental and homeostatic functions [35–38]. Nuclear factor erythroid 2-related factor 1 (Nrf1, also called NFE2L1), nuclear factor erythroid 2-related factor 2 (Nrf2), and nuclear factor erythroid 2-related factor 3 (Nrf3) comprise a subgroup of CNC factors that mediate adaptive responses to cellular stresses [35–38]. The most studied stress-activated CNC factor is Nrf2, responsible for the transcriptional response of cells to oxidative stress mediators [39–43]. There are fewer studies reporting functions of Nrf1 in controlling oxidative stress and estrogen-induced breast cancer. It has been suggested that Nrf1 may play as important a role in human carcinogenesis as Nrf2 [44–47]. Under oxidative stress, Nrf2 is known to be regulated by Kelch-like ECH-associated protein 1 (Keap1) [39, 40, 43]. In contrast, Nrf1 is not regulated by Keap1 [48, 49]. Nrf1, but not Nrf2 or Nrf3, is essential for embryonic development; Nrf1−/− mice die at mid-late gestation, presumably due to anemia-induced hypoxia [38]. In contrast, Nrf3 is known to negatively regulate antioxidant enzymes [50].
Since Nrf1, Nrf2 and Nrf3 control phase II detoxification enzymes that help in detoxification of potential carcinogens, understanding the mechanisms of regulation of these three “Nrfs” might help in development of cancer therapeutics. We report in this study that antioxidants may mediate their protective role in E2-induced breast cancer through a complex interplay of CNC b-zip family of proteins.
MATERIALS AND METHODS
Chemicals
17 β-Estradiol (E2), Resveratrol (Res) and Vitamin C (VC) were purchased from Sigma–Aldrich (St. Louis, MO). 17 β-Estradiol (E2) and Res were dissolved in dimethylsulfoxide (DMSO) and VC in distilled water prior to treatments. The concentration of DMSO in control experiments or in experimental samples was always 1/1000th (v/v) of the final medium volume.
Cell culture
Non-tumorigenic breast epithelial cell line MCF-10A was purchased from American Type Culture Collection (ATCC, Manassas, VA). Experiments were performed in passages two to six of cells sub-cultured from a frozen vial. MCF-10A cells were grown in Dulbecco’s modified Eagle’s medium/F12 (50:50) media (Mediatech, Herndon,VA). Twenty four hours prior to treatment, cells were washed twice with phosphate-buffered saline (PBS) and then grown in phenol red-free Dulbecco’s modified Eagle’s medium/F12 (50:50) supplemented with 5% charcoal-dextran-stripped horse serum (Cocalico Biologicals, Reamstown, PA). Cells were treated with E2 (10 and 50 nM), VC (1 mM), Res (50 μM), VC + E2 and Res + E2 for up to 72 hr. Treated cells were washed with PBS, RNA and protein were isolated and used for RT-PCR, Western blot and Co-Immunoprecipitation (Co-Ip) analyses, respectively, according to established methods.
Reverse transcription and real-time PCR
Real-time PCR was used to quantify mRNA expression levels of Nrf1, Nrf2, Nrf3, Superoxide dismutase 3 (SOD3) and NAD(P)H:quinone oxidoreductase 1(NQO1). Our recently published study suggests that SOD3 and not SOD1 or SOD2 play important role in antioxidant-mediated prevention of E2-induced breast cancer in female ACI rats [51]. After different treatments, total RNA from cultured cells was isolated using TRI Reagent (Molecular Research Center, Inc.), according to the supplier’s protocols. Five μg total RNA was reverse transcribed using the Superscript II reverse transcription system and an oligo-dT18 primer (Invitrogen, Carlsbad, CA). After reverse transcription, RNase H (2 units/μl) was added to all samples to ensure degradation of the remaining RNA. Real-time PCR was performed in duplicate 25 μl reactions using human-specific Nrf1, Nrf2, Nrf3, SOD3 and NQO1 primers (Qiagen, Valencia, CA and Integrated DNA Technologies, Coralville, IA, USA) using the iCycler iQ5 system (Bio-Rad Laboratories,Hercules, CA). The mRNA expression of cyclophilin, a housekeeping gene, was used for quantification and standardization purposes [54]. The expression of the genes under study relative to cyclophilin were determined by dividing the number of cDNA molecules for the gene of interest by the number of cyclophilin cDNA molecules as reported previously [55]. Data were analyzed from at least three different samples in each group.
Western blot analysis
Cell lysates were prepared in RIPA buffer with protease inhibitor cocktail (Sigma–Aldrich, St Louis, MO). Thirty micrograms total protein, isolated from quadruplicates of control or treated cells were size fractionated on a 12% SDS-polyacrylamide gel, and transferred onto PVDF membranes, under standard conditions [28, 51–53]. Membranes were blocked in 5% dry non-fat milk/PBS/0.05% Tween-20 at room temperature for 2 hr. Affinity purified polyclonal antibodies against Nrf1, Nrf2, Nrf3, SOD3 and NQO1 (Santa Cruz Biotechnology, Santa Cruz, CA) were diluted 1:1500 in PBS/0.05% Tween-20 and used for immunodetection. After overnight incubation at room temperature with the primary antibody, membranes were washed four times for 8 min per wash using PBS/0.05%Tween-20. Horse radish peroxidase conjugated IgG was diluted 1:2000 in PBS/0.05% Tween-20 and used as a secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). After incubation for 2 hr at room temperature, the membrane was washed again as described above. Chemiluminescent detection was performed using the BM Chemiluminescence Detection kit (Roche, Indianapolis, IN) and Alpha Innotech FluorChem HD2 (Alpha Innotech, San Leandro, CA) gel documentation system. Membranes probed for Nrf1, Nrf2, SOD3, and NQO1 were washed in PBS/0.05% Tween-20 and re-incubated overnight at room temperature with α-Tubulin mouse monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA), diluted 1:2000 in PBS/0.05%Tween-20. Horse radish peroxidase-conjugated anti-mouse IgG antibody (Santa Cruz Biotechnology) was diluted 1:2500 in PBS/0.05% Tween-20 and used as a secondary antibody for α-Tubulin detection. Secondary antibody was incubated with the the membrane for 2 hr at room temperature prior to chemiluminescent detection using the method described above.
RNA interference
Small interfering RNAs (siRNAs) for Nrf3 and scrambled siRNA were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). MCF-10A cells were transfected with siNrf3 (20 nmol/l) using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA). Scrambled siRNA- (20 nmol/l) transfected MCF-10A cells were used as a negative control.
Co-Immunoprecipitation
A Pierce kit (Pierce Biotechnology, Inc., Rockford, IL; Catalog #26149) was used for co-immunoprecipitation of interacting proteins, Nrf2 and Nrf3. After direct covalent immobilization of the primary antibody (Nrf2 antibody, Santa Cruz, CA) to amino link plus resin, immunoprecipitation of the Nrf2 protein (bait protein), and co-immunoprecipitation of interacting Nrf3 protein (prey protein) were performed using spin columns. The experiments were repeated reversing bait and prey proteins. Now Nrf3 antibody (Santa Cruz, CA) was covalently immobilized to the resin and immunoprecipitated, the blot was probed to detect co-immunoprecipitation of Nrf2 as prey protein. Unspecific interactions were identified by using the provided control gel and substituting IgG for the specific antibody. Eluted fractions were then subjected to Western blotting for identification of the interacting proteins.
Statistical analysis
Statistical analyses were performed by using Sigma Plot 11.0 (Systat Software, San Jose, CA) and IBM SPSS Statistics 19 software (IBM Inc, Armonk, NY). All cell culture treatments were done in quadruplicate. The Student’s t-test was used to calculate p values for comparing Nrf2 mRNA and protein levels for the time course and dose-dependent experiments between control and estrogen treatment groups. One-way analysis of variance (ANOVA) and least significant difference (LSD) post hoc analyses were used to calculate p values for comparisons of Nrf1, Nrf2, Nrf3, SOD3, or NQO1 mRNA and protein expression levels among all groups with E2, Res and VC-treated MCF-10A cells. A p value of <0.05 was considered significant.
RESULTS
Estrogen decreases, whereas antioxidants reverse E2-mediated decrease in Nrf2 mRNA and protein expression levels, and further increase its mRNA and protein expression levels
A dose-dependent change in Nrf2 mRNA and protein expression levels was determined using different concentrations of E2. A maximum decrease in Nrf2 mRNA (24 hr) and protein expression (48 hr) at a dose of 50 nM E2 was observed (Figure 1A and 1B). A time-dependent change in Nrf2 mRNA and protein expression levels was determined using 50 nM E2. A maximal inhibition of Nrf2 mRNA expression at 24 hr and protein expression at 48 hr was observed (Figure 1C and 1D). The effect of Res and VC on the regulation of Nrf2 mRNA and protein expression levels in MCF-10A cells was examined. Vitamin C (1 mM) or Res (50 μM) significantly increased Nrf2 mRNA and protein expression levels and reversed E2-mediated decrease in Nrf2 mRNA and protein expression levels in MCF-10A cells after 48 hr of treatment (Figure 2A and 2B). The fold changes in Nrf2 protein expression levels in MCF-10A cells treated with E2, Res, Res + E2, VC and VC + E2 for 48 hr were 0.43, 2.33, 1.87, 3.32 and 2.84, respectively, compared to vehicle-treated control cells (Figure 2B).
FIGURE 1.
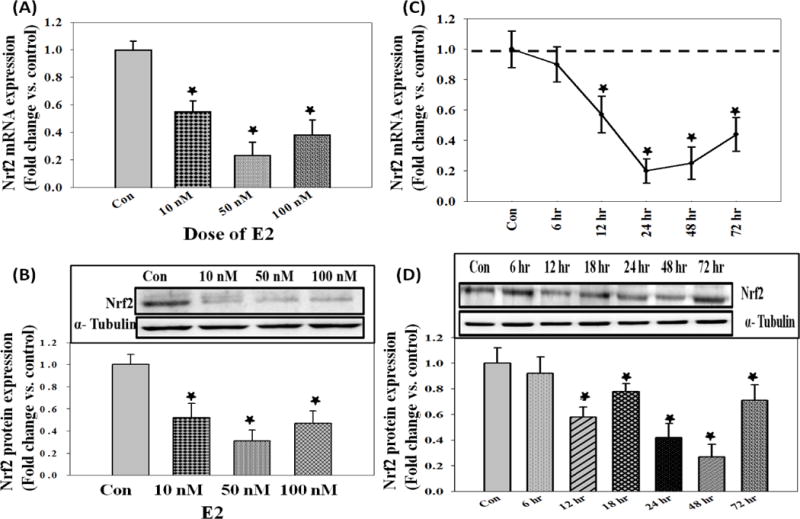
17 β-Estradiol (E2) down-regulates Nrf2 in MCF-10A cells in a time- and dose-dependent manner. (A) Nrf2 mRNA expression levels in MCF-10A cells treated with 10, 50 or 100 nM E2 for 24 hr; (B) Nrf2 protein expression levels in MCF-10A cells treated with increased doses of E2 for 48 hr; (C) Nrf2 mRNA expression levels in MCF-10A cells treated with 50 nM E2 for up to 72 hr; and (D) Nrf2 protein expression levels in MCF-10A cells treated with 50 nM E2 for up to 72 hr.
‘*’ indicates p value ≤ 0.05 compared with respective controls.
FIGURE 2.
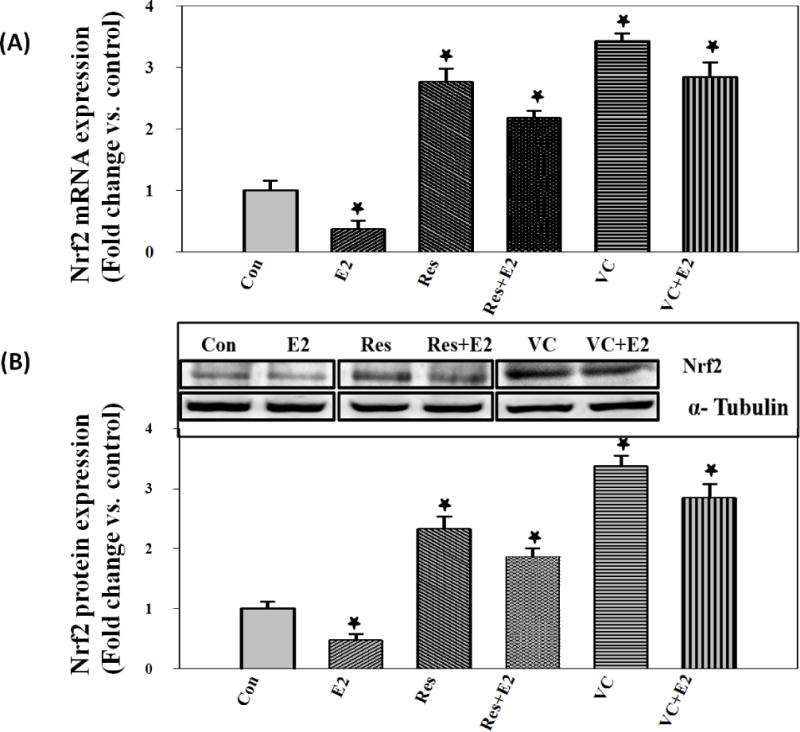
Antioxidants (VC and Res) significantly reverse E2-mediated decrease in Nrf2 mRNA and protein expression levels in MCF-10A cells. (A) Nrf2 mRNA expression levels in MCF-10A cells treated with 50 nM E2, 50 μM Res, Res + E2, 1 mM VC or VC + E2 for 24 hr and (B) Nrf2 protein expression levels in MCF-10A cells treated with 50 nM E2, 50 μM Res, Res + E2, 1 mM VC or VC + E2 for up to 48 hr.
‘*’ indicates p value ≤ 0.05 compared with respective controls.
Estrogen treatment induces Nrf3 and Nrf1, whereas antioxidants VC and Res differentially regulate Nrf3 and Nrf1 in MCF-10A cells
Nrf3 mRNA and protein expression levels were quantified in MCF-10A cells treated with E2 (50 nM) for up to 72 hr. A significant increase in Nrf3 mRNA as well as protein expression levels compared to control was identified at 48 hr after treatment (Figure 3A and 3B). Resveratrol (50 μM) and VC (1 mM) treatment reversed E2-mediated increase in Nrf3 mRNA as well as protein expression levels, and significantly decreased Nrf3 mRNA and protein expression levels in MCF-10A cells compared to controls (Figure 3A and 3B). The fold changes in Nrf3 protein expression levels in MCF-10A cells treated with E2, Res, Res + E2, VC and VC + E2 for 48 hr were 2.68, 0.53, 0.31, 0.61 and 0.78, respectively, compared to vehicle-treated control cells (Figure 3B).
FIGURE 3.
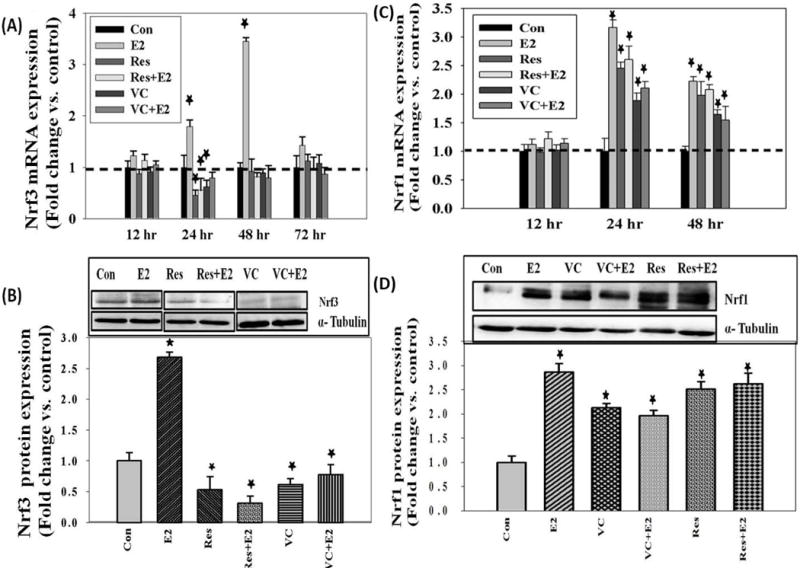
17 β-Estradiol induces Nrf3 as well as Nrf1 mRNA and protein expression levels in MCF-10A cells. Antioxidants (VC and Res) reverse E2-mediated increase in Nrf3 mRNA and protein expression levels and induce Nrf1 mRNA and protein expression levels in MCF-10A cells. (A) Nrf3 mRNA expression levels in MCF-10A cells treated with 50 nM E2, 50 μM Res, Res + E2, 1 mM VC or VC + E2 for up to 72 hr; (B) Nrf3 protein expression levels in MCF-10A cells treated with 50 nM E2 and 50 μM Res, Res + E2, 1 mM VC or VC + E2 for 48 hr; (C) Nrf1 mRNA expression levels in MCF-10A cells treated with 50 nM E2, 50 μM Res, Res + E2, 1 mM VC or VC + E2 for up to 48 hr; and (D) Nrf1 protein expression levels in MCF-10A cells treated with 50 nM E2, 50 μM Res, Res + E2, 1 mM VC or VC + E2 for 48 hr.
Nrf1 mRNA and protein expression levels were quantified in MCF-10A cells treated with E2 (50 nM) for up to 48 hr. A significant increase in Nrf1 mRNA as well as protein expression levels compared to control was detected (Figure 3C and 3D). Antioxidants by themselves also significantly increased Nrf1 mRNA and protein expression levels compared to vehicle-treated control cells (Figure 3C and 3D). This increase was independent of E2 treatment. The fold change in Nrf1 protein expression levels in MCF-10A cells treated with E2, Res, Res + E2, VC and VC + E2 for up to 48 hr were 2.87, 2.51, 2.62, 2.13 and 1.97, respectively, compared to vehicle-treated control cells (Figure 3D).
Antioxidants reverse estrogen-mediated decrease in NQO1 and SOD3 mRNA and protein expression levels
mRNA and Protein expression levels of NAD(P)H:quinone oxidoreductase 1 (NQO1) were quantified in MCF-10A cells treated with E2 (50 nM) for up to 48 hr. Significant decreases in NQO1 mRNA as well as protein expression levels were determined (Figure 4A and 4B). Resveratrol (50 μM) and VC (1 mM) treatment reversed E2-mediated decrease in NQO1 mRNA as well as protein expression levels and significantly increased NQO1 mRNA and protein expression levels in MCF-10A cells treated for up to 48 hr (Figure 4A and 4B). The fold changes in NQO1 protein expression levels in MCF-10A cells treated with E2, Res, Res + E2, VC and VC + E2 for 48 hr were 0.48, 3.28, 3.15, 2.24 and 1.12, respectively, compared to vehicle-treated cells (Figure 4B). mRNA and protein expression levels of SOD3 were quantified in MCF-10A cells treated with E2 (50 nM) for up to 48 hr. A significant decrease in SOD3 mRNA as well as protein expression levels was detected compared to control cells (Figure 4C and 4D). Resveratrol (50 μM) and VC (1 mM) treatment reversed E2-mediated decrease in SOD3 mRNA as well as protein expression levels and further, significantly increased SOD3 mRNA and protein expression levels in MCF-10A cells treated for up to 48 hr (Figure 4C and 4D). The fold changes in SOD3 protein expression levels in MCF-10A cells treated with E2, Res, Res + E2, VC and VC + E2 for 48 hr were 0.42, 3.84, 3.79, 2.32 and 2.18, respectively, compared to vehicle-treated controls (Figure 4D).
FIGURE 4.
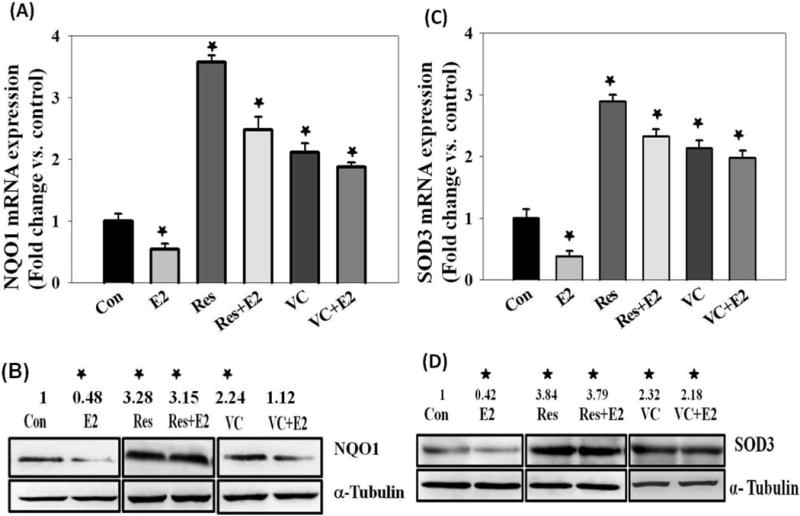
Antioxidants reverse E2-mediated decrease in Phase-II detoxifying enzymes, NQO1 and SOD3, at both mRNA and protein levels. (A) NQO1 mRNA expression levels in MCF-10A cells treated with 50 nM E2, 50 μM Res, Res + E2, 1 mM VC or VC + E2 for 24 hr; (B) NQO1 protein expression levels in MCF-10A cells treated with 50 nM E2, 50 μM Res, Res + E2, 1mM VC or VC + E2 for 48 hr; (C) SOD3 mRNA expression levels in MCF-10A cells treated with 50 nM E2, 50 μM Res, Res + E2, 1 mM VC or VC + E2 for 24 hr; and (D) SOD3 protein expression levels in MCF-10A cells treated with 50 nM E2, 50 μM Res, Res + E2, 1 mM VC or VC + E2 for 48 hr.
‘*’ indicates p value ≤ 0.05 compared with respective controls.
Nrf3 negatively regulates Nrf2 and Nrf2-dependent gene NQO1
Nrf2 is a known positive regulator of antioxidant phase-II detoxifying gene NQO1 (38), whereas Nrf3 is known to negatively regulate NQO1 gene (73). We investigated whether silencing of Nrf3 has any effect on the expression of Nrf2 gene. An increase in Nrf2 protein expression was observed after silencing of Nrf3 in MCF-10A cells (Figure 5A). Additionally an increase in NQO1 protein expression after silencing of Nrf3 was detected (Figure 5A).
FIGURE 5.
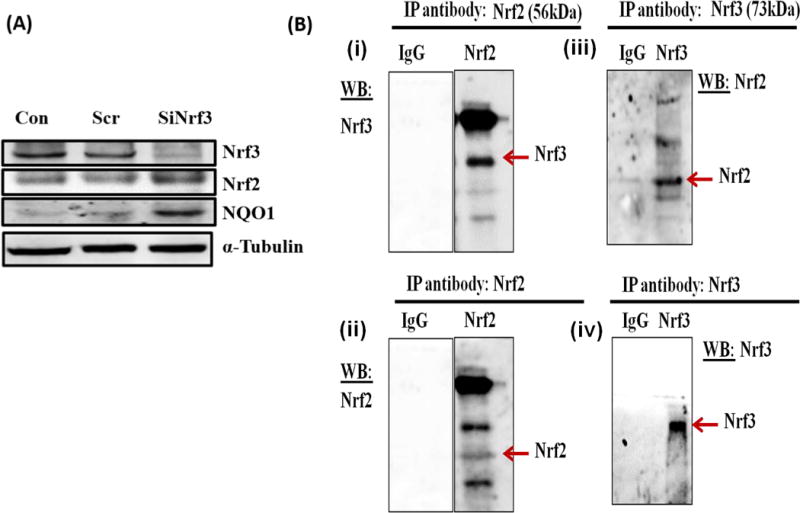
Nrf3 negatively regulates Nrf2 and Nrf2-dependent gene NQO1 and Nrf3 interacts with Nrf2. (A) MCF-10A cells were transfected with 20 nmol/l of scrambled siRNA or siNrf3 for 48 hr, and Western blot analysis was carried out using Nrf3 antibody. The same membrane was reprobed with Nrf2, NQO1 and α-Tubulin antibodies. (B) Co-Immunoprecipitation of Nrf2 and Nrf3 from MCF-10A cells. Co-Immunoprecipitation of Nrf2 and Nrf3 were performed in MCF-10A cell lysates using Pierce Co-Ip kit, according to manufactures protocol with some modifications. The immunoblot in the upper panel in figure 5B (i) was probed for Nrf3 after Nrf2 was pulled down. The same blot was stripped and probed for Nrf2 shown in the lower panel of figure 5B (ii). The immunoblot in the upper panel in figure 5B (iii) was probed for Nrf2 after Nrf3 was pulled down. The same blot was stripped and probed for Nrf3 shown in the lower panel of figure 5B (iv).
Nrf3 interacts with Nrf2
Co-immunoprecipitation is an in vitro method for detecting protein-protein interactions. We used Pierce Co-immunoprecipitation Kit to investigate whether Nrf2 interacts with Nrf3 and vice versa as described in the methods section. After immobilization and immunoprecipitation of the primary antibody (Nrf2), Co-Ip of interacting protein Nrf3 was detected by Western blotting (Figure 5Bi). The same blot was stripped and probed for Nrf2 and a mild band was detected (Figure 5Bii). After immobilization and immunoprecipitation of the primary antibody (Nrf3), Co-Ip of interacting protein Nrf2 was detected by Western blotting (Figure 5Biii). The same blot was stripped and probed for Nrf3 and Nrf3 was detected (Figure 5Biv). Any unspecific interactions were identified by using the provided control gel and substituting IgG for the specific antibody. No nonspecific interaction was observed (Figure 5B).
DISCUSSION
Long-term exposure to the female sex hormone E2 has been associated with the initiation and progression of breast cancer [4–14]. However, the mechanisms of E2-induced breast carcinogenesis are not clear. It has been suggested that estrogen metabolism plays a major role in the onset of estrogen-induced breast cancer [10, 12–14]. Oxidative stress produced by redox cycling between catechol estrogens and estrogen quinones has been suggested to play a critical role in estrogen-induced breast cancer [12–18].
Induction of antioxidant phase-II detoxifying enzymes NQO1 and SOD3 has been suggested to play a role in breast cancer chemoprevention [28, 51]. In the current study, we have investigated the role of Cap’n’collar (CNC) transcription factors in antioxidant-mediated prevention of estrogen-induced breast carcinogenesis. We have demonstrated significant suppression of SOD3 mRNA and protein expression after E2 treatment and this suppression was reversed upon co-treatment with VC or Res (Figure 4). Antioxidant treatment further increased the mRNA and protein expression levels of SOD3 (Figure 4). Additionally, E2-mediated decrease in the expression of NQO1 mRNA and protein was reversed by antioxidants VC or Res (Figure 4). These studies further support the importance of antioxidant phase-II detoxifying enzymes NQO1 and SOD3 in prevention of E2-induced breast cancer [28, 51]. Role of Nrf2 in E2-induced breast cancer has only recently begun to be examined [28, 51–53]. Nrf2-mediated regulation of several antioxidant enzymes has previously been reported [11–14]. In this study we found significant suppression of Nrf2 mRNA and protein expression levels in E2-treated MCF-10A cells (Figure 1). This suppression was reversed upon co-treatment with VC or Res (Figure 2). Antioxidant treatment further increased the mRNA and protein expression levels of Nrf2 (Figure 2). This finding suggested the involvement of Nrf2-pathway in regulation of E2-induced breast cancer.
We further examined the role of Nrf1 in E2-induced breast cancer and effect of antioxidant agents in its regulation. We demonstrated significant increase in Nrf1 mRNA and protein expression levels following E2 treatment in MCF-10A cells (Figure 3C and 3D). Treatment with VC or Res increased Nrf1 mRNA and protein expression levels (Figure 3C and 3D). From these results it could be suggested that Nrf1 has compensatory role in E2-induced stress and antioxidants (Res and VC) play very important chemopreventive role in E2-induced breast cancer by up-regulation of cytoprotective gene Nrf1. However, further studies are needed to demonstrate specific role of Nrf1 in E2-induced breast cancer.
Nrf3 is the third member of the Nrf gene family [35, 37]. In this study we also examined the role of Nrf3 in E2-induced breast cancer and the effect of antioxidant agents, in regulation of Nrf3. We demonstrated that E2 treatment increased Nrf3 mRNA expression levels in a time dependant manner in MCF-10A cells (Figure 3A). Estrogen-mediated increase in the expression of Nrf3 protein was reversed by antioxidants VC or Res (Figure 3B) which further support the importance of Nrf3 in E2-induced breast cancer. The increased expression of Nrf2 and NQO1 following silencing of Nrf3 (Figure 5A) and Co-Ip of Nrf3 with Nrf2 and vice versa (Figure 5B) suggests that possibly Nrf2 is regulated by Nrf3; although further studies are needed to establish this relationship between Nrf2 and Nrf3. In conclusion, we demonstrated that CNC b-zip transcription factors are regulated differently and play important roles in E2-induced breast cancer.
Acknowledgments
This work was supported by the National Institutes of Health Grant (CA 109551), the University of Missouri Research Board Grant, and financial support from the School of Pharmacy, University of Missouri-Kansas City (HKB).
ABBREVIATIONS/FOOTNOTES
- E2
17β-estradiol
- VC
Vitamin C
- Res
Resveratrol
- CNC
Cap’n’collar
- Nrf1
Nuclear factor erythroid 2-related factor 1
- Nrf2
Nuclear factor erythroid 2-related factor 2
- Nrf3
Nuclear factor erythroid 2-related factor 3
- SOD3
Superoxide dismutase 3
- NQO1
NAD(P)H:quinone oxidoreductase 1
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Shinohara MM, Tozbikian G, Wolfe JT, Shin SJ, Mies C, Elenitsas R. Cutaneous metastatic breast carcinoma with clear cell features. Journal of Cutaneous Pathology. 2013;40(8):753–7. doi: 10.1111/cup.12162. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 4.Nelson R. Steroidal oestrogens added to list of known human carcinogens. Lancet. 2002;360:2053. doi: 10.1016/S0140-6736(02)12045-9. [DOI] [PubMed] [Google Scholar]
- 5.Bhat HK, Han X, Gladek A, Liehr JG. Regulation of the formation of the major diethylstilbestrol-DNA adduct and some evidence of its structure. Carcinogenesis. 1994;15(10):2137–42. doi: 10.1093/carcin/15.10.2137. [DOI] [PubMed] [Google Scholar]
- 6.Bhat HK, Calaf G, Hei TK, Loya T, Vadgama JV. Critical role of oxidative stress in estrogen-induced carcinogenesis. Proc Natl Acad Sci U S A. 2003;100:3913–3918. doi: 10.1073/pnas.0437929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavalieri E, Rogan E. Catechol quinones of estrogens in the initiation of breast, prostate, and other human cancers: keynote lecture. Ann N Y Acad Sci. 2006;1089:286–301. doi: 10.1196/annals.1386.042. [DOI] [PubMed] [Google Scholar]
- 8.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344:276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 9.Howell A. The endocrine prevention of breast cancer. Best Pract Res Clin Endocrinol Metab. 2008;22:615–623. doi: 10.1016/j.beem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Mense SM, Remotti F, Bhan A, Singh B, El-Tamer M, Hei TK, Bhat HK. Estrogen-induced breast cancer: alterations in breast morphology and oxidative stress as a function of estrogen exposure. Toxicol Appl Pharmacol. 2008;232(1):78–85. doi: 10.1016/j.taap.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mense SM, Singh B, Remotti F, Liu X, Bhat HK. Vitamin C and alpha-naphthoflavone prevent estrogen-induced mammary tumors and decrease oxidative stress in female ACI rats. Carcinogenesis. 2009;301202(7):1208. doi: 10.1093/carcin/bgp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel MM, Bhat HK. Differential oxidant potential of carcinogenic and weakly carcinogenic estrogens: Involvement of metabolic activation and cytochrome P450. J Biochem Mol Toxicol. 2004;18:37–42. doi: 10.1002/jbt.20005. [DOI] [PubMed] [Google Scholar]
- 13.Roy D, Liehr JG. Temporary decrease in renal quinone reductase activity induced by chronic administration of estradiol to male Syrian hamsters. Increased superoxide formation by redox cycling of estrogen. J Biol Chem. 1988;263:3646–3651. [PubMed] [Google Scholar]
- 14.Russo J, Lareef MH, Tahin Q, Hu YF, Slater C, Ao X, Russo IH. 17Beta-estradiol is carcinogenic in human breast epithelial cells. J Steroid Biochem Mol Biol. 2002;80:149–162. doi: 10.1016/s0960-0760(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 15.Singh B, Mense SM, Remotti F, Liu X, Bhat HK. Antioxidant butylated hydroxyanisole inhibits estrogen-induced breast carcinogenesis in female ACI rats. J Biochem Mol Toxicol. 2009;23:202–211. doi: 10.1002/jbt.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 17.Roy D, Liehr JG. Estrogen, DNA damage and mutations. Mutat Res. 1999;424:107–115. doi: 10.1016/s0027-5107(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 18.Yager JD. Endogenous estrogens as carcinogens through metabolic activation. J Natl Cancer Inst Monogr. 2000:67–73. doi: 10.1093/oxfordjournals.jncimonographs.a024245. [DOI] [PubMed] [Google Scholar]
- 19.Adlercreutz H. Phyto-oestrogens and cancer. Lancet Oncol. 2002;3(6):364–73. doi: 10.1016/s1470-2045(02)00777-5. [DOI] [PubMed] [Google Scholar]
- 20.Probst-Hensch NM, Pike MC, McKean-Cowdin R, Stanczyk FZ, Kolonel LN, Henderson BE. Ethnic differences in post-menopausal plasma oestrogen levels: high oestrone levels in Japanese-American women despite low weight. Br J Cancer. 2000;82(11):1867–70. doi: 10.1054/bjoc.1999.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gikas PD, Mokbel K. Phytoestrogens and the risk of breast cancer: a review of the literature. Int J Fertil Womens Med. 2005;50(6):250–8. [PubMed] [Google Scholar]
- 22.Singh B, Mense SM, Bhat NK, Putty S, Guthiel WA, Remotti F, Bhat HK. Dietary quercetin exacerbates the development of estrogen-induced breast tumors in female ACI rats. Toxicol Appl Pharmacol. 2010;247(2):83–90. doi: 10.1016/j.taap.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinrichs B, Zahid M, Saeed M, Ali MF, Cavalieri EL, Rogan EG. Formation of diethylstilbestrol-DNA adducts in human breast epithelial cells and inhibition by resveratrol. J Steroid Biochem Mol Biol. 2011;127(3–5):276–81. doi: 10.1016/j.jsbmb.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garvin S, Ollinger K, Dabrosin C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006;231:113–22. doi: 10.1016/j.canlet.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 25.Kao CL, Chen LK, Chang YL, et al. Resveratrol protects human endothelium from H(2)O(2)-induced oxidative stress and senescence via SirT1 activation. J Atheroscler Thromb. 2010;17:970–9. doi: 10.5551/jat.4333. [DOI] [PubMed] [Google Scholar]
- 26.Mohammadshahi M, Haidari F, Ghadiri Soufi F. Chronic resveratrol administration improves diabetic cardiomyopathy in part by reducing oxidative stress. Cardiol J. 2014;21(1):39–46. doi: 10.5603/CJ.a2013.0051. [DOI] [PubMed] [Google Scholar]
- 27.Pruthi S, Yang L, Sandhu NP, Ingle JN, Beseler CL, Suman VJ, Cavalieri EL, Rogan EG. Evaluation of serum estrogen-DNA adducts as potential biomarkers for breast cancer risk. J Steroid Biochem Mol Biol. 2012;132:1–2. 73–9. doi: 10.1016/j.jsbmb.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh B, Bhat NK, Bhat HK. Induction of NAD(P)H-quinone oxidoreductase by antioxidants in female ACI rats is associated with decrease in oxidative DNA damage and inhibition of estrogen-induced breast cancer. Carcinogenesis. 2012;33:156–163. doi: 10.1093/carcin/bgr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Q, Li G, Wei X, Zhang J, Chiu JF, Hasenmayer D, Zhang D, Zhang H. Resveratrol-induced apoptosis is enhanced by inhibition of autophagy in esophageal squamous cell carcinoma. Cancer Lett. 2013;(13):S0304–3835. 00251–6. doi: 10.1016/j.canlet.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Garvin S, Ollinger K, Dabrosin C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006;231:113–22. doi: 10.1016/j.canlet.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 31.Kuo SM, Burl LR, Hu Z. Cellular phenotype-dependent and – independent effects of vitamin C on the renewal and gene expression of mouse embryonic fibroblasts. PLoS One. 2012;7(3):e32957. doi: 10.1371/journal.pone.0032957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mamede AC, Tavares SD, Abrantes AM, Trindade J, Maia JM, Botelho MF. The role of vitamins in cancer: a review. Nutr Cancer. 2011;63(4):479–94. doi: 10.1080/01635581.2011.539315. [DOI] [PubMed] [Google Scholar]
- 33.Ullah MF, Khan HY, Zubair H, Shamim U, Hadi SM. The antioxidant ascorbic acid mobilizes nuclear copper leading to a prooxidant breakage of cellular DNA: implications for chemotherapeutic action against cancer. Cancer Chemother Pharmacol. 2011;67(1):103–10. doi: 10.1007/s00280-010-1290-4. [DOI] [PubMed] [Google Scholar]
- 34.Elbekai RH, Duke J, El-Kadi AO. Ascorbic acid differentially modulates the induction of heme oxygenase-1, NAD(P)H:quinone oxidoreductase 1 and glutathione S-transferase Ya by As(3+), Cd(2+) and Cr(6+) Cancer Lett. 2007;246(1–2):54–62. doi: 10.1016/j.canlet.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi A, Ito E, Toki T, Kogame K, Takahashi S, Igarashi K, Hayashi N, Yamamoto M. Molecular cloning and functional characterization of a new Cap‘n’ collar family transcription factor Nrf3. J Biol Chem. 1999;274(10):6443–52. doi: 10.1074/jbc.274.10.6443. [DOI] [PubMed] [Google Scholar]
- 36.Mathers J, Fraser JA, McMahon M, Saunders RD, Hayes JD, McLellan LI. Antioxidant and cytoprotective responses to redox stress. Biochem Soc Symp. 2004;71:157–76. doi: 10.1042/bss0710157. [DOI] [PubMed] [Google Scholar]
- 37.Chevillard G, Blank V. NFE2L3 (NRF3): the Cinderella of the Cap‘n’Collar transcription factors. Cell Mol Life Sci. 2011;68:3337–48. doi: 10.1007/s00018-011-0747-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sykiotis GP, Bohmann D. Stress-activated cap‘n’collar transcription factors in aging and human disease. Sci Signal. 2010;3(112):re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 40.Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free radical biology & medicine. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwak MK, Itoh K, Yamamoto M, Kensler TW. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Molecular and cellular biology. 2002;22:2883–2892. doi: 10.1128/MCB.22.9.2883-2892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug metabolism reviews. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 44.Biswas M, Chan JY. Role of Nrf1 in antioxidant response element-mediated gene expression and beyond. Toxicol Appl Pharmacol. 2010;244:16–20. doi: 10.1016/j.taap.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han W, Ming M, Zhao R, Pi J, Wu C, He YY. Nrf1 CNC-bZIP protein promotes cell survival and nucleotide excision repair through maintaining glutathione homeostasis. J Biol Chem. 2012;287:18788–95. doi: 10.1074/jbc.M112.363614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hernandez-Montes E, Pollard SE, Vauzour D, et al. Activation of glutathione peroxidase via Nrf1 mediates genistein’s protection against oxidative endothelial cell injury. Biochem Biophys Res Commun. 2006;346:851–9. doi: 10.1016/j.bbrc.2006.05.197. [DOI] [PubMed] [Google Scholar]
- 47.Venugopal R, Jaiswal AK. Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene. 1998;17:3145–56. doi: 10.1038/sj.onc.1202237. [DOI] [PubMed] [Google Scholar]
- 48.Wang W, Chan JY. Nrf1 is targeted to the endoplasmic reticulum membrane by an N-terminal transmembrane domain. J Biol Chem. 2006;281:19676–19687. doi: 10.1074/jbc.M602802200. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Crouch DH, Yamamoto M, Hayes JD. Negative regulation of the Nrf1 transcription factor by its N-terminal domain is independent of Keap1: Nrf1, but not Nrf2, is targeted to the endoplasmic reticulum. Biochem J. 2006;399:373–385. doi: 10.1042/BJ20060725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sankaranarayanan K, Jaiswal AK. Nrf3 negatively regulates antioxidant- response element-mediated expression and antioxidant induction of NAD(P)H:quinone oxidoreductase1 gene. J Biol Chem. 2004;279(49):50810–7. doi: 10.1074/jbc.M404984200. [DOI] [PubMed] [Google Scholar]
- 51.Singh B, Bhat HK. Superoxide dismutase 3 is induced by antioxidants, inhibits oxidative DNA damage and is associated with inhibition of estrogen-induced breast cancer. Carcinogenesis. 2012;33(12):2601–10. doi: 10.1093/carcin/bgs300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh B, Chatterjee A, Ronghe AM, Bhat NK, Bhat HK. Antioxidants- mediated upregulation of OGG1 via NRF2 induction is associated with prevention of oxidative DNA damage in estrogen-induced breast cancer. BMC Cancer. 2013;13:253–261. doi: 10.1186/1471-2407-13-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh B, Ronghe AM, Chatterjee A, Bhat NK, Bhat HK. MicroRNA-93 regulates NRF2 expression and is associated with breast carcinogenesis. Carcinogenesis. 2013;34:1165–1172. doi: 10.1093/carcin/bgt026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhat HK, Hacker HJ, Thompson EB, Liehr JG. Differential regulation by estrogen of c-fos in hamster kidney and estrogen-induced kidney tumor cells: receptor mediation vs. metabolic activation. Int J Oncol. 1995;7:527–534. doi: 10.3892/ijo.7.3.527. [DOI] [PubMed] [Google Scholar]
- 55.Bhat HK, Epelboym I. Quantitative analysis of total mitochondrial DNA: Competitive polymerase chain reaction vs. real-time polymerase chain reaction. J Biochem Mol Toxicol. 2004;1118:180–186. doi: 10.1002/jbt.20024. [DOI] [PubMed] [Google Scholar]


