Abstract
Background
Volatile organic compounds (VOCs) from tobacco smoke are associated with cancer, cardiovascular, and respiratory diseases. The objective of this study was to characterize the exposure of nonsmokers to VOCs from secondhand smoke (SHS) in vehicles using mercapturic acid metabolites.
Methods
Fourteen nonsmokers were individually exposed in the backseat to one hour of SHS from a smoker seating in the driver’s seat who smoked 3 cigarettes at 20 minute intervals in a stationary car with windows opened by 10 cm. Baseline and 0-8 h post-exposure mercapturic acid metabolites of 9 VOCs were measured in urine. Air-to-urine VOC ratios were estimated based on respirable particulates (PM2.5) or air nicotine concentration, and lifetime excess risk (LER) of cancer death from exposure to acrylonitrile, benzene, and 1,3-butadiene was estimated for adults.
Results
The greatest increase in 0-8 h post-exposure concentrations of mercapturic acids from baseline was MHBMA-3 (parent, 1,3-butadiene) (2.1-fold), then CNEMA (acrylonitrile) (1.7-fold), PMA (benzene) (1.6-fold), MMA (methylating agents) (1.6-fold), and HEMA (ethylene oxide) (1.3-fold). The LER of cancer death from exposure to acrylonitrile, benzene, and 1,3-butadiene in SHS for 5 hour a week ranged from 15.5×10−6 to 28.1×10−6 for adults, using air nicotine and PM2.5 to predict air VOC exposure, respectively.
Conclusion
Nonsmokers have significant intake of multiple VOCs from breathing SHS in cars, corresponding to health risks that exceed the acceptable level.
Impact
Smoking in cars may be associated with increased risks of cancer, respiratory, and cardiovascular diseases among nonsmokers.
Keywords: secondhand smoke, automobiles, volatile organic compounds, mercapturic acids
INTRODUCTION
Exposure to secondhand smoke (SHS), a combination of the smoke emitted at the burning tip of a cigarette and smoke exhaled by the smoker, is associated with an array of adverse health effects (1). While public health efforts such as smoke-free air policies in workplaces and public places have been successful in reducing exposure to SHS (2) and related diseases (3, 4), 126 million people remain exposed to SHS daily in the U.S., of which 22 million are children (1, 5). Further, the decline in SHS exposure among non-smokers has been slower among children than adults (6, 7). Exposure to SHS in childhood is linked to an increased risk of asthma, sudden infant death syndrome (SIDS), otitis media, upper respiratory tract infections, and behavioral problems (5).
Motor vehicles (cars) are an important source of exposure to SHS in children. On average, people spend more than an hour a day in motor vehicles (8) and smokers often smoke while driving or as passengers. The prevalence of SHS exposure was found to be higher in cars (9.2%) than in homes (6.0%) among adults enrolled in the National Adults Tobacco Survey (9). Also, as many as 48% of smoking parents smoke with children present in the car (10).
Estimation of the health risks associated with SHS in cars depends on accurate measurement of exposure. Although air particulate matter less than 2.5 μm (PM2.5), carbon monoxide (CO), and nicotine have been used to characterize SHS exposure in cars (11, 12), biomarkers constitute the most objective method of measuring intake or dose. Few published studies have reported on biomarkers of exposure to SHS in vehicles. In one study, urine cotinine, the primary proximate metabolite of nicotine, was measured in nonsmoking adults and children after 2 hours of heavy SHS exposure from 78 smoked cigarettes in a tour bus with closed windows (13). This study vastly overestimates most SHS exposure scenarios in automobiles in the U.S. In a recent study we presented the 24 hour time course of biomarkers of nicotine and tobacco-specific nitrosamines in nonsmokers after an hour exposure to SHS from 3 smoked cigarettes in a stationary car (14).
Volatile organic compounds (VOCs) represent an important class of carcinogens, toxicants, and/or irritants present in tobacco smoke (15). It is thought that the gas phase constituents in mainstream tobacco smoke contribute heavily towards tobacco smoke cancer, cardiovascular, and respiratory risk indices (16, 17). Intake of VOCs can be measured using highly specific mercapturic acid metabolites formed from glutathione S-conjugates (GSH) via the mercapturic acid pathway and excreted in the urine (18). VOC mercapturic acid metabolites have been measured in cigarette smokers, water pipe users, and nonsmokers (18-21).
Although a few studies have reported mercapturic acid metabolite excretion in people who may have been exposed to SHS (22), to the best of our knowledge, no study has reported pre- and post-SHS exposure levels of mercapturic acids. The aim of this study was to investigate the simultaneous excretion of mercapturic acid metabolites of nine different VOCs following one hour exposure to SHS in a stationary automobile. As a secondary aim, we used particulate matter (PM2.5) concentrations measured in a concurrent study (23) to estimate air VOC to urine mercapturic acid ratios that can be used in computations of lifetime excess risk (LER) of lung cancer and cancers associated with exposure to benzene and 1,3-butadiene, two known human carcinogens, and acrylonitrile, a probable human carcinogen. The LER is excess cancer risk caused by exposure to an agent that is in addition to any cancer risk carried by an individual not exposed to the agent.
MATERIALS AND METHODS
Overview
The data presented in this paper were collected as part of a study of SHS exposure in motor vehicles, the details of which have been published previously (23). The study was conducted in the Clinical Research Center at San Francisco General Hospital (SFGH) and in an automobile parked in a nearby parking lot. The engine of the vehicle was off for the duration of the exposure period and the vehicle remained stationary. A 1992 Jeep Cherokee owned by a smoker was used in the study. The smoker sat in the driver’s seat and smoked three cigarettes over the course of an hour (at 0, 20, and 40 min). The cigarette was held in the smoker’s right hand. A nonsmoking participant sat in the right rear seat of the car. The front and rear windows of the Jeep were open 10 cm. This opening was selected based on informal discussion with nonsmokers exposed to SHS and appeared to be the minimum opening that would be generally tolerated. Air sampling devices were collocated in the middle of the backseat and tube inlets were placed at the approximate breathing zone. Air concentrations of tobacco smoke constituents have been previously reported (23).
Fourteen nonsmokers and one active smoker participated in the study. The smoker’s role was limited to smoking cigarettes in the car during the 1-hour SHS exposure period. The nonsmoking participants were balanced by sex and were healthy with recent histories of SHS exposure but were asked to avoid SHS exposure 7 days prior to the study day. Prior exposure was required to ensure that we were not exposing subjects to an unfamiliar risk. Nonsmoking status was determined by self-report and confirmed by plasma cotinine concentrations. Exclusion criteria included a history of recent respiratory illness, history of major medical or psychiatric conditions, body mass index (BMI) > 30, pregnancy or lactation, current illicit drug or alcohol abuse, inability to speak English, or a history of fainting.
The study was approved by the Committee on Human Research (CHR) at the University of California, San Francisco. Written, informed consent was obtained from each participant and all participants were financially compensated for their time.
Study procedures and biosampling
The nonsmoking participants arrived at the Tobacco Research Center, (a UCSF outpatient research clinic near San Francisco General Hospital, SFGH) by 7 A.M. An intravenous (IV) line for blood sampling was placed and baseline blood and pre-exposure urine samples were collected. Between 8 and 9 A.M., the participant was escorted to the clinic parking lot and asked to sit in the right back seat of the car, while the smoker sat in the driver’s seat. Three cigarettes in total were smoked at 20 minute intervals (timed by a research coordinator), starting at time 0 when the nonsmoker entered the car. The same brand of cigarettes, Marlboro Regulars, was smoked at each exposure session. There was only one smoking session per study day. The smoker was instructed to smoke each cigarette in the same way. The average weight of cigarettes consumed per session was 1.99 g (min-max, 1.55-2.35) and the mean change in expired carbon monoxide in the smoker was 16.1 ppm (8-31). The nonsmoker exited the car 60 minutes after the lighting of the first cigarette. The subject then went to the SFGH Clinical Research Center (CRC), a research ward, for a 24 hour stay. At the CRC, blood samples were taken at 15, 30, 45, 60 and 90 minutes, and 2, 3, 4, 6, 8, 12, 16 and 24 hours after exiting the vehicle; and plasma was analyzed for concentrations of cotinine. Urine was collected post-exposure in blocks of 0-4, 4-8, 8-12 and 12-24 hours. Urine was analyzed for concentrations of VOC mercapturic acid metabolites and creatinine, as well as biomarkers of other tobacco smoke constituents, the results of which will be reported elsewhere.
Details of air sampling procedures inside the car and ambient (background) for nicotine, CO, and respirable particulate matter (PM2.5) have been described previously (23).
Analytical chemistry
The following mercapturic acid metabolites of VOCs were measured in the pre-exposure, 0-4 and 4-8 h urine samples – shown as mercapturic acid metabolite [abbreviation] (parent compound) by previously described methods (21): 2-carbamoylethylmercapturic acid [AAMA] (acrylamide); 2-cyanoethylmercapturic acid [CNEMA] (acrylonitrile); 2-hydroxyethylmercapturic acid [HEMA] (ethylene oxide); 2-hydroxypropylmercapturic acid [2-HPMA] (propylene oxide); 3-hydroxypropylmercapturic acid [3-HPMA] (acrolein); 4-hydroxy-2-buten-1-yl-mercapturic acid [MHBMA-3] (butadiene); phenylmercapturic acid [PMA] (benzene); 3-hydroxy-1-methyl-propylmercapturic acid [HMPMA] (crotonaldehyde); and, methylmercapturic acid [MMA] (methylating agents such as 4-(methylnitrosamino)-1-(3-pyridyl)-1- butanone (NNK) and N-nitrosodimethylamine (NDMA)). VOC metabolites were not measured beyond the 0-8 hour urine samples because 8 hours is sufficient to capture most of the additive VOC exposure over baseline. Urine samples over 8 hours were expected to be at or near baseline levels given the rapid initial half-lives of VOCs and elimination from the body (24).
Statistical analysis
Descriptive statistics were computed for pre-exposure (baseline), 0-8 h post-exposure, and maximum (peak) post-exposure concentrations of mercapturic acid metabolites (arithmetic means, geometric means, and medians). Within-subject differences in pre- and post-exposure biomarker concentrations were assessed using Wilcoxon signed rank test. Statistical analyses were carried out using SAS v. 9.3 (SAS Institute, Inc., Cary, NC, USA) and statistical tests were considered significant at α = 0.05.
The cancer risk due to inhalation of VOCs in SHS can be computed using Equation 1, which was applied recently to estimate cancer risks from VOC exposure among patrons and servers exposed to SHS in restaurants and bars (25). While only PM2.5 concentrations were used in that analysis to estimate VOC exposure from SHS in the absence of air VOC measurements, air nicotine concentrations can be similarly used.
| (Equation 1) |
Where, CSHS-VOC is the daily average concentration of a SHS-VOC during a lifetime of 70 years and AUR is the Air Unit Risk reported by the U.S. Environmental Protection Agency (U.S. EPA) for carcinogens. AUR is the increase in the lifetime risk for an individual who is exposed to 1 μg/m3 of a chemical for a lifetime (70 years), assuming 20 m3/day of inhalation (26). In predicting CSHS-VOC from PM2.5 or air nicotine, CSHS-PM is the average concentration of SHS-PM2.5 during the exposure period; Cnicotine is the average concentration of nicotine in air during the exposure period; and, EFSHS-VOC, EFSHS-PM, and EFnicotine are the average cigarette emission factors of SHS-VOC, SHS-PM2.5, and nicotine from the literature. F is the adjustment factor, which is 1 h/day × 0.5 m3/h/(20 m3/day) × 5 day/7 day × 51 years/70 years for an adult (≥19 years) who is exposed to SHS in cars for an average of 1 hour a day, with an average respiration rate of 0.5 m3/h at sedentary activity level (27), and is exposed 5 days a week for 51 years (19 to 70 years).
We computed air to urine VOC ratios (VOC ratio(air to urine)) for acrylonitrile, benzene, and 1,3-butadiene using average time-integrated PM2.5 and air nicotine measured over the exposure period, the average emission factors (EFs) computed by Liu and colleagues (28), and the average increase in the respective baseline-corrected mercapturic acid metabolite concentration (VOC(urine)) as shown in Equation 2.
Or,
| (Equation 2) |
The VOC ratio(air to urine) reported here can be used to predict CSHS-VOC as shown in Equation 3 to estimate cancer risks in studies where PM2.5, nicotine, or VOCs were not measured in air.
| (Equation 3) |
Where, study-ΔVOC(urine) is the change in mercapturic acid metabolites following SHS exposure in a study and F is the study’s population-specific adjustment factor.
RESULTS
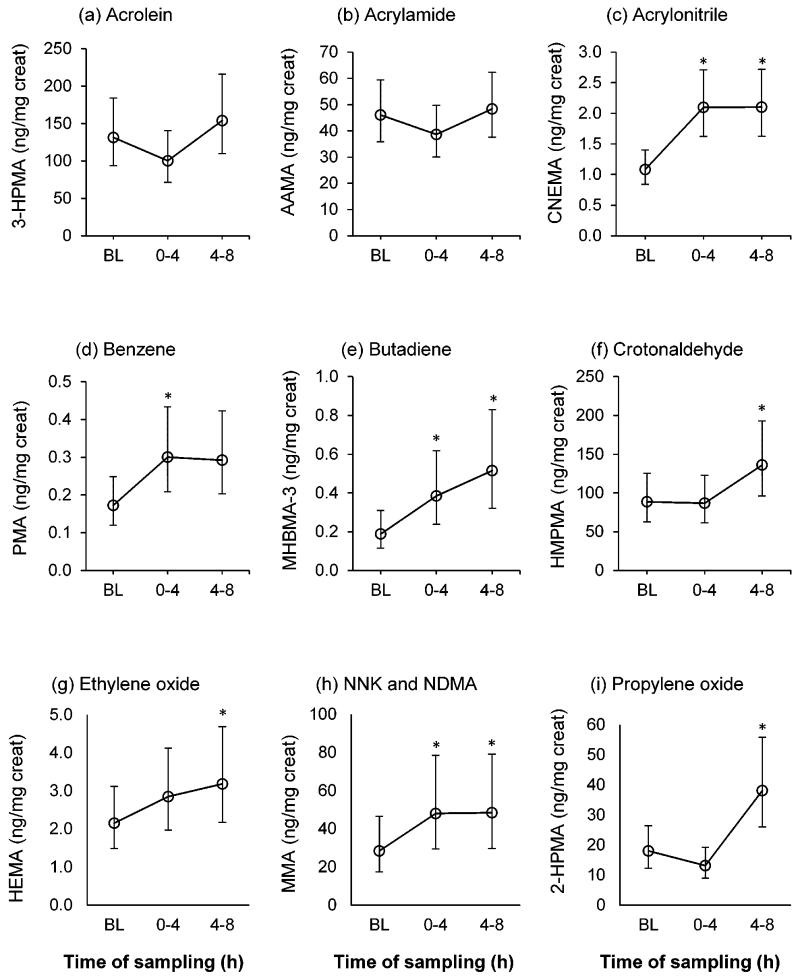
Table 1 presents the average concentrations of PM2.5, CO, and nicotine measured inside and outside the car and the ventilation rates in the car. Air measurements have been reported in greater detail as “Set 2” in a previous manuscript (23). Descriptive statistics (arithmetic mean and median) of pre-exposure, 0-8 hour post-exposure, maximum post-exposure concentrations, and changes in concentrations of nine mercapturic acid metabolites of VOCs are presented in Table 2. Figure 1 presents geometric means and 95% CI of the nine mercapturic acid metabolites measured in baseline, 0-4 hour, and 4-8 hour urine samples.
Table 1.
Average air measurements of respirable particulate matter (PM2.5), carbon monoxide, nicotine, and ventilation rates over the exposure period.
| Air measurement | n | Mean ± S.D. |
|---|---|---|
| PM2.5 (μg/m3) | ||
| inside of the car | 13 | 1172 ± 503 |
| outside of the car | 13 | 17.7 ± 12.8 |
| Carbon monoxide (ppm) | ||
| dashboard | 12 | 3.3 ± 1.7 |
| middle | 12 | 2.5 ± 1.2 |
| back | 12 | 2.5 ± 0.9 |
| Nicotine (μg/m3) | ||
| inside of the car | 13 | 65.6 ± 107.9 |
| outside of the car | 10 | 0.06 ± 0.08 |
| Air changes per hour | ||
| dashboard | 11 | 6.0 ± 1.8 |
| middle | 12 | 5.0 ± 1.7 |
| back | 10 | 3.4 ± 0.9 |
Notes: Measurements of air exposure for this study has been previously published and referred to as “Set 2” (23).
Table 2.
Changes in concentrations biomarkers after 1-hour exposure to secondhand smoke (SHS) in cars.
| Biomarker | Pre [C] (ng/mg creatinine) |
0-8 h post [C] (ng/mg creatinine) |
Max post [C] (ng/mg creatinine) |
0-8 h change (ng/mg creatinine) |
p value | Max change (ng/mg creatinine) |
p value |
|---|---|---|---|---|---|---|---|
| 2-HPMA | |||||||
| Mean (95% CI) | 21.6 (12.7, 30.5) | 37.9 (12.7, 63.0) | 49.0 (22.6, 75.5) | 18.2 (−8.9, 45.3) | 0.127 | 30.0 (1.5, 58.6) | 0.011 |
| Median (IQR) | 16.3 (13.5, 23.0) | 22.1 (14.8, 39.9) | 35.9 (17.1, 63.6) | 4.6 (−3.1, 19.6) | 9.4 (3.7, 36.3) | ||
| 3-HPMA | |||||||
| Mean (95% CI) | 160.5 (101.3, 219.7) | 160.0 (86.3, 233.7) | 187.4 (115.7, 259.2) | −1.6 (−86.4, 82.2) | 0.839 | 26.1 (−57.8, 109.9) | 0.414 |
| Median (IQR) | 113.7 (88.8, 261.7) | 122.1 (103.8, 162.8) | 150.2 (127.8, 191.7) | −6.4 (−56.8, 46.4) | 31.6 (4.9, 57.3) | ||
| AAMA | |||||||
| Mean (95% CI) | 50.8 (36.2, 65.3) | 46.5 (37.6, 55.5) | 54.7 (42.2, 67.10 | −2.3 (−11.6, 7.0) | 0.893 | 6.4 (−3.0, 15.7) | 0.244 |
| Median (IQR) | 49.5 (39.2, 57.5) | 46.2 (33.0, 58.2) | 50.0 (37.4, 66.1) | 1.2 (−8.8, 8.5) | 5.4 (−6.2, 17.5) | ||
| CNEMA | |||||||
| Mean (95% CI) | 1.29 (0.86, 1.73) | 2.22 (1.94, 2.50) | 2.45 (2.11, 2.79) | 0.92 (0.57, 1.27) | <.001 | 1.15 (0.68, 1.62) | <.001 |
| Median (IQR) | 1.12 (0.77, 1.71) | 2.31 (1.94, 2.58) | 2.53 (2.10, 2.88) | 0.82 (0.70, 1.23) | 1.06 (0.75, 1.36) | ||
| HEMA | |||||||
| Mean (95% CI) | 2.73 (1.58, 3.87) | 3.43 (2.30, 4.55) | 3.84 (2.40, 5.28) | 0.81 (0.21, 1.40) | 0.003 | 1.22 (0.30, 2.14) | 0.001 |
| Median (IQR) | 1.91 (1.32, 3.56) | 2.81 (1.87, 4.53) | 2.93 (2.19, 5.12) | 0.54 (0.38, 0.81) | 0.59 (0.46, 1.14) | ||
| HMPMA | |||||||
| Mean (95% CI) | 110.2 (46.3, 174.2) | 140.8 (82.0, 199.6) | 171.4 (114.7, 228.1) | 29.1 (−47.6, 105.9) | 0.147 | 61.1 (−15.2, 137.3) | 0.048 |
| Median (IQR) | 86.3 (70.3, 94.3) | 102.5 (83.8, 156.3) | 154.7 (109.6, 183.1) | 13.5 (−9.9, 71.2) | 73.3 (7.8, 100.3) | ||
| MMA | |||||||
| Mean (95% CI) | 40.1 (19.4, 60.5) | 65.1 (35.5, 94.7) | 74.3 (39.2, 109.5) | 27.2 (12.7, 41.8) | <.001 | 36.7 (17.5, 55.9) | <.001 |
| Median (IQR) | 24.2 (13.2, 52.1) | 40.8 (33.3, 112.4) | 46.9 (33.3, 121.0) | 19.1 (10.6, 31.4) | 24.8 (14.0, 56.5) | ||
| MHBMA-3 | |||||||
| Mean (95% CI) | 0.27 (0.10, 0.47) | 0.58 (0.40, 0.76) | 0.73 (0.46, 0.99) | 0.28 (0.05, 0.50) | 0.009 | 0.43 (0.13, 0.73) | <.001 |
| Median (IQR) | 0.19 (0.11, 0.38) | 0.53 (0.43, 0.73) | 0.65 (0.43, 0.87) | 0.21 (0.10, 0.39) | 0.33 (0.21, 0.47) | ||
| PMA | |||||||
| Mean (95% CI) | 0.23 (0.14, 0.32) | 0.34 (0.26, 0.41) | 0.37 (0.29, 0.46) | 0.11 (0.02, 0.20) | 0.017 | 0.14 (0.04, 0.25) | 0.011 |
| Median (IQR) | 0.22 (0.09, 0.29) | 0.33 (0.24, 0.41) | 0.38 (0.26, 0.42) | 0.11 (−0.05, 0.18) | 0.14 (0.002, 0.22) |
Notes: mean = arithmetic mean; CI = confidence intervals; IQR = interquartile range; [C] = concentration; 0-8 h change = 0-8 h post [C] minus pre [C]; Max change = Max post [C] minus pre [C]; 2-HPMA = 2-hydroxypropylmercapturic acid (propylene oxide); 3-HPMA = 3-hydroxypropylmercapturic acid (acrolein); AAMA = 2-carbamoylethylmercapturic acid (acrylamide); CNEMA = 2-cyanoethylmercapturic acid (acrylonitrile); HEMA = 2-hydroxyethylmercapturic acid (ethylene oxide); MHBMA-3 = 4-hydroxy-2-buten-1-yl-mercapturic acid (1,3-butadiene); PMA = phenylmercapturic acid (benzene); HMPMA = 3-hydroxy-1-methyl-propylmercapturic acid (crotonaldehyde); MMA = methylmercapturic acid (methylating agents such as 4-(methylnitrosamino)-1-(3-pyridyl)-1- butanone (NNK) and N-nitrosodimethylamine (NDMA)).
Figure 1.
Concentrations of mercapturic acid metabolites of volatile organic compounds in baseline (BL) and 0-4 and 4-8 hour post-exposure urine samples. Values are geometric means and 95% confidence intervals. (*significantly different from BL, α < 0.05).
NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; NDMA, N-nitrosodimethylamine
Of nine mercapturic acid metabolites measured, the average 0-8 hour post-exposure concentrations of CNEMA (parent compound, acrylonitrile), HEMA (ethylene oxide), MHBMA (butadiene), MMA (methylating agents), and PMA (benzene) were significantly higher than pre-exposure levels. 0-8 Hour post-exposure CNEMA increased 1.7-fold, HEMA increased 1.3-fold, MHBMA-3 increased 2.1-fold, MMA increased 1.6-fold, and PMA increased 1.6-fold. The maximum post-exposure concentrations of 2-HPMA (propylene oxide) (2.3-fold increase from baseline), CNEMA (1.9-fold), HEMA (1.4-fold), HMPMA (crotonaldehyde) (1.6-fold), MHBMA-3 (2.7-fold), MMA (1.9-fold), and PMA (1.6-fold) were significantly higher than pre-exposure concentrations.
The parameters used to estimate air to urine VOC ratios and LER of death from cancer for acrylonitrile, benzene, and 1,3-butadiene are shown in Table 3. The ratios were estimated using the baseline-corrected 0-8 h post-exposure concentrations (time-weighted average concentrations); the baseline-corrected peak concentrations of the mercapturic acid metabolites (similar to a spot urine concentrations); and, the measured time-integrated PM2.5 and air nicotine. The estimated air to urine ratios of VOCs obtained using PM2.5 were approximately double the estimated ratios using air nicotine. The LER of overall cancer death, representing the sum of risks from exposure to acrylonitrile, benzene, and 1,3-butadiene emitted in SHS for adults, was 15.5×10−6 using air nicotine to estimate air VOC exposure and 28.1×10−6 using PM2.5 to estimate air VOC exposure.
Table 3.
Parameters used to estimate air to urine ratio of volatile organic compound (VOC) and computations of lifetime excess risk (LER) of cancer from exposure to acrylonitrile, benzene, and 1,3-butadiene.
| Parameters | Acrylonitrile | Benzene | 1,3-Butadiene |
|---|---|---|---|
| Background-corrected PM2.5 measured in car, (μg/m3)a | 1155 | 1155 | 1155 |
| Background-corrected nicotine measured in car, (μg/m3)a | 65.5 | 65.5 | 65.5 |
| Baseline-corrected 0-8 h urine VOC, (ng/mg creat) | 0.915 | 0.109 | 0.275 |
| Baseline-corrected max urine VOC, (ng/mg creat) | 1.150 | 0.144 | 0.429 |
| PM2.5 emission factor, (μg/cig)b | 12471 | 12471 | 12471 |
| Nicotine emission factor, (μg/cig)b | 1274 | 1274 | 1274 |
| VOC emission factor, (μg/cig)b | 170 | 431 | 279 |
| Adult respiration rate - sedentary (≥19 yrs), (m3/h) | 0.5 | 0.5 | 0.5 |
| Average hours per day exposed in car, (h/day) | 1 | 1 | 1 |
| Days per week exposed to SHS in car, (days/week) | 5 | 5 | 5 |
| Number of years exposed to SHS | 51 | 51 | 51 |
| A. Estimation of air to urine VOC ratio using PM2.5 | |||
| Estimated air VOC levels based on air PM2.5 (μg/m3) | 15.7 | 39.9 | 25.8 |
| Air VOC to 0-8h urine VOC ratio (μg/m3)•(ng/mg creat)−1 | 17.2 | 66.2 | 94.0 |
| Air VOC to max urine VOC ratio (μg/m3)•(ng/mg creat)−1 | 13.7 | 277.4 | 60.3 |
| B. Estimation of air to urine VOC ratio using air nicotine | |||
| Estimated air VOC levels based on air nicotine (μg/m3) | 8.74 | 22.2 | 14.3 |
| Air VOC to 0-8h urine VOC ratio (μg/m3)•(ng/mg creat)−1 | 9.55 | 203.3 | 52.2 |
| Air VOC to max urine VOC ratio (μg/m3)•(ng/mg creat)−1 | 7.60 | 154.0 | 33.4 |
| C. Lifetime Excess Risk (LER) for cancer deaths | |||
| EPA Air Unit Risk (×10−6) | 68 | 7.8 | 30 |
| LER for VOC predicted from PM2.5 (×10−6) | 13.9 | 4.1 | 10.1 |
| LER for VOC predicted from air nicotine (×10−6) | 7.7 | 2.2 | 5.6 |
Corrected particulate matter (PM2.5) and nicotine = inside concentration minus outside concentration;
Emission factors are average values of published secondhand smoke emission factors and summarized by Liu and colleagues in a Supplementary Material (25); U.S. EPA Air Unit Risk (AUR) are obtained on EPA’s Integrated Risk Information System at http://www.epa.gov/iris/
DISCUSSION
We present novel data on the concentrations of nine mercapturic acid metabolites of toxic or carcinogenic VOCs following 1 hour of individual exposure to SHS in a stationary car with all windows partially opened by 10 cm. Of 9 mercapturic acid metabolites measured in urine, 7 increased significantly following SHS exposure (we assessed changes in biomarkers as either within-subject 0-8 hour post-exposure concentration minus baseline concentration or within-subject peak post-exposure concentration minus baseline concentration). These include 2-HPMA (parent compound, propylene oxide), CNEMA (acrylonitrile), HEMA (ethylene oxide) HMPMA (crotonaldehyde), MHBMA-3 (butadiene), MMA (methylating agents), and PMA (benzene). The greatest increase in 0-8 h post-exposure compared to baseline was for MHBMA-3 (1,3-butadiene) (2.1-fold), followed by CNEMA (acrylonitrile) (1.7-fold), PMA (benzene) (1.6-fold), MMA (methylating agents) (1.6-fold), and HEMA (ethylene oxide) (1.3-fold). These findings provide evidence that smoking in cars leads to systemic exposure of toxic and/or carcinogenic VOCs in nonsmokers. Further, we provide the first estimates of air to urine VOC ratios that can be used to compute LERs for cancer deaths from exposure to acrylonitrile, benzene, and 1,3-butadiene based on urine VOC metabolite data. The LER of overall cancer death from exposure to these three VOCs for adults ranged from 15.5×10−6 to 28.1×10−6, depending on whether air nicotine or PM2.5 was used to estimate air VOC exposure.
Exposure to SHS in various settings including bars, casinos, and outdoor locations results in absorption of toxic tobacco smoke constituents such as tobacco-specific nitrosamines (29-31) which are known to be associated with increased risk of lung cancer (32). Besides lung cancer, SHS causes stroke, nasal irritation, reproductive effects in women, and coronary heart disease in adults, and middle ear disease, impaired lung function, lower respiratory illness, and SIDS in children (33). Given the wide array of SHS-related diseases, data on intake of tobacco smoke constituents other than tobacco-specific nitrosamines are essential to assessing SHS health risks beyond lung cancer.
The emergence of VOCs as an important class of toxicants in tobacco smoke due to their biologic activity and overall high levels in tobacco smoke underscores the need for data on VOC exposure from SHS (25). Risk assessment models show that four VOCs, namely, 1,3-butadiene, acrylonitrile, acetaldehyde, and benzene, are among the top five constituents of mainstream cigarette smoke with the highest cancer risk indices, arsenic being the other, and acrolein has the highest non-cancer risk index for respiratory effects (16). Benzene and 1,3-butadiene are known human carcinogens (Group A, U.S. EPA cancer classification). Benzene, an aromatic compound formed through incomplete combustion, is generated at average levels of 431 μg per cigarette (range, 263-590 μg per cigarette) while 1,3-butadiene, an unsaturated hydrocarbon, is generated at an average of 279 μ per cigarette (range, 157-400 μg per cigarette) [(25) Supplementary Materials]. Benzene is known to cause leukemia (34) and 1,3-butadiene causes lymphohematopoietic cancers in humans (35). Acrylonitrile (Group B1), ethylene oxide (Group B1), and propylene oxide (Group B2) are probable human carcinogens; and crotonaldehyde is a possible human carcinogen (Group C) (26). Acrylonitrile, which is suspected of causing lung cancer in humans (36, 37), is emitted at 170 μg per cigarette (range, 99-250 μg per cigarette) (25).
Our finding of substantial increases of mercapturic acid metabolites of known or suspected human carcinogens in this study supports the biological plausibility that nonsmokers exposed to SHS in cars are at increased risk of cancers of various types. We estimated an LER for overall death from cancers associated with acrylonitrile, benzene, and 1,3-butadiene under our study’s exposure conditions to range from 15.5×10−6 to 28.1×10−6 for adults, which are comparable to the LER for cancer deaths estimated from exposure to these three SHS VOCs among restaurant and bar servers in Minnesota, USA (21.4×10−6) (25). Exposure to these three chemicals alone is sufficient to increase the LER substantially above the de minimis risk of 1×10−6 (the level of risk at which regulation is not warranted).
Acrolein and acrylamide have not been shown to be carcinogenic in humans. Nonetheless, acrolein is of special interest as an etiological agent for cigarette-smoke related cancers because it causes DNA damage in the p53 tumor suppressor genes and inhibits DNA repair (38), and also because of its major effects on the respiratory tract (16). Acrolein is emitted from tobacco smoke at similar rates as benzene and 1,3-butadiene (25, 32). Despite the relatively high concentrations of acrolein in tobacco smoke, we did not find significant changes in its metabolite, 3-HPMA, following SHS exposure. This may be due to the high reactivity of acrolein such that air concentrations decline quickly due to reactions with other chemicals in air or on surfaces (39). The acrylamide metabolite, AAMA, did not increase post-SHS exposure also. It is likely that sources other than tobacco smoke, such as diet, contributed to acrolein and acrylamide exposure to a greater extent than SHS (40, 41).
VOCs are ubiquitous in the environment, as demonstrated by the measurable levels of mercapturic acids at baseline (before SHS exposure) in this study. For example, vehicle exhaust is a source of VOCs. Emission rates of select VOCs from vehicle tailpipes include: benzene, 11,900 μg/km; acrolein, 60 μg/km; crotonaldehyde, 1,760 μg/km (42); and 1,3-butadiene, 2,100 μg/km (43). In our study, baseline levels of 2-HPMA, 3-HPMA, AAMA, CNEMA, HMPMA, MHBMA-3, and PMA in our subjects were at least 2-fold lower than levels measured in nonsmokers in the U.S. general population; HEMA was 4-fold higher at baseline in the current study participants than in the U.S. general population (44). Given that tobacco smoke is a major source of VOCs to nonsmokers, lower baseline mercapturic acid levels in our subjects compared to the U.S. general population is consistent with lower smoking rates and lower levels of SHS exposure in California (particularly in the San Francisco Bay area) compared to other states (45). Comparisons of background levels of mercapturic acids across studies should be done cautiously due to differences in dietary and environmental exposures in different populations and differences in assay performance at low concentrations.
While cancer is a major concern for adults, diseases such as asthma and other respiratory outcomes are primary concerns for children. The average 1-hour time-integrated, background-corrected PM2.5 (1155 μg/m3) measured concurrently in this study exceeds threshold levels that are considered hazardous by the U.S. EPA National Ambient Air Quality Standard (NAAQS). Even when averaged over 24 h (since the NAAQS is a 24 h standard), the average PM2.5 levels correspond to an Air Quality Index that is deemed unhealthy for sensitive groups. Respiratory outcomes are expected at these high PM2.5 levels. Exposure to VOCs is also known to be associated with asthma in both children and adults (46, 47).
Compared to the mercapturic acid concentrations reported here, 24-hour post-exposure concentrations of AAMA, CNEMA, 3-HPMA, 2-HPMA, MHBMA, and PMA ranged from ~2-fold (PMA) to ~35-fold (CNEMA) higher among cigarette smokers in a recent study from our research group (21). It should be noted however, that there is no risk-free level of carcinogens, and all 7 biomarkers which increased significantly post-exposure are mercapturic acid metabolites of human or animal carcinogens.
A limitation of our study is that our exposure scenario may not be representative of most smoking situations in cars. We employed a stationary car with partially opened windows, and ventilation in this scenario is lower than most moving cars with windows in various configurations and/or air conditioning system on. We also used only one type of cigarette. While it is likely that we have overestimated SHS exposure for some people using our exposure scenario, namely, cars being driven with windows opened, air concentrations of PM2.5, CO, and nicotine previously reported from this study (23) are consistent with other studies of PM2.5, CO, and air nicotine after cigarettes are smoked in closed cars and at various ventilation system configurations (11, 48, 49). Given the lower SHS levels in cars being operated with opened windows, the LER of lung cancer may be lower than what we have estimated under the current scenario. Further, the LER of cancer risk was estimated from exposure to just the three VOCs for which we had AUR data and are human or probable human carcinogens. SHS contains a number of other carcinogenic chemicals, so our risk estimate is low. Likewise our risk estimate is low because we did not consider potential childhood exposure to VOCs. We did not do that because of difficulty modeling changing ventilatory rates across the years of childhood. We know that children experience even higher levels of systemic exposure to chemicals in SHS than do adults, and LER would have been higher had we included childhood exposure.
CONCLUSION
This is the first study, to the best of our knowledge, which shows increased excretion of mercapturic acid metabolites of toxic or carcinogenic VOCs after brief exposure to SHS. The greatest increase in 0-8 h post-exposure compared to baseline was for MHBMA-3 (1,3-butadiene) (2.1-fold), then CNEMA (acrylonitrile) (1.7-fold), PMA (benzene) (1.6-fold), MMA (methylating agents) (1.6-fold), and HEMA (ethylene oxide) (1.3-fold). These results support the idea that smoking in cars may be associated with increased risks of cancer, respiratory, and cardiovascular diseases among nonsmokers. Children and nonsmoking adults with pre-existing conditions such as asthma and a history of cardiovascular diseases should be protected from SHS exposure in cars.
ACKNOWLEDGEMENTS
The authors thank Cotys Winston for assistance in conducting the clinical studies, Charles Perrino and Minjiang Duan, Chris Havel, Lita Ramos and Lisa Yu for performing analytical chemistry, Faith Allen for data management and Scott Rostler for editorial assistance.
Authors’ research support: This study was supported by the Flight Attendants Medical Research Institute (FAMRI) (N.L. Benowitz) and US Public Health Service grant DA12393 (R.T. Jones) and grant R25CA113710 (S.A. Glantz) from the National Institutes of Health. The study was carried out in part at the General Clinical Research Center at San Francisco General Hospital Medical Center (NIH/NCRR UCSF-CTSI UL1 RR024131).
Footnotes
Potential conflict(s) of interest: NL Benowitz served on smoking cessation advisory boards for Pfizer and has been an occasional consultant to McNeil and GlaxoSmithKline, and has served as a paid expert witness in litigation against tobacco companies. The other authors declare no conflicts of interest.
REFERENCES
- 1.USDHHS . The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Centers for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2006. [PubMed] [Google Scholar]
- 2.Bondy SJ, Zhang B, Kreiger N, Selby P, Benowitz N, Travis H, et al. Impact of an indoor smoking ban on bar workers’ exposure to secondhand smoke. Journal of Occupational and Environmental Medicine. 2009;51:612–9. doi: 10.1097/JOM.0b013e31819cb222. [DOI] [PubMed] [Google Scholar]
- 3.Sargent RP, Shepard RM, Glantz SA. Reduced incidence of admissions for myocardial infarction associated with public smoking ban: before and after study. British Medical Journal. 2004;328:977–80. doi: 10.1136/bmj.38055.715683.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herman PM, Walsh ME. Hospital admissions for acute myocardial infarction, angina, stroke, and asthma after implementation of Arizona’s comprehensive statewide smoking ban. Journal Information. 2011;101:491–6. doi: 10.2105/AJPH.2009.179572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.USDHHS . Children and secondhand smoke exposure. Excerpts from the health consequences of involuntary exposure to tobacco smoke: A report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2007. [Google Scholar]
- 6.Pirkle JL, Bernert JT, Caudill SP, Sosnoff CS, Pechacek TF. Trends in the exposure of nonsmokers in the US population to secondhand smoke: 1988–2002. Environ Health Perspect. 2006;114:853–8. doi: 10.1289/ehp.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dempsey DA, Meyers MJ, Oh SS, Nguyen EA, Fuentes-Afflick E, Wu AH, et al. Determination of tobacco smoke exposure by plasma cotinine levels in infants and children attending urban public hospital clinics. Arch Pediatr Adolesc Med. 2012;166:851–6. doi: 10.1001/archpediatrics.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol. 2001;11:231–52. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 9.King BA, Dube SR, Homa DM. Smoke-free rules and secondhand smoke exposure in homes and vehicles among US adults, 2009-2010. Preventive Chronic Disease. 2013;10:E79. doi: 10.5888/pcd10.120218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nabi-Burza E, Regan S, Drehmer J, Ossip D, Rigotti N, Hipple B, et al. Parents Smoking in Their Cars With Children Present. Pediatrics. 2012;130:e1471–e8. doi: 10.1542/peds.2012-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones MR, Navas-Acien A, Yuan J, Breysse PN. Secondhand tobacco smoke concentrations in motor vehicles: a pilot study. Tob Control. 2009;18:399–404. doi: 10.1136/tc.2009.029942. [DOI] [PubMed] [Google Scholar]
- 12.Semple S, Apsley A, Galea KS, MacCalman L, Friel B, Snelgrove V. Secondhand smoke in cars: assessing children’s potential exposure during typical journey conditions. Tob Control. 2012;21(6):578–83. doi: 10.1136/tobaccocontrol-2011-050197. [DOI] [PubMed] [Google Scholar]
- 13.Willers S, Skarping G, Dalene M, Skerfving S. Urinary cotinine in children and adults during and after semiexperimental exposure to environmental tobacco smoke. Archives of Environmental Health: An International Journal. 1995;50:130–8. doi: 10.1080/00039896.1995.9940890. [DOI] [PubMed] [Google Scholar]
- 14.Jones IA, St Helen G, Meyers MJ, Dempsey DA, Havel C, Jacob P, 3rd, et al. Biomarkers of secondhand smoke exposure in automobiles. Tob Control. 2014;23:51–7. doi: 10.1136/tobaccocontrol-2012-050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nature Reviews Cancer. 2003;3:733–44. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 16.Fowles J, Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob Control. 2003;12:424–30. doi: 10.1136/tc.12.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie J, Marano KM, Wilson CL, Liu H, Gan H, Xie F, et al. A probabilistic risk assessment approach used to prioritize chemical constituents in mainstream smoke of cigarettes sold in China. Regul Toxicol Pharmacol. 2012;62:355–62. doi: 10.1016/j.yrtph.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Ding YS, Blount BC, Valentin-Blasini L, Applewhite HS, Xia Y, Watson CH, et al. Simultaneous determination of six mercapturic acid metabolites of volatile organic compounds in human urine. Chem Res Toxicol. 2009;22:1018–25. doi: 10.1021/tx800468w. [DOI] [PubMed] [Google Scholar]
- 19.Carmella SG, Chen M, Zhang Y, Zhang S, Hatsukami DK, Hecht SS. Quantitation of acrolein-derived (3-hydroxypropyl) mercapturic acid in human urine by liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry: effects of cigarette smoking. Chem Res Toxicol. 2007;20:986–90. doi: 10.1021/tx700075y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckert E, Schmid K, Schaller B, Hiddemann-Koca K, Drexler H, Göen T. Mercapturic acids as metabolites of alkylating substances in urine samples of German inhabitants. International journal of hygiene and environmental health. 2011;214:196–204. doi: 10.1016/j.ijheh.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Jacob P, Raddaha AHA, Dempsey D, Havel C, Peng M, Yu L, et al. Comparison of Nicotine and Carcinogen Exposure with Water pipe and Cigarette Smoking. Cancer Epidemiology Biomarkers & Prevention. 2013;22:765–72. doi: 10.1158/1055-9965.EPI-12-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schettgen T, Müller J, Fromme H, Angerer J. Simultaneous quantification of haemoglobin adducts of ethylene oxide, propylene oxide, acrylonitrile, acrylamide and glycidamide in human blood by isotope-dilution GC/NCI-MS/MS. Journal of Chromatography B. 2010;878:2467–73. doi: 10.1016/j.jchromb.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 23.Northcross AL, Trinh M, Liu J, Jones IA, Myers M, Dempsey D, et al. PM2.5 and polyaromatic hydrocarbons exposure from secondhand smoke in the backseat of a vehicle Tob Control. 2014;23:14–20. doi: 10.1136/tobaccocontrol-2012-050531. [DOI] [PubMed] [Google Scholar]
- 24.Ashley DL, Bonin MA, Cardinali FL, McCraw JM, Wooten JV. Measurement of volatile organic compounds in human blood. Environ Health Perspect. 1996;104:871. doi: 10.1289/ehp.96104s5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu R, Bohac DL, Gundel LA, Hewett MJ, Apte MG, Hammond SK. Assessment of risk for asthma initiation and cancer and heart disease deaths among patrons and servers due to secondhand smoke exposure in restaurants and bars. Tob Control. doi: 10.1136/tobaccocontrol-2012-050831. 2013.10.1136/tobaccocontrol-2012-050831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.US EPA [accessed May 14, 2014];Integrated Risk Information System (IRIS): A-Z List of Substances. 2014 May; cited 21 March 2014. http://www.epa.gov/iris/
- 27.US EPA . Exposure Factors Handbook (Final Report) Agency USEP; Washington, DC: 1997. [Google Scholar]
- 28.Liu R, Jiang Y, Li Q, Hammond SK. An assessment of health risks and mortality from exposure to secondhand smoke in Chinese restaurants and bars. PLoS One. 2014;9:e84811. doi: 10.1371/journal.pone.0084811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson KE, Kliris J, Murphy L, Carmella SG, Han S, Link C, et al. Metabolites of a tobacco-specific lung carcinogen in nonsmoking casino patrons. Cancer Epidemiology Biomarkers & Prevention. 2003;12:1544. [PubMed] [Google Scholar]
- 30.Hecht SS, Ye M, Carmella SG, Fredrickson A, Adgate JL, Greaves IA, et al. Metabolites of a tobacco-specific lung carcinogen in the urine of elementary school-aged children. Cancer Epidemiology Biomarkers & Prevention. 2001;10:1109–16. [PubMed] [Google Scholar]
- 31.St.Helen G, Bernert JT, Hall DB, Sosnoff CS, Xia Y, Balmes JR, et al. Exposure to secondhand smoke outside of a bar and a restaurant leads to increases in tobacco exposure biomarkers in non-smokers. Environ Health Perspect. 2012;120:1010–6. doi: 10.1289/ehp.1104413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.USDHHS . A report of the Surgeon General: How tobacco smoke causes disease: The biology and behavioral basis for smoking-attributable disease. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Centers for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2010. [PubMed] [Google Scholar]
- 33.USDHHS . The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2014. [PubMed] [Google Scholar]
- 34.Hoffmann D, Brunnemann KD, Hoffmann I. Advances in Modern Environmental Toxicology Benzene: Occupational and Environmental Hazards-Scientific Update. Princeton Scientific Publishing Co; Princeton: 1989. Significance of benzene in tobacco carcinogenesis. [Google Scholar]
- 35.Baan R, Grosse Y, Straif K, Secretan B, El Ghissassi F, Bouvard V, et al. A review of human carcinogens—Part F: chemical agents and related occupations. The lancet oncology. 2009;10:1143–4. doi: 10.1016/s1470-2045(09)70358-4. [DOI] [PubMed] [Google Scholar]
- 36.O’Berg MT. Epidemiologic study of workers exposed to acrylonitrile. J Occup Environ Med. 1980;22:245–52. [PubMed] [Google Scholar]
- 37.Scélo G, Constantinescu V, Csiki I, Zaridze D, Szeszenia-Dabrowska N, Rudnai P, et al. Occupational exposure to vinyl chloride, acrylonitrile and styrene and lung cancer risk (Europe) Cancer Causes Control. 2004;15:445–52. doi: 10.1023/B:CACO.0000036444.11655.be. [DOI] [PubMed] [Google Scholar]
- 38.Feng Z, Hu W, Hu Y, Tang M-s. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proceedings of the National Academy of Sciences. 2006;103:15404–9. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicol Sci. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- 40.Watzek N, Scherbl D, Feld J, Berger F, Doroshyenko O, Fuhr U, et al. Profiling of mercapturic acids of acrolein and acrylamide in human urine after consumption of potato crisps*. Molecular nutrition & food research. 2012;56:1825–37. doi: 10.1002/mnfr.201200323. [DOI] [PubMed] [Google Scholar]
- 41.Boettcher MI, Bolt HM, Angerer J. Acrylamide exposure via the diet: influence of fasting on urinary mercapturic acid metabolite excretion in humans. Arch Toxicol. 2006;80:817–9. doi: 10.1007/s00204-006-0123-z. [DOI] [PubMed] [Google Scholar]
- 42.Schauer JJ, Kleeman MJ, Cass GR, Simoneit BR. Measurement of emissions from air pollution sources. 5. C1-C32 organic compounds from gasoline-powered motor vehicles. Environmental Science & Technology. 2002;36:1169–80. doi: 10.1021/es0108077. [DOI] [PubMed] [Google Scholar]
- 43.Ye Y, Galbally I, Weeks I. Emission of 1, 3-butadiene from petrol-driven motor vehicles. Atmos Environ. 1997;31:1157–65. [Google Scholar]
- 44.Alwis KU, Blount BC, Britt AS, Patel D, Ashley DL. Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS) Anal Chim Acta. 2012;750:152–60. doi: 10.1016/j.aca.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lightwood J, Glantz SA. The effect of the California tobacco control program on smoking prevalence, cigarette consumption, and healthcare costs: 1989–2008. PLoS One. 2013;8:e47145. doi: 10.1371/journal.pone.0047145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arif AA, Shah SM. Association between personal exposure to volatile organic compounds and asthma among US adult population. Int Arch Occup Environ Health. 2007;80:711–9. doi: 10.1007/s00420-007-0183-2. [DOI] [PubMed] [Google Scholar]
- 47.Rumchev K, Spickett J, Bulsara M, Phillips M, Stick S. Association of domestic exposure to volatile organic compounds with asthma in young children. Thorax. 2004;59:746–51. doi: 10.1136/thx.2003.013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ott W, Klepeis N, Switzer P. Air change rates of motor vehicles and in-vehicle pollutant concentrations from secondhand smoke. Journal of Exposure Science and Environmental Epidemiology. 2007;18:312–25. doi: 10.1038/sj.jes.7500601. [DOI] [PubMed] [Google Scholar]
- 49.Sendzik T, Fong GT, Travers MJ, Hyland A. An experimental investigation of tobacco smoke pollution in cars. Nicotine & Tobacco Research. 2009;11:627–34. doi: 10.1093/ntr/ntp019. [DOI] [PMC free article] [PubMed] [Google Scholar]