Abstract
Astrocytes are an attractive cell target for gene therapy but the validation of new therapeutic candidates is needed. We determined whether adeno-associated viral (AAV) vector-mediated overexpression of glutamine synthetase (GS) or excitatory amino acid transporter 2 (EAAT2), or expression of microRNA targeting adenosine kinase (miR-ADK) in hippocampal astrocytes in the rat brain could modulate susceptibility to kainate-induced seizures and neuronal cell loss. Transgene expression was found predominantly in astrocytes following direct injection of glial-targeting AAV9 vectors by 3 weeks post-injection. ADK expression in miR-ADK vector-injected rats was reduced by 94–96% and was associated with a ~50% reduction in the duration of kainate-induced seizures and greater protection of dentate hilar neurons but not CA3 neurons compared to miR-control vector-injected rats. In contrast, infusion of AAV-GS and EAAT2 vectors did not afford any protection against seizures or neuronal damage as the level of transcriptional activity of the glial-fibrillary acidic promoter was too low to drive any significant increase in transgenic GS or EAAT2 relative to the high endogenous levels of these proteins. Our findings support ADK as a prime therapeutic target for gene therapy of temporal lobe epilepsy and suggest alternative approaches including the use of stronger glial promoters are needed to increase transgenic GS and EAAT2 expression to levels that may be required to affect seizure induction and propagation.
Keywords: Adenosine kinase, animal model, temporal lobe epilepsy, EAAT2, glutamine synthetase, kainate
Introduction
Gene therapy is an attractive prospect for treatment of neurological diseases because it has the potential to produce long-term therapeutic benefits for diseases that are typically chronic in nature.1, 2 Outcomes from gene therapy clinical trials including Parkinson’s disease3–5 and Canavan disease6 have been promising. These studies suggest that gene therapy can be conducted safely, is well tolerated, and that symptomatic relief or modification of disease progression is achievable. However, further optimization of these and other existing strategies, and identification of new approaches are still needed. To date, neurons have been the main cellular target for gene therapy in the brain, with strategies primarily centered on modification of neuronal function to enable a therapeutic outcome. Glial cell types such as astrocytes and microglia have received less attention, to a large extent due to the lack of vectors capable of efficient and selective targeting of transgenes to these cell types. This landscape has changed in the last few years with advances in viral vector technology; lentiviral7 and recombinant adeno-associated viral (AAV) vectors based on AAV serotypes 5, 8 or 9 (AAV5, AAV8, AAV9)8–10 which are capable of astrocytic transduction. Combined with the clear recognition that astrocytes play a number of diverse and important roles in the healthy brain and can contribute to disease progression in neurodegenerative diseases,11 attention has now turned to exploring astrocytes as an alternative cell target for CNS gene therapy. Their abundance in the face of neuronal depletion in neurodegenerative diseases makes them an attractive choice for gene therapy.
The identification of molecular targets and the development of strategies aimed at altering astrocyte function to achieve therapeutic benefit have not been fully explored. One disease amenable to an astrocyte-targeting gene therapy approach is mesial temporal lobe epilepsy (TLE), one of the most common forms of drug-resistant epilepsy that is characterized by the unpredictable and recurrent occurrence of seizures. Spontaneous seizures often originate from a spatially restricted focal region of neuronal hyperexcitability in the antero-medial temporal lobe that includes the epileptic hippocampus. Surgical resection of this brain region is highly effective in controlling seizures in about 85% of these patients, thereby validating this as a specific target for therapeutic intervention.12 The main neuropathological hallmark of TLE is Ammon’s horn sclerosis, defined by a selective pattern of neuronal cell loss in the hippocampus, aberrant axonal sprouting and reorganization of neuronal circuitry, and reactive gliosis.13 Whilst the past two decades have largely focused on unravelling the contribution of neurons as the underlying substrate for generation of chronic recurrent seizures, in recent years, research in this field has shifted to gaining an understanding of the contribution of astrocytes to epilepsy.
Reactive gliosis, whereby astrocytes undergo structural and biochemical changes, is a prominent feature of TLE in humans and many animal seizure models, and there are several mechanisms by which reactive astrocytes could contribute to the evolution of abnormal network excitability in TLE.14, 15 Two possible contributing processes could involve the altered ability of astrocytes to regulate synaptic glutamate and adenosine levels.
Increased extracellular glutamate is strongly linked to the initiation of spontaneous seizures in humans,16 with proconvulsant effects replicated in animal models by administration of glutamate agonists.17 Astrocytes are largely responsible for preventing glutamate receptor overactivation by mediating glutamate clearance from the synapse via the EAAT1 and astrocyte-specific EAAT2 glutamate transporters.18–20 Astrocytic glutamate is then converted to glutamine by glutamine synthetase (GS) before being shunted back to neurons to act as a precursor for glutamate or GABA synthesis. Alterations in EAAT2 and GS expression in sclerotic hippocampal tissue removed during epilepsy surgery,21–25 and the demonstration that elevated extracellular glutamate levels appear to trigger spontaneous seizures in human TLE16 has provided some impetus into establishing whether these proteins would be suitable gene therapy targets. However, whether glutamate transporters are altered in human epilepsy is controversial; no change in transporter expression in resected tissue from TLE patients was found in two studies21, 24 while regionally specific changes in EAAT1-3 expression have also been reported.21–23, 25 Differences in tissue processing or disease severity might explain these discrepancies. GS expression in astrocytes is also downregulated in resected epileptic tissue.21, 25 Although the changes in glutamate transporter expression in human TLE warrants further investigation, studies in animal models provide compelling evidence that impaired glutamate uptake and processing by astrocytes has the potential to contribute to the development of seizures. Genetic deletion of either EAAT2 or GS expression alone, or pharmacological inhibition of glutamine synthesis by methionine sulfoximine is sufficient to cause the development of spontaneous seizures in rats and mice.26–29
An alternative target is adenosine kinase (ADK), an enzyme expressed exclusively in astrocytes and involved in regulating extracellular concentrations of adenosine. Adenosine is a potent natural anticonvulsant and protective molecule that plays a pivotal role in seizure termination and contributes to post-ictal refractoriness to further seizures.30–32 Work led by Boison and colleagues has shown ADK expression is a key molecular link between astrogliosis and neuronal dysfunction in epilepsy.30, 33 Transgenic mice engineered with increased ADK activity are prone to development of spontaneous seizures and conversely, boosting local adenosine concentration by transplantation of ADK-deficient cells are sufficient to prevent epileptogenesis.34, 35 Pharmacological activation of adenosine A1 receptors by adenosine analogs provides effective seizure control in various experimental seizure models including mice resistant to treatment with conventional antiepileptic drugs.36–38 Moreover, AAV8-mediated astrocytic expression of an ADK cDNA in antisense orientation prevents the occurrence of spontaneous recurrent seizures in Adk transgenic mice.39
These results highlight EAAT2, GS and ADK as potential therapeutic targets for TLE, but whether they are suitable in a gene therapy context has not been fully investigated. In this study, we determine whether AAV9-mediated overexpression of EAAT2 and GS or silencing of ADK expression using an alternative RNA interference-mediated mechanism in astrocytes can mitigate against kainate-induced seizures and neuronal cell death in rats.
Results
AAV8 and AAV9 vectors mediate widespread transduction of astrocytes in the rat hippocampus
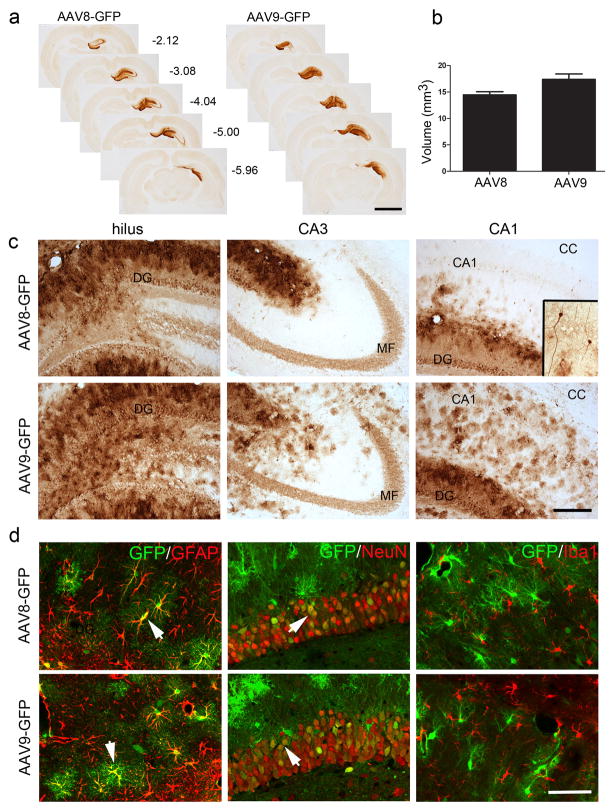
Previous studies have demonstrated that systemic administration of AAV9 vectors expressing green fluorescent protein (GFP) under control of the strong constitutive chicken beta-actin/CMV hybrid (CAG) promoter directs GFP expression to astrocytes40, 41 and neurons.41 In contrast, direct injection of this vector into the brain leads predominantly to neuronal transduction.40 We and others showed previously that use of cell-specific promoters in AAV8 vectors can direct transgene expression in glial cell types including astrocytes and oligodendrocytes following direct injection of vector into the rat brain.9, 39 This prompted us to test the capacity of the astrocyte-specific glial fibrillary acidic protein (GFAP) promoter to direct and restrict transgene expression to astrocytes following direct infusion into the rat brain. We infused titer-matched AAV9 or AAV8 vectors expressing GFP into the dorsal hippocampus of rats and analyzed the spatial distribution and cellular transduction patterns of transgene expression at 3 weeks. A highly sensitive anti-GFP antibody was used to detect GFP expression by immunohistochemistry. GFP expression was widely distributed throughout the dorsal hippocampus, spreading approximately 4 mm over the rostral-caudal axis with both vector serotypes, and was restricted to the injected hemisphere (Fig 1a). The total volume of the hippocampus transduced by these vectors was similar (Fig 1b); but notably, GFP-immunoreactive cells were more homogenously distributed throughout the dorsal hippocampus, spreading up to the border with the corpus callosum and beyond the lateral border of the mossy fibre pathway in AAV9 vector-injected rats (Fig 1c). The large majority of GFP-immunoreactive cells were astrocytes based on their location away from the hippocampal dentate granule and pyramidal neuron cell body layers and cellular morphology.42 Immunostained cells with an intricate bushy-like morphology, characteristic of hippocampal astrocytes, were clearly visualized towards the outer edges of the transduced zone, such as the dendritic fields of the CA1 region. GFP-positive cells were found in abundance in the granule cell molecular layer and hilus adjacent to the infusion site (Fig 1c). GFP-immunoreactivity was also found in a subset of dentate granule neurons that was supported by staining of the mossy fibre pathway, the axonal projections from these cells (Fig 1c). A few GFP-positive CA1 neurons were also found (Fig 1c inset) but neuronal transduction was not found in the CA3 region with either vector serotype (Fig 1c). Immunofluorescent labeling with antibodies to cell-specific markers confirmed the identity of transduced cells. GFP-positive cells co-localised with the astrocyte marker GFAP, and with NeuN-immunoreactive neurons scattered throughout the granule cell layer but not with the microglial marker Iba1 (Fig 1d). Overall, the transduction patterns were very comparable between the two AAV serotypes, and shows that use of an astrocyte-specific promoter in AAV9 vectors can cause a significant shift in transgene expression to astrocytes.
Fig. 1. Widespread transduction of the dorsal hippocampus following AAV8 and AAV-mediated gene transfer.
(a) Representative images showing the rostral-caudal distribution of GFP immunoreactivity in the hippocampus following AAV8 and AAV9 vector infusion. The rostral-caudal coordinate (in mm) for each section relative to bregma is shown. (b) Transduction volumes (mm3) in the hippocampus. Bars represent the mean + SEM, n = 3–4. Unpaired t-test, P = 0.08 (c) GFP immunoreactivity in the hilar region, CA3 and CA1 regions in representative AAV8 and AAV9-injected brains (upper dentate granule cell layer (DF), mossy fibre pathway (MF), corpus callosum (CC) are shown as reference points, with the inset showing a higher magnification view of GFP-positive CA1 neurons. (d) Double-immunofluorescent GFP labeling with GFAP, NeuN and Iba1 showing co-localisation of GFP signal in both astrocytes and some neurons (arrowheads) with both vector serotypes but not microglia. Scale bar, (a) 1 mm, (c) 250 um, (d) 80 um
Transgene expression in the rat hippocampus following AAV9-mediated gene transfer
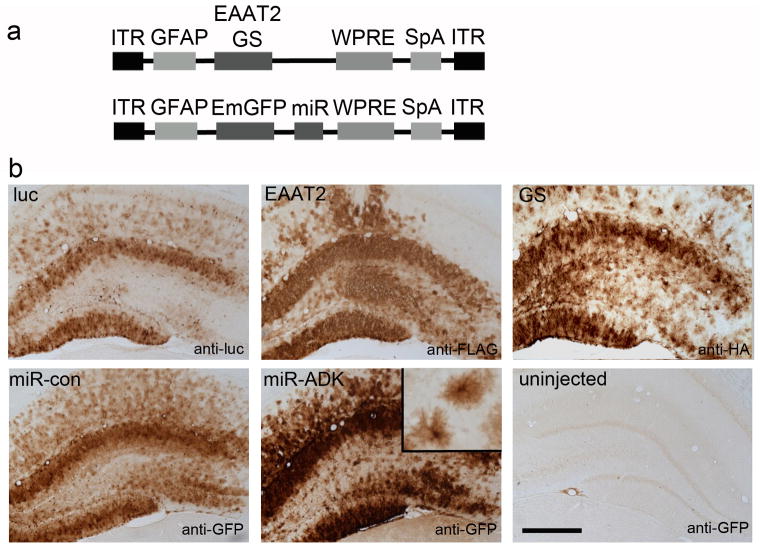
Next we developed AAV plasmids expressing the putative therapeutic transgenes, a hemagglutinin (HA)-tagged-GS and a FLAG-tagged EAAT2 (Fig 2a). The functionality of both these epitope-tagged transgenes was confirmed using in vitro models (Supplementary Data). A luciferase construct was generated as a control. Two AAV plasmids expressing miRNA sequences targeting rat ADK mRNA or a control miRNA linked to an Emerald GFP reporter to facilitate cell tracking of miRNA sequences were also developed (Fig 2a). Because we observed marginally more widespread astrocyte transduction with the AAV9-GFP vector, we packaged these constructs into AAV9 vectors. Subgroups of rats were injected with these vectors and we confirmed expression of these transgenes 3 weeks later. The cellular expression patterns of anti-HA, anti-FLAG, anti-luciferase and anti-GFP immunoreactivity which was used to visualize cells transduced with the AAV-miRNA vectors was consistent with that found with the AAV9-GFP vector, with robust transgene staining in astrocytes with all vectors (Fig 2b). Highly dense transgene expression was found in the dentate granule cell molecular layer, with fewer transduced astrocytes across the CA2 and lateral CA3 regions on the injected side and no transgene expression observed in the contralateral non-injected hippocampus.
Fig. 2. Transgene expression following AAV9-mediated gene transfer.
(a) Schematic diagrams showing the AAV expression cassettes expressing EAAT2 or GS (luciferase not shown), and or miR sequences (miR-con, miR-ADK). (b) Transgene expression in the hippocampus following infusion of AAV9 vectors expressing luciferase (luc), EAAT2, GS, miR-con and miR-ADK. Inset shows high power image of the astrocyte morphology of transduced cells. Transgene expression was not detected in the contralateral uninjected hemisphere of any vector treatment group (anti-GFP staining of contralateral miR-ADK vector-injected brain shown as example). Scale bar, 300 um
Anticonvulsant efficacy of AAV9-mediated overexpression of EAAT2 and GS and ADK knockdown in astrocytes
We then asked whether overexpression of EAAT2, GS, or suppression of ADK expression in astrocytes could modulate seizure development, duration or severity in an experimental rat model of TLE. We used the intrahippocampal kainate infusion model that has been shown to elicit a characteristic pattern of recurrent and/or continuous status epilepticus that persists for approximately 2 h, but is associated with a low mortality rate.43
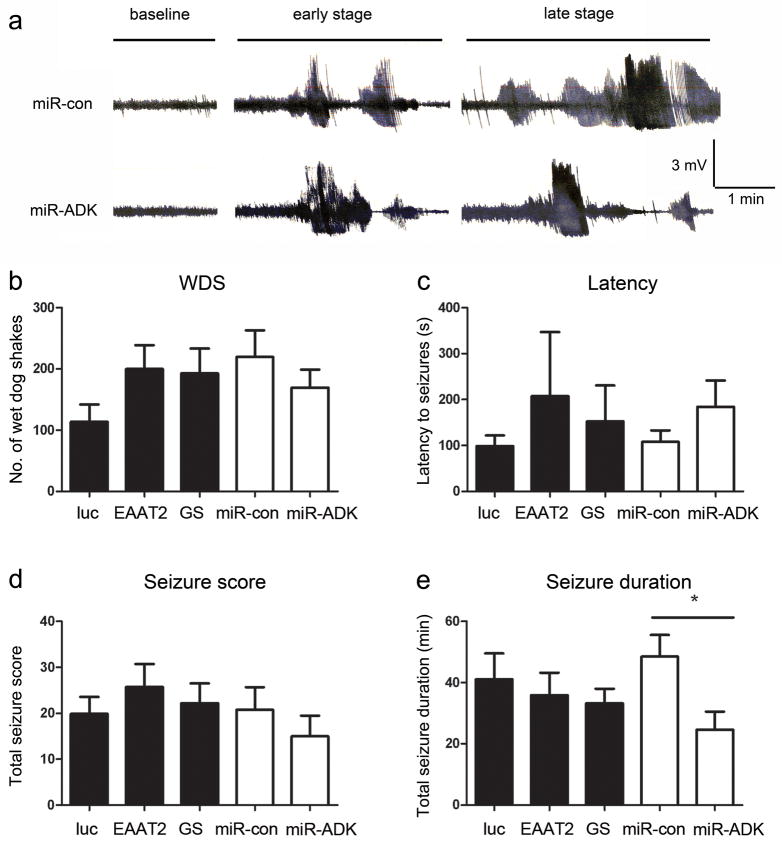
Subgroups of rats were generated in two cohorts: rats in the first group received a bilateral hippocampal infusion of EAAT2, GS or luciferase vectors, while the second group of rats received mir-ADK and miR-con vectors. Four weeks later, rats received an infusion of kainate, electroencephalographic (EEG) recordings were made and rats scored for seizure-associated motor behaviours.44 Electrographic seizures characterized by a recycling pattern of high frequency, synchronised ictal spiking of 1–2 min duration were elicited in all animals (Fig 3a). There was no difference in the latencies to development of seizures (One-way ANOVA, F2,24 = 0.36, P = 0.70 for luc, EAAT2, GS animals; unpaired t-test, t15 = 1.17, P = 0.26 for miR-con versus miR-ADK), the frequency of wet dog shakes (F2,24 = 1.67, P = 0.21 for luc, EAAT2, GS rats; unpaired t-test, t15 = 0.96, P = 0.35 for miR-con versus miR-ADK) during the 90 min monitoring period or the cumulative seizure score (F2,24 = 0.35, P = 0.71 for luc, EAAT2, GS rats; unpaired t-test, t15 = 0.86, P = 0.40 for miR-con versus miR-ADK) indicative of the severity of motor seizures between the treatment groups and their respective controls in the two studies (Fig 3b–d). The frequency of seizures and overall total duration of seizures experienced by control rats (luciferase and miR-con) in the two studies were similar, with rats spending 40–50 min of the monitoring period in seizures. There was no difference in the total seizure duration between the EAAT2, GS and luciferase vector-injected animals (One-way ANOVA, F2,24 = 0.35, P = 0.71). In contrast, the total duration of seizures experienced by miR-ADK vector-injected rats was reduced by ~50% compared to the miR-con vector-injected rats (unpaired t-test, t15 = 2.6, P = 0.02) suggesting potent anti-seizure effects can be achieved following modulation of ADK function (Fig 3e).
Fig. 3. Reduced seizure durations in miR-ADK-treated animals.
(a) Representative EEG traces at baseline and during electrographic seizures at early (~20 min) and late stages (~45 min) following kainate injection. Traces from a miR-con and miR-ADK vector injected rat are shown as examples. No differences in (b) total numbers of wet-dog shakes (WDS), (c) the latency to seizures, (d) cumulative seizure score using the Racine seizure rating scale was found between treatment groups over 90 min. (d) Total durations of electrographic seizures in the miR-ADK treatment group were reduced compared to the miR-con. Bars represent mean + SEM, n = 8–10, unpaired t-test, *P = 0.02.
miR-ADK protects against kainate-induced neurotoxicity
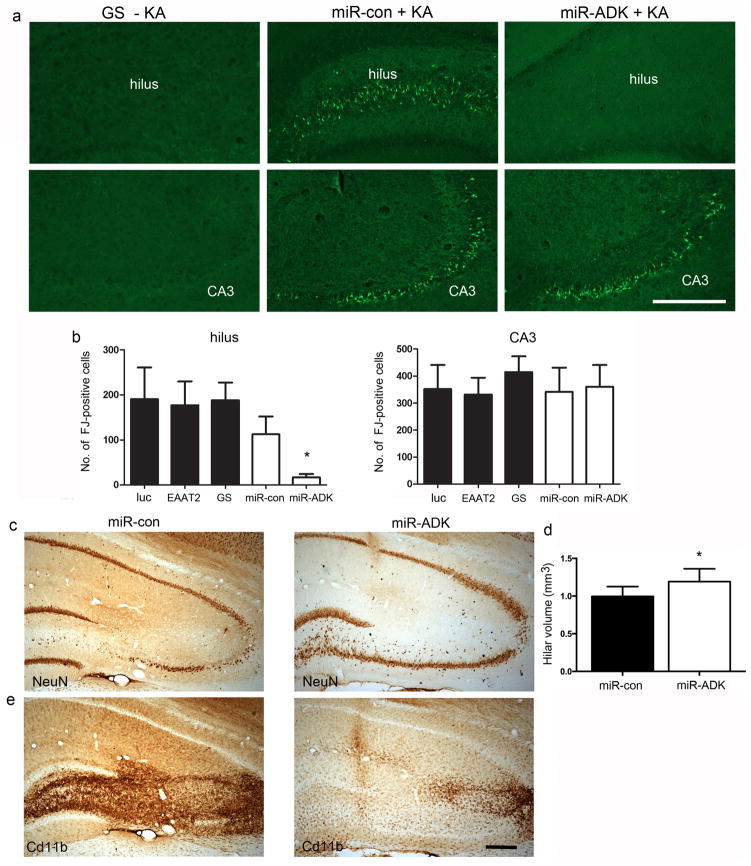
Intrahippocampal injection of kainate elicits epileptic seizures in the CA3 region and causes local neurodegeneration at the infusion site. 41, 43 Seizures that propagate to distal limbic structures can also induce neurodegeneration if they are of sufficient duration and intensity.43, 45 To determine whether the vector treatments could afford neuroprotection against kainate and seizure-induced neurotoxicity, we stained sections with Fluorojade B (FJ) to label degenerating neurons.46 No FJ-positive cells were found in the hippocampus in any vector-treatment group in the absence of kainate treatment (Fig 4a). In contrast, numerous FJ-positive cells were clearly evident in the CA3 pyramidal region in the hippocampus ipsilateral to kainate injection (Fig 4a), with one or two FJ-positive cells observed in the contralateral hippocampus in some animals (results not shown). We quantified numbers of FJ-positive cells in the dentate hilus and CA3 region (Fig 4b) ipsilateral to kainate injection and found FJ-positive cell numbers in the CA3 region were similar between all treatment groups relative to their respective controls (One-way ANOVA, F2,24 = 0.38, P = 0.68 for luc, EAAT2, GS animals; unpaired t-test, t15 = 0.15, P = 0.88 for miR-con versus miR-ADK). However, hilar cell loss in the miR-ADK treated rats was significantly attenuated relative to the miR-con-injected rats whilst the extent of hilar cell loss between EAAT2, GS and luciferase-overexpressing rats was similar (One-way ANOVA, F2,24 = 0.02, P = 0.98 for luc, EAAT2, GS animals; unpaired t-test, t15 = 2.5, P = 0.02 for miR-con versus miR-ADK). Immunohistochemistry with the NeuN antibody confirmed that miR-ADK expression was associated with a neuroprotective effect in the hilus, with a greater preservation of NeuN-immunoreactive hilar neurons in the miR-ADK vector-injected rats (Fig 4c). This was confirmed by volume measurements of the hilus in NeuN-immunolabelled sections, with significant atrophy of this region in the miR-con compared to miR-ADK-vector-injected rats (unpaired t-test, t15 = 2.6, P = 0.02) (Fig 4d). Reduced neuroinflammatory responses as visualized by attenuated Cd11b-immunoreactivity indicative of reduced microgliosis was also found in the miR-ADK rats (Fig 4e). The differential protection between the hilar and CA3 neurons in the miR-ADK injected animals can be attributed to the differences in the spatial distribution of transgene expression, as we observed a higher density of transduced astrocytes in the hilar/dentate granule cell region and fewer transduced astrocytes in the CA3 region.
Fig. 4. Attenuated neuronal cell loss in the hippocampus in miR-ADK-treated rats following intrahippocampal kainate.
(a) Representative images of Flurojade-B (FJ) staining in the dentate hilus and CA3 region in brains injected with vector alone (section from GS vector injected brain shown as example (GS –KA)) or miR-con or miR-ADK vector-injected brain 6 days following kainate infusion (miR-con + KA, miR-ADK + KA). (b) Total numbers of FJ-positive cells in the hilus and CA3 region. (c) NeuN immunostaining in the hippocampus and (d) stereological quantification of hilar volumes in NeuN-stained sections in the miR-con and miR-ADK treated rats. (d) Reduced microglial proliferation as visualized by Cd11b-immunoreactivity. For all graphs, bars represent mean + SEM, n = 8–10, Unpaired t-test, *P = 0.02. Scale bar, 300 um
Significant attenuation of ADK expression by miR-ADK but low level expression of transgenic GS and EAAT2
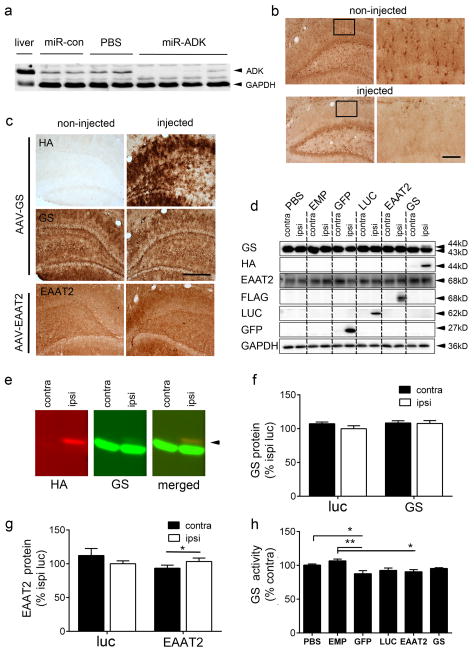
We next determined whether the anti-seizure and neuroprotective effects observed in the miR-ADK vector-injected rats, and lack of effect in the AAV-GS and EAAT2 vector-injected rats, could be explained by the overall change in protein level and/or function that was attained. The extent of knockdown of ADK expression achieved following infusion of the AAV9-miR-ADK vector was analysed by Western blot analysis of hippocampal homogenates prepared from additional rats injected with AAV9-miR-ADK and miR-con vectors or PBS vehicle. ADK protein levels were significantly reduced by 94–96% in three out of four miR-ADK animals relative to the miR-con and PBS-injected controls, and down to 56% in the remaining miR-ADK animal (Fig 5a). The smaller effect observed in the last animal was likely due to an off-target infusion. Anti-ADK immunohistochemistry showed ADK expression was reduced in astrocytes in the regions transduced by the miR-ADK vector (Fig 5b) as identified by anti-GFP immunoreactivity in an adjacent brain section (not shown). Although we did not measure adenosine concentrations, it is highly likely that the seizure and neuroprotective effects observed in our study are attributable to increased extracellular levels of adenosine in the transduced hippocampus given that previous studies have shown an 80% reduction in ADK expression is associated with increased extracellular adenosine levels in an ex vivo paradigm.47
Fig. 5. Significant knockdown of ADK expression but no increase in GS or EAAT2 expression with AAV vector infusions.
(a) Western blot analysis of hippocampal homogenates from individual PBS, miR-con and miR-ADK vector-injected rats, and positive control liver homogenate probed with anti-ADK antibody. GAPDH was used as the loading control. (b) Immunolabelling with anti-ADK antibody showed loss of ADK staining in hippocampal astrocytes in the zone transduced by AAV9-miR-ADK vector (inset, shows high magnificent image) compared to the same region in a non-injected brain. Note, in this miR-ADK vector-injected hippocampal section, the dentate hilar region was not transduced by the AAV-miR-ADK vector. (c) Representative images of hippocampal HA, GS and EAAT2-immunostaining in AAV9-GS or AAV9-EAAT2-injected rats. (d) Western blot analysis of hippocampal lysates from rats injected unilaterally with PBS or AAV vectors at 4 weeks probed with antibodies to GS, HA, EAAT2, FLAG, LUC, GFP or GAPDH. (e) Western blots showing individual fluorescent signals detected by anti-HA and GS antibodies and colocalisation of HA and GS transgenic bands. (f) Semi-quantitative analysis of band intensities from Western blots of hippocampal lysates from luciferase (luc) or GS vector-injected rats or (g) EAAT2 vector-injected rats probed with GS or EAAT2 antibodies, respectively. (h) Hippocampal GS activity (μM/mg protein/h) in PBS and AAV vector-injected rats. Bars represent the mean + SEM, n = 4–7 per treatment, * P<0.5, **P<0.01 (Tukey’s post-hoc analysis). Scale bar for (b) 250 um for low power image and 63 um for inset; (c) 250 um.
We then looked at the relationship between endogenous and transgenic GS and EAAT2 protein levels in the hippocampus of additional subgroups of rats unilaterally infused with AAV vectors. Anti-GS immunohistochemistry showed high levels of endogenous GS expression in astrocytes but no obvious difference in the intensity of GS immunoreactivity between non-injected and vector-injected sides, despite the strong detection of transgenic GS using an anti-HA antibody (Fig 5c). Similarly, high endogenous levels of EAAT2 were also found highly but a low level increase in EAAT2 immunoreactivity on the vector–injected side compared to the non-injected side was observed (Fig 5c).
To further investigate these findings, we conducted western blot analyses to determine the relative contribution of transgenic GS and EAAT2 to endogenous levels of these proteins in vector-injected hippocampal lysates. The higher molecular weight transgenic HA-tagged GS protein band was detected with both GS and HA antibodies in AAV9-GS-vector injected rats, but band intensities suggested transgenic GS was in low abundance relative to endogenous GS levels (Fig 5d,e). Transgenic EAAT2 was detected with the anti-FLAG and EAAT2 antibodies but no clear distinction between endogenous and transgenic forms of EAAT2 was observed (Fig 5d). We also sought to determine whether astrocytic expression of either of these transgenes would alter expression levels of the other protein as they are functionally linked. AAV-mediated GS expression did not appear to alter EAAT2 protein levels and conversely, GS protein band intensities in AAV-EAAT2 vector-injected samples appeared similar when comparing between the contralateral and ipsilateral vector-infused sides. We also investigated whether reporter gene expression might also affect GS expression, as previous studies have shown astrocytic GFP expression can downregulate GS expression.48 GS protein levels across all transgene-expressing vector treatment groups were similar to that of PBS injected hippocampal lysates and lysates prepared from rats injected with an AAV vector expressing no transgene (AAV empty), however GFP overexpression appeared to attenuate GS expression levels consistent with previous reports.48
We then conducted a semi-quantitative analysis of Western blot band intensities in the GS vector and EAAT2 vector-injected animals, and compared this to the AAV-luciferase group (as used in our seizure study). There were no signficant differences in GS protein levels between the vector-injected and non-injected hemispheres, nor between the luciferase vector compared to the GS vector-injected sides (luc ipsi 100.0 ± 4.3% vs GS ipsi 107.7 ± 4.3%; unpaired t-test, t9=1.18, P = 0.26) (Fig 5f). Similarly, no differences in EAAT2 levels were found in the EAAT2 versus luciferase vector -injected hippocampus (luc ipsi 100.0 ± 4.4% versus EAAT2 ipsi 103.3 ± 5.2%, t6 = 0.4886, P = 0.642). However there was a small but significant increase in EAAT2 levels on the vector-injected side relative to the non-injected side (EAAT2 ipsi 103.3 ± 5.2% versus EAAT2 contra 93.45 ± 4.5%; paired t-test t3 = 4.542, P = 0.02), consistent with the slight increase in EAAT2 immunolabelling (Fig 5c,g).
As an additional approach, we also looked to see if we could detect a functional change in GS activity across all AAV vector-injected treatment groups (Fig 5h). We found no difference in GS activity between the GS and luciferase vector group consistent with the protein expression data. Surprisingly, there was an overall attenuation in GS activity across the transgene expression groups relative to PBS-injected animals (One way ANOVA, F5,26 = 4.76, P = 0.003) and rats that received an infusion of AAV vector that does not express a transgene (AAV empty) suggesting that transgene expression per se in astrocytes causes this effect.
Discussion
Astrocytes are an attractive cell target for new genetic therapies given their prevalence in the brain and potential contribution to the pathophysiology of neurodegenerative diseases. However the identification and validation of astrocyte-specific therapeutic targets is still needed. Here, we report that miR-mediated knockdown of hippocampal astrocytic ADK expression showed anti-seizure and neuroprotective efficacy in the kainate model of epilepsy in rats, whereas expression levels of transgenic GS or EAAT2 transgene were too low to exert any therapeutic effect in this model.
Astrocyte-specific targeting with AAV vectors
Advances in viral vector technology have enabled transgene expression in astrocytes, with lentiviral and AAV vectors leading the way. An expanded repertoire of AAV vectors has been developed on the back of the discovery of more than 100 novel AAV capsid variants, with much of the work to date centered on the development and characterization of vectors derived from serotypes AAV1-9. In comparison to the AAV2 vectors used in early pioneering CNS gene therapy studies, most of these new generation vectors have superior transduction properties and vary in their tropism for specific cell populations in different brain regions, providing a more versatile toolkit that will enable the development of targeted and optimized therapeutic strategies.49–51 Moreover, these newer generation AAV vectors have opened up avenues for achieving cell-specific targeting through the engineering of novel capsids,52, 53 or the optimization of viral-delivered vector genomes. Differential expression of transgenes in neurons, astrocytes and oligodendrocytes in the brain can be achieved using cell–specific promoters to drive transgene expression in the context of AAV58, AAV8 vectors,9 and chimeric AAV1/2 vectors,54 with the promoter specificity generally over-riding the effect of capsid tropism, unlike AAV2 vectors that are highly neurotropic regardless of promoter used.55
We were intrigued by reports that intravenous delivery of AAV9 vectors expressing GFP under control of the CAG promoter transduces astrocytes throughout the CNS in the adult mice and non-human primates,40, 41 with neuronal transduction also observed in one study.41 Paradoxically, our lab and others have found that direct injection of the same vector into the brain leads predominantly to neuronal transgene expression.40 This raises the possibility that coupling a transgene to the GFAP promoter might lead to a vector optimized for astrocytic expression of transgenes in much the same way as we had previously observed with AAV8.9 Indeed, we found that this combination of promoter and AAV9 capsid led to transgene expression to astrocytes, with an almost identical transduction pattern following vector infusion into the hippocampus.9, 39 Our vector stocks were highly pure as visualized by detection of only AAV viral capsid proteins on protein gels, eliminating the possibility that cellular protein contaminants in our vector stocks influenced the cell transduction patterns observed.56
A few dentate granule neurons were also transduced, with GFP noticeably filling granule cell axonal projections to the CA3 pyramidal neurons. This is in line with reports that the 2.2 kb human GFAP promoter can also direct low level transgene expression in neurons,57 a feature that is more evident using sensitive anti-GFP immunohistochemical detection methods. It is unclear whether this residual neuronal expression would influence the anti-seizure results we obtained. Nevertheless, one approach that could be used to increase the stringency of astrocyte-specific targeting, if required, is to take advantage of the differential expression of endogenous miRNA in specific cell types. By engineering miRNA binding sites for mir124, a neuron-specific miRNA into lentiviral vector genomes, Colin et al.,58 demonstrated an effective reduction in the number of transduced neurons in the striatum from 18% to 6%.
Anti-seizure effects found with astrocytic knockdown of ADK expression but not GS or EAAT2 overexpression
Gene delivery to the human brain still presents a significant hurdle, particularly for diseases that are associated with widespread neuropathology. Systemic delivery of a gene delivery agent is seen as a promising minimally-invasive approach that could be used to achieve global gene transfer, provided that vector systems capable of passage across the blood brain barrier are available. AAV9 vectors might fulfill some of these criteria as a systemic gene delivery agent but there are still some barriers to overcome. Moreover, although intravenous delivery is less invasive than direct intracerebral injection, the systemic approach is likely to require much greater doses of vector, immunological responses, and risk of transduction of non-target organs. While the concept of miR-mediated de-targeting can be extended to silencing of transgene expression in peripheral organs such as the liver,59 the presence of neutralizing antibodies to AAV capsid proteins might pose a significant limitation in the clinical translation of this approach.41 Gray et al., have also described a vector capable of crossing the seizure-compromised blood brain barrier.60 While systemic delivery approaches have therapeutic applicability for generalized forms of epilepsy that involve large areas of the brain, targeting of therapies to defined site of seizure origin may be more desirable to minimize the possibility of perturbation of normal brain function.
Gene therapy strategies for epilepsy to date have focused on modulating neuronal excitability by overexpressing inhibitory neuromodulatory peptides such as Neuropeptide Y61, 62 and galanin63 or reducing the strength of excitatory signaling by modulating NMDA receptor function.64 Adenosine is a potent natural anticonvulsant65, 66 but attempts to develop adenosine-based therapies suitable for systemic administration have been thwarted because adenosinergic drugs are associated with sedative and cardiovascular side-effects that limit their clinical usefulness.37 Strategies allowing augmentation of adenosine levels locally within a specific target site will circumvent these problems. We show here that local miR-mediated downregulation of ADK expression in hippocampal astrocytes is sufficient to limit the duration of kainate-induced seizures. At the vector dose used, a >90% reduction in ADK levels was achieved. Future studies will be required to confirm that ADK knockdown in vivo using this miR-based approach is associated with increased extracellular adenosine concentrations at baseline and/or during the course of seizures using microdialysis approaches.32 We speculate that this seems likely given that a lentiviral-mediated knockdown of ADK transcripts in human mesenchymal stem cells by 80% is sufficient to increase adenosine release and our results are consistent with the observation that mice that received hippocampal grafts of these ADK-modified cells showed reduced seizure durations and attenuated cell loss following kainate injection.47 Similarly, AAV8-mediated expression of ADK-antisense sequences in astrocytes also completely abolishes spontaneous seizures in mice.39 Transplantation of co-polymers or cell types engineered to release adenosine by manipulating ADK activity provides effective seizure control in several experimental seizure paradigms including kindling, and spontaneous seizures in the CA3 region.34, 67, 68 We found that the greater neuroprotection afforded to neurons in the hilus compared to CA3 neurons which was consistent with the extent of transgene spread observed, with miR-ADK sequences predominantly expressed in the medial dorsal hippocampus, while expression was weaker towards the lateral CA3 region. Further refinements of this strategy could involve determining whether stronger therapeutic efficacy could be achieved by specifically targeting transgene expression to the CA3 region in light of studies demonstrating complete protection against seizures and injury in this region in forebrain-deficient ADK transgenic mice following intramygdaloid challenge with kainate.69 Moreover, the long-term effects of miR-overexpression on endogenous gene expression and ADK knockdown will need to be determined. Our results lend support to the concept that astrocytes are a good alternative cell target for gene therapy for epilepsy, with ADK being a prime therapeutic target.
The selection of GS and EAAT2 as potential therapeutic targets stemmed from observations that reduced astrocytic levels of GS21, 25 and potentially glutamate transporters22, 23 in the epileptogenic hippocampus, could lead to perturbed glutamate regulation and provide a potential mechanism for elevated synaptic glutamate levels that are involved in triggering seizures.70 Therefore, increasing the astrocytic capacity for glutamate clearance and breakdown in the face of extracellular glutamate concentrations that can be up to 6-fold higher than basal levels during spontaneous seizures in rats71 and in the human epileptic hippocampus16 might be sufficient to suppress seizures.
We found AAV-mediated expression of GS or EAAT2 had no effect on kainate-induced seizures nor did it protect against neuronal cell loss. Contrasting with the robust detection of transgenic GS and EAAT2 using antibodies to the respective epitope tags, it was surprising to observe that transgenic GS and EAAT2 did not contribute to any significant increase over endogenous levels of these proteins, particularly in light of the substantial knockdown of endogenous ADK achieved with miR-ADK. Thus the level of transcriptional activity of the GFAP promoter, while sufficient to drive production of miR sequences at concentrations capable of powerfully suppressing ADK expression, was not sufficient to drive a substantial increase in GS or EAAT2 expression at levels that might be required to impact on glutamate processing and seizure protection. Astrocyte-specific EAAT272 or Aldh1L173 promoters could be tested as alternatives to see if these might produce higher levels of GS expression. Higher vector titers might possibly also be tested although our results suggest a cautious approach should be taken. GS expression is sensitive to AAV vector-mediated GFP expression in a titer-dependent manner as observed in astrocytes in the CA1 region, with high titer (3×1010 genome copies/injection) almost leading to complete loss of GS expression and reactive gliosis in the absence of neuronal cell loss.48 Reactive gliosis results in deficits in neuronal inhibition, that would further perpetuate a shift towards increased hyperexcitability in hippocampal circuits,48 an unwanted effect in an epileptogenic hippocampus. Moreover, high viral loads are more likely to elicit cell-mediated and humoral immune responses.74 We also found transgene expression in astrocytes leads to a slight attenuation in GS activity levels by some unknown mechanism, an effect not found in hippocampal lysates from rats injected with AAV vector that does not express a transgene (AAV empty). Whether this is of significance and whether there is a functional consequence on hippocampal excitability remains to be investigated.
In contrast to the GS vector injected rats, a low level increase in EAAT2 expression was observed in the vector-injected hemisphere but only relative to the contralateral non-injected side. A recent study found pilocarpine-induced seizure induction and propagation parameters were not different in transgenic mice expressing 1.5–2-fold increased EAAT2 protein levels compared to control mice.75 This suggests that approaches may be needed to increase EAAT2 levels by at least 2-fold to potentially exert any effect on seizures. Downstream chronic epileptogenic processes including mossy fibre sprouting, seizure-induced neurogenesis and frequency of spontaneous seizures were reduced in EAAT2 overexpressing mice.75 This suggests that EAAT2 may be useful for preventing specific epileptogenic processes.
An increased capacity for glutamate uptake needs to be matched with an increased capacity to metabolise glutamate. In fact, the rapid metabolism of intracellular glutamate is a prerequisite for efficient glutamate clearance from the extracellular space by glutamate transporters – thus high intracellular concentrations of glutamate can slow net transport of glutamate into the cell76 and therefore the capacity to metabolise glutamate may be the rate-limiting step. Thus GS may be a better therapeutic target than EAAT2 or a combination of both may be required to provide effective seizure control. Our results suggest that alternative strategies aimed at increasing GS and EAAT2 levels should be tested before any definitive conclusion on their suitability as therapeutic targets can be made.
Our studies show that prophylactic expression of miR-ADK in the naïve rat brain can affect seizure durations; future studies will require evaluation of the miR-ADK knockdown approach in seizure models with existing hippocampal neuropathology and spontaneous seizures. The cellular environment into which the vectors will be introduced could influence cell-targeting, as AAV5 vectors expressing GFP under CAG promoter appeared to switch from preferentially transducing neurons in the naïve hippocampus to reactive astrocytes in a hippocampus showing extensive seizure-induced neuronal loss and reactive astrogliosis.10 It is unclear whether reactive astrocytes assume altered phenotypes that passively or actively allow cell uptake of viral vector, but nevertheless it suggests that viral vector tropism could be influenced by pathological changes in expression levels of receptors on astrocytes. It will be interesting to determine whether astrocyte-specific targeting in the epileptic brain can be maintained using a GFAP promoter driven transgene, and whether upregulation of GFAP gene activity in astrocytes that become reactive following injury can be mimicked by increased transgene expression levels.77
Finally, from a broader perspective, glutamate receptor overactivation and neuronal cell loss are overarching concepts applicable to other neurodegenerative disease, suggesting that ADK knockdown gene therapy could have therapeutic potential for other neurodegenerative disorders. Our study adds to a growing list of astrocyte-specific therapeutic strategies including utilization of these cells as reservoirs for local secretion of growth factors such as glial-derived neurotrophic factor that might enhance protection of dopamine neurons in Parkinson’s disease8 or inhibitory neurochemicals such as adenosine as shown in this study and those of others.39 Modulation of astrocytic processes that contribute to neuroinflammation could be another strategy. VIVIT, a peptide that interferes with the immune/inflammatory calcineurin/NFAT (nuclear factor of activated T-cells) signaling pathway78 in astrocytes is one example shown to have efficacy in the APP/PS1 transgenic mouse model of Alzheimer’s disease by improving cognitive and synaptic function and reducing glial activation. The availability of selective astrocyte-specific targeting viral vectors has opened up a pathway that facilitates the identification of new therapeutic targets in astrocytes.
Materials and Methods
Development of AAV plasmids
Enhanced green fluorescent protein (GFP) from jellyfish (Aequorea victoria), FLAG epitope-tagged human EAAT2, haemaglutinin (HA)-tagged-human GS, and firefly luciferase cDNA were cloned into the poly-linker site an AAV backbone plasmid under the control of a 2.2kb human GFAP promoter (GenBank accession number M67446), and containing a truncated woodchuck hepatitis post-transcriptional regulatory element (WPRE) and short polyadenylation (SpA) signal flanked by AAV2 inverted terminal repeats to generate pAM-GFAP-GFP, pAM-GFAP-EAAT2, pAM-GFAP-GS, and pAM-GFAP-luciferase plasmids. Three microRNA (miR) targeting rat adenosine kinase (nucleotides 241–261, UCCACGCAGAAUUCAAUGAAA; 458–478 ACCUUGCUGCCGCCAAUUGUU; 844–864 CAAGGGAGAGAUGACACUAUA) were designed using BLOCK-iT RNAi designer (Invitrogen) or miR negative control (GUCUCCACGCGCAGUACAUUU) and inserted into an AAV plasmid containing the GFAP promoter, WPRE and SpA, as well as an Emerald GFP reporter to facilitate cell tracking of miR sequences, to yield pAM-GFAP-miR-ADK and pAM-GFAP-miR-con.
AAV vector packaging
The AAV expression plasmids were packaged into AAV8 or AAV9 serotype vectors and purified by iodixanol density ultracentrifugation and genomic titers determine by real-time PCR using primers to WPRE as described previously.9, 79, 80 Highly pure vector stocks were produced as only bands corresponding to the AAV viral capsid proteins VP1, -2, -3 were visible by Coomassie blue staining on SDS-PAGE gels. The vector titers used for the expression and seizure study were (in vector genomes/mL); AAV8-GFP, 1 × 1012; AAV9-GFP, 1 × 1012; AAV9-miR-ADK, 9.48 × 1011; AAV9-miR-con, 4.12 × 1012; AAV9-GS, 2.64 × 1012; AAV9-EAAT2, 1.68 × 1012; AAV9-luciferase, 2.0 × 1012. The AAV backbone plasmid consisting of the GFAP promoter, poly-linker site, truncated WPRE and SpA was also to generate AAV9 vector containing these regulatory elements but no transgene (AAV9-empty), vector titer, 3 × 1012.
Vector infusions
All studies were conducted under ethics approval and guidelines from the University of Auckland Animal Ethics Committee. Male Sprague-Dawley rats (250–300 g) were anaesthetized with pentobarbital (70 mg/kg i.p) and rats positioned in a Kopf stereotaxic frame (David Kopf Instruments, Tujunga, CA). Rats were randomly assigned to receive 3uL of AAV vector infused unilaterally (for the expression and functional studies) or bilaterally (for the seizure analysis) into the hippocampus (coordinates: anterior-posterior −3.6 mm, medial-lateral +/− 2.1 mm, dorsal-ventral −4.3 mm from skull surface, bregma = 0,81 at a rate of 70 nL/min controlled by a microinfusion pump as described previously.9
Immunohistochemistry
For analysis of transgene expression, rats were euthanized 3 weeks following AAV infusion by pentobarbital overdose (100 mg/kg i.p) and transcardially perfused with 100 mL 0.9% (w/v) NaCl followed by 10% (w/v) neutral-buffered formalin (Sigma Aldrich, St Louis, MO). The brains were post-fixed in 10% formalin overnight at 4 °C and then cryoprotected in 30% (w/v) sucrose in phosphate-buffered saline before cryosectioning. Forty micron coronal hippocampal sections were taken for immunohistochemistry as described previously.9, 82 Antibodies used were rabbit anti-GFP (1:100,000; ab290; Abcam, Cambridge, UK), rabbit anti-luciferase (1:1,000; 70C-CR2029RAP; Fitzgerald, North Acton, MA), rabbit anti-HA (1:4000, ab9110, Abcam), mouse anti-FLAG (1:2,000, F3165, Sigma Aldrich), mouse anti-CD11b (1:10,000; CBL1512 Millipore, Billerica, MA), rabbit anti-GS (1:2,500, ab49873, Abcam), goat anti-EAAT2 (1:100, sc7760 Santa Cruz Biotechnology, Dallas, TX) or mouse anti-NeuN (1:2,000, MAB377, Millipore), mouse anti-ADK (1:1,500clone AK6.87, gifted by Jozef Spychala, University of North Carolina), diluted in PBS containing 0.2% (v/v) Triton-X100 and 10% (v/v) horse serum. Biotinylated secondary antibodies used were goat anti-rabbit or anti-mouse (1:250, Sigma Aldrich), or donkey anti-rabbit, anti-mouse or donkey anti-goat antibodies (1:1000, Jackson ImmunoResearch, West Grove, PA), followed by incubation with ExtrAvidin Peroxidase (1:250, Sigma Aldrich). Immunoreactivity was visualized by incubation with diaminobenzidine, and images were captured on an Olympus AX70 microsope using a BG CX9000 digital camera and Picture Frame image capture software (Optronics, v 2.2).
For immunofluorescent labeling, 1%(v/v) H2O2 treatment was omitted and rabbit anti-GFP (1:200,000; ab290, Abcam) was applied in combination with either anti-GFAP (1:5,000, G3893, Sigma Aldrich), anti-NeuN (1:2,000), goat anti-Iba1 (1:500, ab5076, Abcam) and incubated at 4°C for 48 h. Donkey anti-rabbit Alexa-488 secondary antibody (1:1000, Invitrogen) was applied to sections in combination with either donkey anti-mouse Alexa-594 (1:1000, Invitrogen) or biotinylated donkey anti-goat sencondary antibody (1:1000, Jackson ImmunoResearch) for 24 h at 4°C. For detection of Iba1, sections were further incubated with streptavidin Alexa-594 (1:1000, Invitrogen) for 24 h at 4°C. Images were captured using an Olympus FV1000 confocal laser scanning microscope with a oil immersion 20x oil objective.
Stereology
The volume of the hippocampus transduced by AAV-GFP vectors or hilar volumes following kainate injection in the miR-vector-injected rats was quantified stereologically using the Cavalieri estimator in Stereo Investigator 7 (MBF Bioscience, Willeston, VT) as described previously.9 The area of GFP-immunoreactivity in every 12th hippocampal section was outlined and markers placed at a grid size of 100 um to estimate the area of transduction. Hilar volumes were quantified using the Cavalieri estimator over 4 sections (12 sections apart) covering the dorsal hippocampus.
Kainate model
The seizure analysis was conducted in two separate rat cohorts: Group 1 consisted of rats injected with AAV9-miR-con (n = 8) and AAV9-miR-ADK (n = 9); Group 2 consisted of rats injected with AAV9-luciferase (n = 9), AAV9-EAAT2 (n = 8) and AAV9-GS (n = 10). Three weeks after bilateral AAV vector infusion, rats were implanted with an indwelling cannula and electrode. The tip of a guide cannula (C313G/SP; Plastics One, Roanoke, VA) was positioned above the left cortex (AP −3.6 mm, ML −3.0 mm, bregma = 0) and the tip of a bipolar recording electrode (MS333-2B; Plastics One) was lowered into the right hippocampus (AP −3.6 mm, ML – 3.0 mm, DV −4.0 mm). A ground wire was secured to a skull screw inserted into the posterior skull surface and the unit secured in place with dental cement and skull screws. After a minimum 1-week post-surgical recovery period, kainate was administered. Baseline EEG (Grass Instruments, Middleton, WI) was recorded for 5 min before kainate (40 ng in 0.5 uL, Sigma Aldrich) was injected into the hippocampus via the guide cannulae and EEG recordings taken for a further 90 min. Electrographic seizure activity was defined as high frequency and/or multi-spike complexes and/or high-voltage synchronized activity with amplitudes that exceeded twice that of baseline EEG recordings. Seizure latencies and the total duration of seizures experienced by each animal was quantified from EEG recordings. Seizure behaviours (staring and immobility (Stage 1), mastication (Stage 2), unilateral forelimb clonus (Stage 3), bilateral forelimb clonus (Stage 4), bilateral forelimb clonus with rearing and falling (Stage 5) as defined previously44 and wet-dog shakes were recorded for the 90 min by an observer blinded to the treatments. All animals received pentobarbital (30 mg/kg i.p) at the end of 90 min to terminate any residual seizure activity. Rats were euthanized one week later and transcardially perfused as described above.
Fluorojade-B staining
Every 12th section spanning the dorsal hippocampus (6 sections, 480 μm apart) was stained with 0.00004% Flurojade-B as described previously.46, 63 An investigator blinded to the treatment groups quantified Fluorojade-B-positive cells, from all sections, in two regions of the hemisphere ipsilateral to kainate infusion: the hilar region, defined as the zone between the upper and lower blades of the dentate granule cell layer, and the hippocampal CA3 pyramidal neuron region. Data was expressed as the total numbers of Flurojade-B positive cells per region over six sections.
Western blotting
Five weeks following infusion of AAV9-miR-con (n = 2), miR-ADK vector (n = 4) or PBS (n = 2), rats were euthanased, the brains removed, and hippocampi dissected. Each hippocampus was homogenized by sonication in ice-cold lysis buffer (50 mM Tris-HCl, 2 mM EDTA, 0.05% Triton-X100, pH 7.5) containing protease inhibitors (Complete Protease inhibitor Cocktail; Roche Diagnostics, Mannheim, Germany). Lysates were centrifuged at 20,800g for 15 min at 4°C and supernatants taken for analysis as described in Supplementary Information.
For Western blot analysis of GS and EAAT2 levels, 10 μg of hippocampal lysate (as prepared for the Glutamine synthetase assay) was resolved by 10% SDS-PAGE, transferred to Hybond-ECL membrane (GE Healthcare), and probed as described as described in Supplementary Information.
Glutamine synthetase assay
Additional subgroups of rats received unilateral hippocampal infusions of PBS (n = 6), AAV9-empty (n = 4), AAV9-luciferase (n = 4), AAV9-GFP (n = 7), AAV9-GS (n = 7) or AAV9-EAAT2 vector (n = 4), and at 4 weeks, rats were euthanized and the hippocampus on the injected and non-injected sides was dissected. Individual hippocampi were homogenized in 40 mM Tris, 1mM EDTA, pH 7.4, centrifuged at 1,500 g for 10 min at 4°C and supernatant diluted to a protein concentration of 1 mg/mL. GS activity was assayed as described previously83 and in Supplementary Information.
Statistical analysis
Data are expressed as mean ± SEM. A one-way ANOVA with Tukey’s post hoc test was used to compare different group means. Unpaired or paired Students t-tests where appropriate were performed using Prism (Graphpad 5.02 software, La Jolla, CA), with significance levels set at *P<0.05, ** P<0.01 and ***P<0.001.
Supplementary Material
Acknowledgments
Support: Supported by grants from Lottery Health Research (NZ) and the Royal Society of New Zealand Marsden Fund to DY, PAL and MJD, and NIH NINDS RO1 award NS44576 to MJD.
This work was supported by funding from Lottery Health Research and the Royal Society of New Zealand Marsden Fund to DY, PAL and MJD, and NIH NINDS RO1 award NS44576 to MJD. We thank M. Brenner for providing the 2.2 kb GFAP promoter.
Footnotes
Conflict of interest
No competing financial interests exist in relation to this work.
Supplementary Information is available at Gene Therapy’s website
References
- 1.Gelfand Y, Kaplitt MG. Gene therapy for psychiatric disorders. World neurosurgery. 2013;80(3–4):S32 e11–8. doi: 10.1016/j.wneu.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 2.Simonato M, Bennett J, Boulis NM, Castro MG, Fink DJ, Goins WF, et al. Progress in gene therapy for neurological disorders. Nature reviews Neurology. 2013;9(5):277–91. doi: 10.1038/nrneurol.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LeWitt PA, Rezai AR, Leehey MA, Ojemann SG, Flaherty AW, Eskandar EN, et al. AAV2-GAD gene therapy for advanced Parkinson’s disease: a double-blind, sham-surgery controlled, randomised trial. Lancet neurology. 2011;10(4):309–19. doi: 10.1016/S1474-4422(11)70039-4. [DOI] [PubMed] [Google Scholar]
- 4.Eberling JL, Jagust WJ, Christine CW, Starr P, Larson P, Bankiewicz KS, et al. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology. 2008 doi: 10.1212/01.wnl.0000312381.29287.ff. [DOI] [PubMed] [Google Scholar]
- 5.Christine CW, Starr PA, Larson PS, Eberling JL, Jagust WJ, Hawkins RA, et al. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology. 2009;73(20):1662–9. doi: 10.1212/WNL.0b013e3181c29356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leone P, Shera D, McPhee SW, Francis JS, Kolodny EH, Bilaniuk LT, et al. Long-term follow-up after gene therapy for canavan disease. Science translational medicine. 2012;4(165):165ra163. doi: 10.1126/scitranslmed.3003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon JR, Sew T, Montero L, Burton EA, Greenamyre JT. Pseudotype-dependent lentiviral transduction of astrocytes or neurons in the rat substantia nigra. Exp Neurol. 2011;228(1):41–52. doi: 10.1016/j.expneurol.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drinkut A, Tereshchenko Y, Schulz JB, Bahr M, Kugler S. Efficient gene therapy for Parkinson’s disease using astrocytes as hosts for localized neurotrophic factor delivery. Mol Ther. 2012;20(3):534–43. doi: 10.1038/mt.2011.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawlor PA, Bland RJ, Mouravlev A, Young D, During MJ. Efficient gene delivery and selective transduction of glial cells in the mammalian brain by AAV serotypes isolated from nonhuman primates. Mol Ther. 2009;17(10):1692–702. doi: 10.1038/mt.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinberg MS, Blake BL, Samulski RJ, McCown TJ. The influence of epileptic neuropathology and prior peripheral immunity on CNS transduction by rAAV2 and rAAV5. Gene Ther. 2011;18(10):961–8. doi: 10.1038/gt.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seifert G, Schilling K, Steinhauser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7(3):194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- 12.de Lanerolle NC, Kim JH, Williamson A, Spencer SS, Zaveri HP, Eid T, et al. A retrospective analysis of hippocampal pathology in human temporal lobe epilepsy: evidence for distinctive patient subcategories. Epilepsia. 2003;44(5):677–87. doi: 10.1046/j.1528-1157.2003.32701.x. [DOI] [PubMed] [Google Scholar]
- 13.de Lanerolle NC, Lee TS, Spencer DD. Histopathology of Human Epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. 4. Bethesda (MD): 2012. [Google Scholar]
- 14.Binder DK, Steinhauser C. Functional changes in astroglial cells in epilepsy. Glia. 2006;54(5):358–68. doi: 10.1002/glia.20394. [DOI] [PubMed] [Google Scholar]
- 15.Wetherington J, Serrano G, Dingledine R. Astrocytes in the epileptic brain. Neuron. 2008;58(2):168–78. doi: 10.1016/j.neuron.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 1993;341(8861):1607–10. doi: 10.1016/0140-6736(93)90754-5. [DOI] [PubMed] [Google Scholar]
- 17.Chapman AG. Glutamate and epilepsy. The Journal of nutrition. 2000;130(4S Suppl):1043S–5S. doi: 10.1093/jn/130.4.1043S. [DOI] [PubMed] [Google Scholar]
- 18.Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, et al. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13(3):713–25. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 19.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16(3):675–86. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 20.Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci. 1995;15(3 Pt 1):1835–53. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eid T, Thomas MJ, Spencer DD, Runden-Pran E, Lai JC, Malthankar GV, et al. Loss of glutamine synthetase in the human epileptogenic hippocampus: possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet. 2004;363(9402):28–37. doi: 10.1016/s0140-6736(03)15166-5. [DOI] [PubMed] [Google Scholar]
- 22.Mathern GW, Mendoza D, Lozada A, Pretorius JK, Dehnes Y, Danbolt NC, et al. Hippocampal GABA and glutamate transporter immunoreactivity in patients with temporal lobe epilepsy. Neurology. 1999;52(3):453–72. doi: 10.1212/wnl.52.3.453. [DOI] [PubMed] [Google Scholar]
- 23.Proper EA, Hoogland G, Kappen SM, Jansen GH, Rensen MG, Schrama LH, et al. Distribution of glutamate transporters in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain. 2002;125(Pt 1):32–43. doi: 10.1093/brain/awf001. [DOI] [PubMed] [Google Scholar]
- 24.Tessler S, Danbolt NC, Faull RL, Storm-Mathisen J, Emson PC. Expression of the glutamate transporters in human temporal lobe epilepsy. Neuroscience. 1999;88(4):1083–91. doi: 10.1016/s0306-4522(98)00301-7. [DOI] [PubMed] [Google Scholar]
- 25.van der Hel WS, Notenboom RG, Bos IW, van Rijen PC, van Veelen CW, de Graan PN. Reduced glutamine synthetase in hippocampal areas with neuron loss in temporal lobe epilepsy. Neurology. 2005;64(2):326–33. doi: 10.1212/01.WNL.0000149636.44660.99. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276(5319):1699–702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 27.Demarque M, Villeneuve N, Manent JB, Becq H, Represa A, Ben-Ari Y, et al. Glutamate transporters prevent the generation of seizures in the developing rat neocortex. J Neurosci. 2004;24(13):3289–94. doi: 10.1523/JNEUROSCI.5338-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell SL, Hablitz JJ. Glutamate transporters regulate excitability in local networks in rat neocortex. Neuroscience. 2004;127(3):625–35. doi: 10.1016/j.neuroscience.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Zaveri HP, Lee TS, Eid T. The development of recurrent seizures after continuous intrahippocampal infusion of methionine sulfoximine in rats: a video-intracranial electroencephalographic study. Exp Neurol. 2009;220(2):293–302. doi: 10.1016/j.expneurol.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boison D. Adenosine Augmentation Therapy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. 4. Bethesda (MD): 2012. [PubMed] [Google Scholar]
- 31.Dragunow M. Purinergic mechanisms in epilepsy. Progress in neurobiology. 1988;31(2):85–108. doi: 10.1016/0301-0082(88)90028-7. [DOI] [PubMed] [Google Scholar]
- 32.During MJ, Spencer DD. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann Neurol. 1992;32(5):618–24. doi: 10.1002/ana.410320504. [DOI] [PubMed] [Google Scholar]
- 33.Li T, Lytle N, Lan JQ, Sandau US, Boison D. Local disruption of glial adenosine homeostasis in mice associates with focal electrographic seizures: a first step in epileptogenesis? Glia. 2012;60(1):83–95. doi: 10.1002/glia.21250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huber A, Padrun V, Deglon N, Aebischer P, Mohler H, Boison D. Grafts of adenosine-releasing cells suppress seizures in kindling epilepsy. Proc Natl Acad Sci U S A. 2001;98(13):7611–6. doi: 10.1073/pnas.131102898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, et al. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Invest. 2008 doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gouder N, Fritschy JM, Boison D. Seizure suppression by adenosine A1 receptor activation in a mouse model of pharmacoresistant epilepsy. Epilepsia. 2003;44(7):877–85. doi: 10.1046/j.1528-1157.2003.03603.x. [DOI] [PubMed] [Google Scholar]
- 37.Dunwiddie TV, Worth T. Sedative and anticonvulsant effects of adenosine analogs in mouse and rat. The Journal of pharmacology and experimental therapeutics. 1982;220(1):70–6. [PubMed] [Google Scholar]
- 38.Zhang G, Franklin PH, Murray TF. Anticonvulsant effect of N-ethylcarboxamidoadenosine against kainic acid-induced behavioral seizures in the rat prepiriform cortex. Neuroscience letters. 1990;114(3):345–50. doi: 10.1016/0304-3940(90)90588-z. [DOI] [PubMed] [Google Scholar]
- 39.Theofilas P, Brar S, Stewart KA, Shen HY, Sandau US, Poulsen D, et al. Adenosine kinase as a target for therapeutic antisense strategies in epilepsy. Epilepsia. 2011;52(3):589–601. doi: 10.1111/j.1528-1167.2010.02947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27(1):59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gray SJ, Matagne V, Bachaboina L, Yadav S, Ojeda SR, Samulski RJ. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther. 2011;19(6):1058–69. doi: 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogata K, Kosaka T. Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience. 2002;113(1):221–33. doi: 10.1016/s0306-4522(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 43.Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23(11):580–7. doi: 10.1016/s0166-2236(00)01659-3. [DOI] [PubMed] [Google Scholar]
- 44.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32(3):281–94. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 45.Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14(2):375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- 46.Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874(2):123–30. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- 47.Ren G, Li T, Lan JQ, Wilz A, Simon RP, Boison D. Lentiviral RNAi-induced downregulation of adenosine kinase in human mesenchymal stem cell grafts: a novel perspective for seizure control. Exp Neurol. 2007;208(1):26–37. doi: 10.1016/j.expneurol.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, et al. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci. 2010;13(5):584–91. doi: 10.1038/nn.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cearley CN, Vandenberghe LH, Parente MK, Carnish ER, Wilson JM, Wolfe JH. Expanded repertoire of AAV vector serotypes mediate unique patterns of transduction in mouse brain. Mol Ther. 2008;16(10):1710–8. doi: 10.1038/mt.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol Ther. 2006;13(3):528–37. doi: 10.1016/j.ymthe.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 51.Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14(3):316–27. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 52.Li C, Diprimio N, Bowles DE, Hirsch ML, Monahan PE, Asokan A, et al. Single amino acid modification of adeno-associated virus capsid changes transduction and humoral immune profiles. J Virol. 2012;86(15):7752–9. doi: 10.1128/JVI.00675-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pulicherla N, Shen S, Yadav S, Debbink K, Govindasamy L, Agbandje-McKenna M, et al. Engineering liver-detargeted AAV9 vectors for cardiac and musculoskeletal gene transfer. Mol Ther. 2011;19(6):1070–8. doi: 10.1038/mt.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.von Jonquieres G, Mersmann N, Klugmann CB, Harasta AE, Lutz B, Teahan O, et al. Glial promoter selectivity following AAV-delivery to the immature brain. PloS one. 2013;8(6):e65646. doi: 10.1371/journal.pone.0065646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu R, Janson CG, Mastakov M, Lawlor P, Young D, Mouravlev A, et al. Quantitative comparison of expression with adeno-associated virus (AAV-2) brain-specific gene cassettes. Gene Ther. 2001;8(17):1323–32. doi: 10.1038/sj.gt.3301529. [DOI] [PubMed] [Google Scholar]
- 56.Klein RL, Dayton RD, Tatom JB, Henderson KM, Henning PP. AAV8, 9, Rh10, Rh43 Vector Gene Transfer in the Rat Brain: Effects of Serotype, Promoter and Purification Method. Mol Ther. 2007 doi: 10.1038/sj.mt.6300331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su M, Hu H, Lee Y, d’Azzo A, Messing A, Brenner M. Expression specificity of GFAP transgenes. Neurochemical research. 2004;29(11):2075–93. doi: 10.1007/s11064-004-6881-1. [DOI] [PubMed] [Google Scholar]
- 58.Colin A, Faideau M, Dufour N, Auregan G, Hassig R, Andrieu T, et al. Engineered lentiviral vector targeting astrocytes in vivo. Glia. 2009;57(6):667–79. doi: 10.1002/glia.20795. [DOI] [PubMed] [Google Scholar]
- 59.Xie J, Xie Q, Zhang H, Ameres SL, Hung JH, Su Q, et al. MicroRNA-regulated, systemically delivered rAAV9: a step closer to CNS-restricted transgene expression. Mol Ther. 2011;19(3):526–35. doi: 10.1038/mt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gray SJ, Blake BL, Criswell HE, Nicolson SC, Samulski RJ, McCown TJ, et al. Directed evolution of a novel adeno-associated virus (AAV) vector that crosses the seizure-compromised blood-brain barrier (BBB) Mol Ther. 2010;18(3):570–8. doi: 10.1038/mt.2009.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richichi C, Lin EJ, Stefanin D, Colella D, Ravizza T, Grignaschi G, et al. Anticonvulsant and antiepileptogenic effects mediated by adeno-associated virus vector neuropeptide Y expression in the rat hippocampus. J Neurosci. 2004;24(12):3051–9. doi: 10.1523/JNEUROSCI.4056-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noe F, Nissinen J, Pitkanen A, Gobbi M, Sperk G, During M, et al. Gene therapy in epilepsy: the focus on NPY. Peptides. 2007;28(2):377–83. doi: 10.1016/j.peptides.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 63.Lin EJ, Richichi C, Young D, Baer K, Vezzani A, During MJ. Recombinant AAV-mediated expression of galanin in rat hippocampus suppresses seizure development. Eur J Neurosci. 2003;18(7):2087–92. doi: 10.1046/j.1460-9568.2003.02926.x. [DOI] [PubMed] [Google Scholar]
- 64.Haberman R, Criswell H, Snowdy S, Ming Z, Breese G, Samulski R, et al. Therapeutic liabilities of in vivo viral vector tropism: adeno-associated virus vectors, NMDAR1 antisense, and focal seizure sensitivity. Mol Ther. 2002;6(4):495–500. doi: 10.1006/mthe.2002.0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dragunow M. Adenosine: the brain’s natural anticonvulsant. Trends in pharmacological sciences. 1986;7:128–130. [Google Scholar]
- 66.Dragunow M. Adenosine and seizure termination. Ann Neurol. 1991;29(5):575. doi: 10.1002/ana.410290524. [DOI] [PubMed] [Google Scholar]
- 67.Boison D, Scheurer L, Tseng JL, Aebischer P, Mohler H. Seizure suppression in kindled rats by intraventricular grafting of an adenosine releasing synthetic polymer. Exp Neurol. 1999;160(1):164–74. doi: 10.1006/exnr.1999.7209. [DOI] [PubMed] [Google Scholar]
- 68.Boison D. Engineered adenosine-releasing cells for epilepsy therapy: human mesenchymal stem cells and human embryonic stem cells. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2009;6(2):278–83. doi: 10.1016/j.nurt.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, et al. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Invest. 2008;118(2):571–82. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cavus I, Kasoff WS, Cassaday MP, Jacob R, Gueorguieva R, Sherwin RS, et al. Extracellular metabolites in the cortex and hippocampus of epileptic patients. Ann Neurol. 2005;57(2):226–35. doi: 10.1002/ana.20380. [DOI] [PubMed] [Google Scholar]
- 71.Kanamori K, Ross BD. Chronic electrographic seizure reduces glutamine and elevates glutamate in the extracellular fluid of rat brain. Brain Res. 2011;1371:180–91. doi: 10.1016/j.brainres.2010.11.064. [DOI] [PubMed] [Google Scholar]
- 72.Su ZZ, Leszczyniecka M, Kang DC, Sarkar D, Chao W, Volsky DJ, et al. Insights into glutamate transport regulation in human astrocytes: cloning of the promoter for excitatory amino acid transporter 2 (EAAT2) Proc Natl Acad Sci U S A. 2003;100(4):1955–60. doi: 10.1073/pnas.0136555100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28(1):264–78. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ciesielska A, Hadaczek P, Mittermeyer G, Zhou S, Wright JF, Bankiewicz KS, et al. Cerebral infusion of AAV9 vector-encoding non-self proteins can elicit cell-mediated immune responses. Mol Ther. 2013;21(1):158–66. doi: 10.1038/mt.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kong Q, Takahashi K, Schulte D, Stouffer N, Lin Y, Lin CL. Increased glial glutamate transporter EAAT2 expression reduces epileptogenic processes following pilocarpine-induced status epilepticus. Neurobiology of disease. 2012;47(2):145–54. doi: 10.1016/j.nbd.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Otis TS, Jahr CE. Anion currents and predicted glutamate flux through a neuronal glutamate transporter. J Neurosci. 1998;18(18):7099–110. doi: 10.1523/JNEUROSCI.18-18-07099.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brenner M, Kisseberth WC, Su Y, Besnard F, Messing A. GFAP promoter directs astrocyte-specific expression in transgenic mice. J Neurosci. 1994;14(3 Pt 1):1030–7. doi: 10.1523/JNEUROSCI.14-03-01030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Furman JL, Sama DM, Gant JC, Beckett TL, Murphy MP, Bachstetter AD, et al. Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer’s disease. J Neurosci. 2012;32(46):16129–40. doi: 10.1523/JNEUROSCI.2323-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lawlor PA, Bland RJ, Das P, Price RW, Holloway V, Smithson L, et al. Novel rat Alzheimer’s disease models based on AAV-mediated gene transfer to selectively increase hippocampal Abeta levels. Mol Neurodegener. 2007;2:11. doi: 10.1186/1750-1326-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.During MJ, Young D, Baer K, Lawlor P, Klugmann M. Development and optimization of adeno-associated virus vector transfer into the central nervous system. Methods Mol Med. 2003;76:221–36. doi: 10.1385/1-59259-304-6:221. [DOI] [PubMed] [Google Scholar]
- 81.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- 82.Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5(4):448–53. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- 83.Petito CK, Chung MC, Verkhovsky LM, Cooper AJ. Brain glutamine synthetase increases following cerebral ischemia in the rat. Brain Res. 1992;569(2):275–80. doi: 10.1016/0006-8993(92)90639-q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.