Abstract
The dry-season biology of malaria vectors is poorly understood, especially in arid environments when no surface waters are available for several months, such as during the dry season in the Sahel. Here we reappraise results on the dry-season physiology of members of the Anopheles gambiae s.l. complex in the broad context of dormancy in insects and especially in mosquitoes. We examine evidence on seasonal changes in reproduction, metabolism, stress tolerance, nutrition, molecular regulation, and environmental conditions and determine if the current results are compatible with dry-season diapause (aestivation) as the primary strategy for persistence throughout the dry season in the Sahel. In the process, we point out critical gaps in our knowledge that future studies can fill. We find compelling evidence that members of the An. gambiae s.l. complex undergo a form of aestivation during the Sahelian dry season by shifting energetic resources away from reproduction and towards increased longevity. Considering the differences between winter at temperate latitudes, which entails immobility of the insect and hence reliance on physiological solutions, as opposed to the Sahelian dry season, which restricts reproduction exclusively, we propose that behavioral changes play an important role in complementing physiological changes in this strategy.
Keywords: aestivation, diapause, dormancy, dry season, geographic variation, malaria, physiology, vector biology, vector ecology
1. Introduction
The burden of malaria has lessened over the past decade, yet it is still very high with hundreds of millions of cases and over half a million deaths annually, most of which occur in sub-Saharan Africa (WHO, 2013). The principal malaria vectors in Africa are members of the Anopheles gambiae s.l. complex, which includes seven or eight sibling species. Three brackish-water species are confined to a relatively narrow range (Anopheles bwambae, Anopheles melas, and Anopheles merus) and four or five freshwater species that include Anopheles arabiensis, Anopheles gambiae s.s., and Anopheles coluzzii (which was recently split from An. gambiae s.s., formerly known as the S and M molecular forms, respectively; Coetzee et al., 2013). Malaria is vectored by all sibling species except the two freshwater species that typically feed on animals: Anopheles quadriannulatus and Anopheles amharicus (formerly known as species B of An. quadriannulatus; Coetzee et al., 2013). Unlike the brackish-water vectors, An. gambiae s.s., An. coluzzii, and An. arabiensis transmit malaria over vast ranges of sub-Saharan Africa, including dry savannahs and semi-arid areas of the Sahel. The means by which these mosquitoes (and malaria) persist in areas without surface waters for three to eight months a year has been one of the long-standing questions in malariology because no stage of the vector is known to survive for over 1-2 months under such conditions (Coluzzi, 1964; Davidson, 1964; Donnelly et al., 2002; Gillies and De Meillon, 1968; Omer and Cloudsley-Thompson, 1968). The rapid build-up of mosquito density after the first rains indicates that they persist locally, possibly via aestivation (summer diapause), or arrive shortly after the rains by long-distance migration (Adamou et al, 2011; Lehmann et al., 2010, 2014). Here we review the current knowledge of the eco-physiological mechanisms that allow persistence of mosquitoes, and thus malaria, in such dry habitats and identify key gaps that future research might fill.
The African Sahel is a belt ∼1,000 km wide and ∼5,400 km long between the Sahara desert in the north and the Sudan Savannah in the south. It has a short wet season (June-October), when 90% of the annual rain (∼500 mm) falls, and a long dry season (November-May, Figure 1). The ephemeral surface waters that abound from June to October may last until December, based on local conditions. From November to May, rainfall is negligible and no surface waters are available over vast expanses of land. The conditions in most of the Sahel are drier than those depicted in Figure 1, which illustrates conditions around the Sahel's southern border (Segou, Mali). The dry season is divided into cold (November-February) and hot (March-May) periods, but outdoor temperatures rarely fall below 15°C or above 40°C (Figure 1) and the temperature range is considerably narrower indoors, in tree holes, or in burrows underground. The daily fluctuation in air temperature is greater during the dry season (Figure 1). Air moisture (measured as dew point) is elevated from late April to October, but is very low (RH∼20%) during much of the dry season (Figure 1). For mosquito activity, the absence of surface water is by far the most restrictive element in this environment. The low humidity during the dry season probably confines activity to short flights during more humid nights, but water is available in every house (e.g., pots with water, fruits), in wells and seepages nearby, and in flowers' nectar and woody-plant juices. Without suitable larval sites, the eggs, larvae, and pupae cannot survive beyond a few days (Beier et al., 1990; Minakawa et al., 2001). The adults typically survive for only a few weeks, which would spell doom to the population, unless a different strategy can be used by mosquitoes to cope with the 5-7 month-long dry season. The only strategy that has been supported by direct evidence is a ῀7-fold extension of (normal) adult life span (Holstein, 1954; Lehmann et al., 2010; Omer and Cloudsley-Thompson, 1968, 1970). This life-span extension has often been referred to as aestivation (Box 1), but, until recently, had not been subjected to rigorous physiological analysis. The main aim of this review is to evaluate how new results (mostly over the last decade) on the ecophysiology of Anopheles gambiae fit within the framework of aestivation as a mechanism of dry-season survival.
Figure 1.
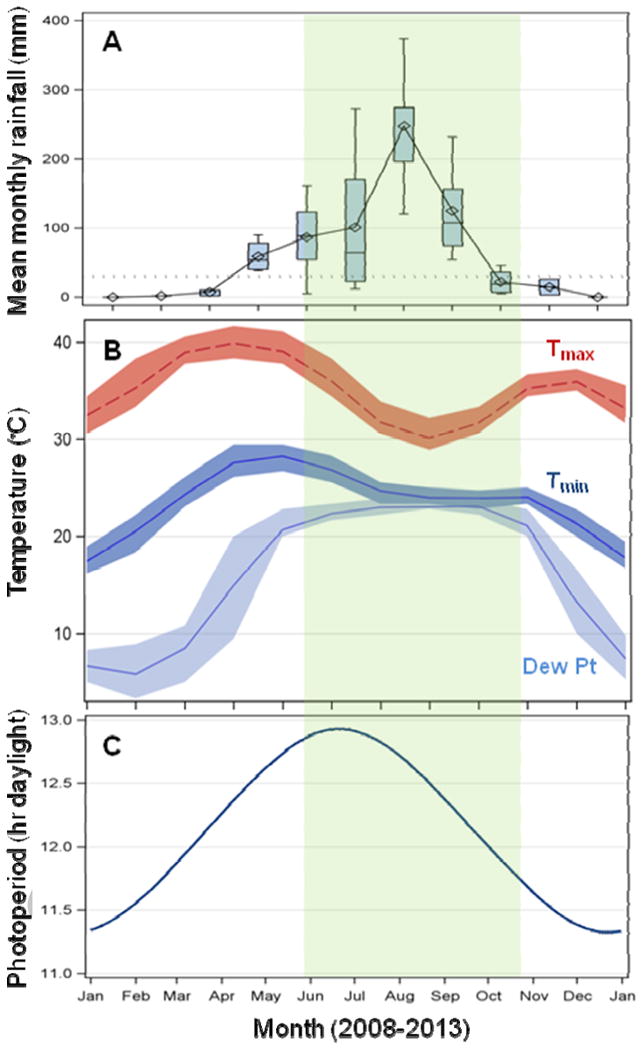
Climatic conditions recorded from 2008-2013 by a governmental weather station at Segou, Mali (13.45°N, 6.26°W), a village at the southern edge of the Sahel; north of this location, conditions are drier. The period defined as the wet season (June through mid-October) is shaded in light green throughout. A) Total monthly rainfall (in mm), shown as a box-whisker plot (box stretches from the 1st to the 3rd quartiles and whiskers extend to the extreme values up to 1.5 times the inter-quartile range). Mean monthly rainfall depicted with diamonds; mean annual rainfall is 570 mm with a range of 225-964 over the 5-year period. Dotted line marks 30 mm rain, representing the minimal single rain event providing enough water to keep some larval sites for the duration of complete development of the aquatic stages of Anopheles gambiae s.l. B) Daily maximum temperature (red; measured at 15:00), minimum temperature (dark blue; measured at 06:00), and dew point (light blue; measured at 06:00). Shaded bands represent the range between 1st and 3rd quartiles. C) Daily photoperiod, with the number of daylight hours (sunrise to sunset) shown.
Box 1. Terminology.
-
Dormancy is a broad term used for the ephemeral state of depressed growth, development, reproduction (in reproductively mature organisms), metabolic rate, and activity linked to unfavorable environmental conditions. Both quiescence and diapause are different forms of dormancy which are well known in insects.
-
1.1.
Diapause is a pre-programmed dormant state, initiated by token stimuli (e.g., photoperiod) in anticipation of future unfavorable environmental conditions and requires a minimum period of latency before termination. The term ‘diapause syndrome’ expresses its many phenotypic and physiological manifestations.
-
1.1.1.
Overwintering diapause (hibernal diapause) is the diapause syndrome associated with cold temperatures typical of the winter in temperate latitudes and high altitudes. It involves a build-up of nutritional reserves ahead of time, reduced metabolism, little or no feeding, cessation of reproduction (in reproductively mature adults), and increased desiccation- and cold-tolerance.
-
1.1.2.
Aestivation (summer diapause) is the form of diapause associated with low humidity and/or high temperatures prevailing through the dry season in tropical and sub-tropical climatic zones. It may involve some or all of the following: a build-up of nutritional reserves ahead of time, reduced metabolism, little or no feeding, cessation of reproduction (in reproductively mature adults), and increased desiccation- and heat-tolerance.
-
1.1.1.
-
1.2.
Reproductive diapause is the seasonality-related arrest of ovarian development in an early previtelogenic stage, used as an indicator of diapause in adult insects and typically accompanied by depressed metabolism, activity, and feeding. In diapausing (overwintering) mosquitoes, two forms of this condition are often cited (gonotrophic dissociation and gonotrophic concordance).
-
1.2.1.
Gonotrophic dissociation is a form of reproductive diapause in which females continue to blood-feed, but eggs remain undeveloped.
-
1.2.2.
Gonotrophic concordance is a form of reproductive diapause in which females stop blood-feeding and eggs remain undeveloped.
-
1.2.1.
-
1.3.
Quiescence is a dormant state that is initiated as a direct response to unfavorable environmental conditions (e.g., desiccation, extreme temperatures) and ends when favorable conditions resume (e.g., the sleeping midge, Polypedilum vanderpanki, can survive extreme desiccation and lose >95% of its water, yet resume its life processes upon rehydration; Keilin, 1959).
-
1.1.
Migration is the intentional, directional movement away from an area in anticipation of future unfavorable conditions (similar to those which initiate diapause) and into a favorable environment, often located a considerable distance away.
Seasonal polyphenism refers to seasonal phenotypic changes in morphology, coloration, physiology, and/or behavior without the dramatic suppression of metabolism, activity, growth, development, or reproduction that characterize dormancy (above). Although distinct from acclimation, some of the physiological changes might be augmented by acclimation.
Seasonality in insects (and invertebrates) refers to suites of phenotypic changes that increase the prospects of survival through an inhospitable period until favorable conditions resume (Andrewartha, 1952; Denlinger and Armbruster, 2014; Dingle, 1996; Kostal, 2006; Masaki, 1980; Tauber et al., 1986). These include migration away from the inhospitable environment and forms of dormancy such as quiescence and diapause (Box 1). The characteristic diversity in the expression of diapause (Andrewartha, 1952) led to the term “diapause syndrome,” which highlights the challenge in classifying these physiological states. Whether the physiology of aestivation is similar to that of overwintering diapause is difficult to resolve, because very few studies have addressed this topic in aestivating insects and less than a handful have addressed this subject in mosquitoes. Thus, in recent reviews of diapause in mosquitoes, aestivation was mentioned with respect to a single (or two) species as opposed to over 50 with respect to hibernation (Denlinger and Armbruster, 2014; Vinogradova et al., 2007). This is surprising, given the overwhelming evidence that seasonal adaptations are critical for mosquitoes and thus for disease transmission (Denlinger and Armbruster, 2014; Jetten and Takken, 1994). Here we will draw broadly on the ecophysiology of mosquitoes living through inhospitable seasons, and consider strategies relevant for mosquito survival of the Sahelian dry season in-situ. We emphasize recent findings on the seasonal ecophysiology of anophelines, and (i) explore the physiological mechanisms used to survive the long dry season, (ii) assess whether these known mechanisms sufficiently explain mosquito persistence, (iii) evaluate if these strategies are in accordance with different forms of dormancy, and (iv) propose, based on a synthesis of i-iii above, mechanisms used by African anophelines. In the process, we identify key gaps in our knowledge of the dry-season ecophysiology of African anophelines, which, if filled, could yield novel vector control methods in these seasonal habitats.
2. Environmental cues used to initiate diapause
A key hallmark of diapause, whether overwintering diapause or aestivation, is that it is a preprogrammed suite of physiological changes in response to one or more token external stimuli that predict the onset of future environmental changes which will require these changes for survival (Box 1). However, the diapause-inducing cues for many insects remain unknown. Here we will describe some cases in which the induction cues have been well-studied, and propose some that could be utilized by An. gambiae s.l. in the Sahel.
2.1 Cues used by insects to induce winter diapause
Depending on latitude, extreme cold and lack of nutritional resources during winter are predictable by decreasing photoperiod (Danilevskii, 1965). As such, much research has explored the role of photoperiod in the induction, maintenance, and termination of winter diapause in a wide variety of taxa, including several mosquito species. For example, in Culex tarsalis, decreased photoperiod induces adult females to shift into a hibernal diapause initiation phase by accumulating fat reserves while simultaneously decreasing ovary size (Harwood and Halfhill, 1964). These findings were further supported by a study comparing the simultaneous effects of photoperiod and temperature on Cx. tarsalis and Culex restuans, which found that short-day photoperiod was more influential than temperature in determining (adult) winter diapause for both species (Buth et al., 1990). However, temperature was found to be of secondary importance, as under long days, the coolest temperature produced diapausing females as compared with the 2 warmer treatments (Buth et al., 1990). Similarly, short-day photoperiod stimulated the production of winter-diapausing eggs of Aedes albopictus both in its native habitat in Japan and across its newly acquired range in the United States (Focks et al., 1994; Lounibos et al., 2003, 2011; Urbanski et al., 2012). This response varied significantly with latitude, such that northern populations had a higher diapause incidence in response to differences in photoperiod than southern populations (Focks et al., 1994; Lounibos et al., 2003, 2011; Urbanski et al., 2012). Similarly, populations of introduced Ae. albopictus in southern Brazil have evolved photoperiod-induced diapause despite having arisen from non-diapausing colonizers (Lounibos et al., 2003). Geographic variation in photoperiodic induction of winter diapause was also found across the range of the mosquito Aedes atropalpus in North America (Beach, 1978).
Photoperiod as a cue to enter winter diapause has also been studied in several anopheline species. For example, short photoperiod was found to cue overwintering diapause in adult Anopheles freeborni (Depner and Harwood, 1966). Importantly, the critical photoperiod for entering winter diapause varied among populations from different latitudes (Depner and Harwood, 1966; Washino, 1970), providing appropriate, fine-tuned diapause responses across the geographic range of the species. Photoperiod has also been implicated as one of the key factors to stimulate winter diapause in Anopheles punctipennis (Washino and Bailey, 1970) and Anopheles messeae (Jaenson and Ameneshewa, 1991).
Other environmental factors, including temperature and nutritional quality, are also used by arthropods to indicate oncoming seasonal environmental changes (Danilevskii, 1965). In deep aquatic environments, photoperiod may not be as detectable as temperature, and temperature was found to be an important cue for winter diapause in aquatic copepods (Hairston and Kearns, 1995). However, for the terrestrial environments that adult mosquitoes inhabit, photoperiod appears to be the most common cue for initiating winter diapause. It is not clear if studies on cues of diapause induction in mosquitoes ignored other factors, such as larval nutrition quality/composition, water salinity, acidity, and hormonal changes in their vertebrate hosts; however, the consensus from these studies remains that short photoperiod (with length depending on latitude) is the key signal used to induce overwintering diapause in mosquitoes.
2.2 Cues used by insects to induce aestivation
For insects in tropical and sub-tropical climates, where variation in daylength is smaller, several environmental conditions (not excluding photoperiod) can be reliable cues used to initiate aestivation. For example, summer diapause in some lepidopterans is presumed to have evolved in anticipation of a decrease in food availability, and is triggered in response to long photoperiods and high temperatures. Specifically, long daylengths induce aestivation of the cabbage butterfly, Pieris brassicae, from southern Spain, while short daylengths cause other populations from northern Europe to enter winter diapause (Held and Spieth, 1999). Thus, long and short daylengths induce summer diapause and overwintering diapause, respectively, across the large geographic range of this species and these phenomena serve to synchronize the resulting generation while protecting them from inhospitable conditions. Similarly, a short summer aestivation is induced by long daylength in the tiger moth Cymbalophora pudica, a species which also undergoes a long winter diapause in response to short daylengths (Kostal and Hodek, 1997; Kostal et al., 1998). In another moth species, Helicoverpa armigera, summer diapause is induced by high summer temperatures, not long photoperiods; however, winter diapause in this species is induced by short photoperiods (Liu et al., 2006). The manifestation of both summer and winter diapause in the same species (by the same or different populations) has contributed to the presumption that similar physiological processes are involved in each case. Yet, empirical evidence to compare these forms of diapause within species and with others manifesting diapause only in one season is still lacking.
The butterfly Bicyclus anynana in sub-Saharan Africa experiences distinct wet and dry seasons, and reproduction is suppressed during the dry season, described as reproductive summer diapause by researchers. Temperature was found to affect reproductive output while food availability affected both reproduction and longevity of these butterflies (Brakefield et al., 2007). Additionally, those butterflies reared in cool and dry conditions are more stress-resistant than those that are not; combined, this species appears to use several environmental cues (temperature, humidity, and food availability) to initiate diapause. Similarly, tropical grasshoppers which experience a dry season reduce their fecundity in response to a low-quality diet, presumably as a mechanism to enhance their own survival and that of the few offspring produced (Luker et al., 2002). In this study, both shortened photoperiod and lower food quality were required to stimulate this reproductive change (Luker et al., 2002), possibly to ensure that aestivation would only be entered at the appropriate season. High temperatures, low plant nutrients, and crowding have been shown as cues used by the sycamore aphid, Drepanosiphum platanodis, to shift into aestivation expressed as reproductive suppression, low activity, and changes in nutrient composition (Chambers, 1982; Dixon, 1966; Dixon et al., 1993; Douglas, 2000).
Aestivation by adult female Culiseta inornata mosquitoes has been observed in southern California, presumably as a mechanism to avoid the hottest part of the summer (Barnard and Mulla, 1977, 1978). This species also undergoes winter diapause in response to shortened photoperiod (Buth et al., 1990; Hudson, 1977). Although long daylengths and short daylengths induced fat body hypertrophy and hypotrophy, respectively, the cues which initiate aestivation of Cs. inornata are not as clear (Barnard and Mulla, 1977, 1978; Denlinger and Armbruster, 2014) and have not yet been fully modeled in the laboratory setting (Reisen et al., 1989).
Moisture availability is a critical, but often overlooked, factor in studies investigating the environmental triggers of diapause, but often is more reliable than either temperature or photoperiod in tropical and sub-tropical environments (Tauber et al., 1998), such as that experienced by mosquitoes in the Sahel. However, changes in moisture (in the form of surface-water availability and/or humidity) may act as a cue to reinforce or maintain diapause, rather than initiate it, because it lacks the predictive properties of photoperiod, given that a decrease in moisture means the environmental stress has already begun. Several studies have indirectly measured the effects of changing oviposition habitat on life-history traits of An. gambiae. Female An. gambiae avoid oviposition in otherwise acceptable larval sites which contained eggs or larvae of Culex quinquefasciatus (Wachira et al., 2010). Therefore, the disappearance of typical larval sites and use of potential alternative, albeit atypical, larval sites (e.g., wells, pit latrines) by competitors could act as another possible cue to initiate aestivation, although, like moisture, oviposition-deprivation is a result of the dry season rather than a predictor of it. Contrary to expectations, oviposition-deprivation of female mosquitoes of a laboratory colony of An. gambiae (G3), with or without multiple bloodmeals, did not increase female longevity and instead reduced it by a few days relative to females with the opportunity for regular gonotrophic cycles (Artis et al., 2014). Notably, this strain has been in colony for about 60 years, and therefore may not reflect the behavior of wild mosquitoes from areas with an annual dry season.
In the case of An. coluzzii, aestivation has been proposed as a mechanism for adult mosquitoes to extend their longevity over the long Sahelian dry season in order to reproduce at the beginning of the next rainy season (Adamou et al., 2011; Huestis et al., 2012; Lehmann et al., 2010, 2014; Yaro et al., 2012). Our recent studies revealed that this species declines rapidly about a month before larval sites disappeared (Lehmann et al. 2010, 2014; Dao et al., in prep), in accordance with anticipation of the coming dry season based on an unknown) token stimulus. Although Sahelian anopheline populations are located within tropical latitudes, photoperiod may still act as an important cue for aestivation; previous studies have suggested that any latitude above 10° has a large enough seasonal change in photoperiod to significantly affect the life-history traits of insects (Denlinger, 1986; Denlinger and Armbruster, 2014). The seasonal change in photoperiod experienced by these mosquito populations is around two hours (Figure 1) and the daily rate of change in photoperiod may also be used as a cue. Just as a shortened photoperiod precedes the oncoming winter and can act as a cue for winter-diapausing insect species, photoperiod decreases at the end of the wet season in the Sahel and could be used by anophelines to predict the upcoming dry season. Previous research has shown that decreased daylength increased longevity of both Anopheles crucians (Lanciani, 1993) and Anopheles quadrimaculatus (Lanciani and Anderson, 1993) from Florida, despite being nondiapausing in their native habitat. Research recently completed in our lab indicated a modest increase in longevity under a photoperiod which mimicked dry-season conditions in the field (Huestis et al., in prep). Additionally, under short photoperiod (11.5 hrs), mosquitoes from a well-known laboratory colony of An. gambiae (G3; a mix of M- and S-forms) exhibited a larger body size and a greater total amount of cuticular hydrocarbons than mosquitoes under long photoperiod (13.5 hrs; Wagoner et al., 2014). Therefore it seems possible, if not likely, that a decreased photoperiod, perhaps in conjunction with lower humidity and/or other factors, acts as a cue to initiate aestivation of Sahelian anophelines. However, much future research in this area is required.
3. Changes in reproduction and blood-feeding
Another key hallmark of mosquito diapause (overwintering or aestivation) is a dramatic shift in feeding (sugar vs. bloodmeals, or avoiding both) and a decrease in reproduction (for those species which diapause as adults; Box 1).
3.1. Reproduction and blood-feeding during insect winter diapause
Other than extended survival, reproductive arrest is arguably the ultimate hallmark of diapause in adult mosquitoes (Bates, 1949; Clements, 1963; Rao, 1947; Swellengrebel, 1929; Washino, 1977). Typically, both mosquito reproduction and blood-feeding are depressed during diapause (Bates, 1949; Clements, 1992; Washino, 1977). Blood-feeding during or prior to diapause is an avenue that pathogens taken in the bloodmeal may use to persist in diapausing mosquitoes throughout the winter (or the summer), especially if transmission halts and the pathogen is cleared from vertebrate hosts, as has been suggested for certain viruses (WEE, JE, and SLE) in Culex spp. For example, in Cx. tarsalis, decreased photoperiod induces adult females to shift into the hibernal-diapause initiation phase by accumulating fat reserves while simultaneously decreasing ovary size (Harwood and Halfhill, 1964). Notably, feeding response prior to diapause did not change with photoperiod, but rather the way in which nutritional resources are used; this shift in resource distribution was reinforced and strengthened by lower temperatures (Harwood and Halfhill, 1964). The terms reproductive- or ovarian-diapause typically refer to arrested ovarian development in an early previtelogenic stage as an indicator of diapause (Vinogradova, 1960; Vinogradova et al., 2007). However, it also implies that other indicators of diapause such as depressed metabolism, flight activity, sugar-feeding and even blood-feeding, may not be manifested by females in reproductive diapause. Egg size (e.g., follicular length) below a species-specific threshold has often been used as a marker of diapause especially in Culex spp. (Eldridge, 1987; Washino, 1977). Female mosquitoes in winter diapause may (i) avoid feeding altogether (e.g., An. maculipennis messae), (ii) feed on sugars exclusively (e.g., Culex pipiens), or (iii) take blood-meals with or without sugars (e.g., Anopheles labranchiae atroparvus). In diapausing anautogenous mosquitoes (which require a bloodmeal to mature even their first egg batch), “gonotrophic concordance,” refers to state i or ii (above) where eggs remain undeveloped because no blood-meal is taken, whereas “gonotrophic dissociation,” only known in anophelines, refers to state iii, where eggs remain undeveloped even after taking blood-meals (Vinogradova et al., 2007; Washino, 1977; Box 1).
3.2. Reproduction and blood-feeding during insect aestivation
For aestivating mosquitoes, the situation is less clear. Laboratory studies on Cs. inornata showed that under long photoperiod (summer daylight), blood-feeding rates in females from southern populations dropped from 60 to 20% (Barnard and Mulla, 1977), suggesting that gonotrophic concordance was the primary mechanisms of aestivation. All females that blood-fed developed eggs normally, but, unlike females raised under short photoperiod, most retained their eggs (for over 2 weeks) when offered water for oviposition, suggesting that gravid females may also aestivate (Barnard and Mulla, 1977, 1978). Results of field studies suggested that all aestivating females took at least one blood-meal and were parous (Barnard and Mulla, 1978). There is large variation in mode and degree of diapause among populations of Cs. inornata. In southern California, this species is active during the cool winter and it aestivates during the summer as described above, while in central California, populations exhibited no (reproductive) diapause during the hot summer although their activity was markedly depressed (Reisen et al., 1989). In contrast, Canadian populations of Cs. inornata are active through the summer yet undergo winter diapause (Buth et al., 1990; Hudson, 1977).
Populations of Anopheles arabiensis in the Sudan, over 20 km away from the Nile with no surface water available, exhibited gonotrophic dissociation, whereas females from populations along the Nile developed eggs normally and larvae were found throughout the dry season in puddles along the river (Omer and Cloudsley-Thompson, 1970). When raised under conditions similar to the natural environment away from the river, survival was extended up to 206 days, providing further evidence for aestivation (Omer and Cloudsley-Thompson, 1968; Table 1). However, results of similar studies in other parts of Africa have not corroborated these findings (Charlwood et al., 2000; Ramsdale and Fontaine, 1970a, b). Recent studies on An. coluzzii (previously known as the M molecular form of An. gambiae; Coetzee et al., 2013) showed that in a Sahelian population, reproduction was depressed sharply during the dry season, while the blood-feeding response did not change seasonally (Yaro et al., 2012; Table 1). Furthermore, a high rate of blood feeding in this population was noted among mosquitoes collected indoors throughout the dry season (Adamou et al., 2011; Huestis et al., 2012; Lehmann et al., 2010). The oviposition rate dropped from 70% (wet season) to 20% (dry season), and in those females that actually laid eggs, the mean number of eggs per female fell significantly, from 173 to 101 (Yaro et al., 2012). Correspondingly, the fraction of females that exhibited gonotrophic dissociation increased over the dry season from 5% to 45%, while a similar fraction of the population retained developed eggs despite having access to water (Yaro et al., 2012). Notably, less extreme changes were measured in a population from along the Niger River. Finally, artificial larval sites constructed and maintained during the Sahelian dry season were used by culicine mosquitoes but not by An. coluzzii or other Anopheles spp.), consistent with latency of this physiological state which is not easily broken (Lehmann et al., 2010). As noted above, studies using the G3 colony of An. gambiae showed that oviposition-site deprivation alone was insufficient to shift mosquitoes into reproductive quiescence and extended longevity (Artis et al., 2014; Dieter et al., 2012). During the dry season, Anopheles funestus exhibited a reduced proportion of gravid females and a higher proportion of partly blood-fed females compared with wet season, suggesting that reproduction is suppressed in at least a fraction of the population (Charlwood et al., 2013).
Table 1.
Summary of physiological comparisons relevant to the dry-season persistence of the members of Anopheles gambiae s.l. complex.
| Diapause trait | Appearance in An. gambiae s.l. complex | Field/Laboratory | Agreea | Reference |
|---|---|---|---|---|
| Longevity extension | >7 months in the dry season in Sudan - An. arabiensis | Field: insectary | Yes | Omer and Cloudsley-Thompson, 1968 |
| ∼7 months in the dry season in Mali - An. coluzzii | Field: mark recatpure | Yes | Lehmann et al., 2010 | |
| Reproductive arrest | Gonotrophic dissociation -An. arabiensis | Field | Yes | Omer and Cloudsley-Thompson, 1970 |
| ∼70% reduction in female reproductive output - An. coluzzii | Field | Yes | Yaro et al., 2012 | |
| Continued male presence and swarming -An. coluzzii | Field | No | Yaro et al., 2012 | |
| Suppression of activity | Suppression of flight - An. coluzzii | Field | Yes | Huestis et al., 2012 |
| Metabolic suppression | Elevated in the late dry season - An. coluzzii | Field | No | Huestis et al., 2012 |
| Photoperiod induction | ∼1.6-fold extension of life in fresh laboratory colony - An. coluzzii | Laboratory | Partly | Huestis et al., in prep |
| ∼1.7-fold extension of life of wild mosquitoes in field insectary | Field: insectary | Partly | Kassogue et al., unpublished | |
| Stress tolerance: desiccation | ∼1.3-fold higher in An. coluzzii vs. An. gambiae | Laboratory (F1s) | No | Lee et al., 2009 |
| ∼1.3-fold higher in <24 hr-old 2 La vs. 2La+ homozygotes | Laboratory | No | Fouet et al., 2012; Gray et al., 2009 | |
| ∼1.5-fold higher in dry-season vs. wet-season An. coluzzii | Field | Partly | Dao et al., unpublished | |
| Stress tolerance: high temperature | ∼1.8-fold increase in An. arabiensis vs. An. gambiae | Laboratory | Yes | Kirby and Lindsay, 2004 |
| No difference in larvae and pupae of 2La vs. 2La+ homozygotes | Laboratory | No | Rocca et al., 2009 | |
| No increased tolerance to high temperature - An. coluzzii. | Field | No | Dao et al., unpublished | |
| Cuticular hydrocarbons | 28% increase in G3 females (virgin only) under short photoperiod | Laboratory | No | Wagoner et al., 2014 |
| 5-fold increase in dry-season vs. wet-season An. coluzzii in Mali | Field | Yes | Huestis et al., in prep |
Indicates overall agreement with aestivation predictions in direction, biological magnitude (arbitrarily defined as ≥1.5-fold), and statistical significance. “Partly” refers tocases where the effect's direction and statistical significance were in agreement with aestivation, but the magnitude of the effect was below this expectation.
As far as we know, aestivation in mosquitoes has been studied only in Cs. inornata, An. arabiensis, and An. coluzzii. The degree of reproductive inhibition exhibited by apparently aestivating An. arabiensis, An. coluzzii, and Cs. inornata varied greatly between populations only tens of kilometers apart, but was evident during the dry season in at least one population of each species. Gonotrophic dissociation and gonoactive females that retained eggs (when offered water for oviposition) were observed under field conditions even within the same population (Barnard and Mulla, 1978; Omer and Cloudsley-Thompson, 1970; Yaro et al., 2012). Such heterogeneity may represent different bet-hedging strategies with respect to reproductive-diapause strength and duration. Heterogeneity along similar lines was noted in overwintering Cx. pipiens, Cx. tarsalis, and Culex tritaeniorhynchus (Reisen et al., 2010; Spielman, 2001; Spielman and Wong, 1973; Tsuda and Kim, 2008), suggesting that such variation is not unique to aestivation. Yaro et al. (2012) proposed that the composition of “weak aestivators” and “strong aestivators” differs among populations, reflecting the severity and prospects of reproductive opportunities during the dry or cold seasons. These findings parallel earlier studies on overwintering larval diapause of Wyeomia smithii, in which the “depth of diapause” was found to vary latitudinally among populations, such that southern populations entered dormancy later in development and could emerge from diapause faster and with less environmental input than northern populations (Bradshaw and Lounibos, 1977).
Unlike Cs. inornata and even An. arabiensis, An. coluzzii males were detected throughout the dry season, albeit in very small numbers (Adamou et al., 2011; Huestis et al., 2012; Lehmann et al., 2010; Yaro et al., 2012). Small swarms were occasionally observed with 1-6 males per swarm, indicating that male reproductive activity is not arrested (Yaro et al., 2012; Table 1), although it might be greatly reduced. The presence of males throughout the dry season is a radical deviation from expectations for mosquito overwintering diapause. The significance of this is unclear, as is whether males were missed in studies of aestivation of Cs. inornata or An. arabiensis. An. coluzzii females exhibited a lower rate of gonotrophic dissociation (Yaro et al., 2012) compared with that of An. arabiensis (Omer and Cloudsley- Thompson, 1970), although both species exhibited high rate of blood-feeding. It is unclear if these are species-specific or population-specific differences.
4. Metabolic rate, nutritional reserves, and activity level during diapause
For many species, a decrease in metabolic rate is a key trait of overwintering diapause, often accompanied by an increase in nutritional reserves to sustain the individual during the diapause period and a decrease in activity level so that these reserves will last longer.
4.1 Metabolic rate during diapause
During winter diapause, a dramatic decrease in metabolic rate occurs in many insects (Clarke and Fraser, 2004; Denlinger, 2002; Guppy and Withers, 1999; Hahn and Denlinger, 2011). However, the magnitude of the decrease cannot be attributed fully to low temperatures alone; rather it is lowered by 50-90% beyond what is expected by the scaling with temperature (Guppy, 2004; Guppy and Withers, 1999; Storey and Storey, 1990, 2004), not including those organisms which enter a cryptobiotic state (extreme slow down/arrest of life processes that accompanies survival over many years). A few well-documented examples include a decrease in metabolic rate in the fly Rhagoletis pomonella (Ragland et al., 2009) and in adult female Cx. pipiens mosquitoes (Benoit and Denlinger, 2007). The decrease in metabolic rate during insect winter diapause has been previously reviewed thoroughly (e.g., Clarke and Fraser, 2004; Denlinger and Armbruster, 2014; Guppy, 2004; Hahn and Denlinger, 2011; MacRae, 2010); therefore, we will focus on metabolic processes during aestivation and use winter diapause for comparative purposes only.
Some species undergo both summer and winter diapause. For example, adults of the alfalfa weevil Hypera postica aestivate in the summer, actively feed and reproduce in the fall, and their eggs undergo winter diapause until the next spring (Tombes, 1964). During adult aestivation, the mean metabolic rate drops to about one-quarter of its mean during the active phase, and remains low for 3 months (Tombes, 1964). This drop in metabolic rate is also associated with a linear decrease in the size of the fat body during aestivation and a decrease in water content; together, these results suggest that these aestivating weevils are inactive and conserve resources - similar to winter diapause. Likewise, the leaf beetle Zygogramma suturalis has a short aestivation in summer and a long winter diapause, both in the adult phase and found at different frequencies in different years (Vinogradova and Pantyuchov, 1995). The metabolic rate (measured as oxygen consumption) of female beetles was approximately 2-5 times lower in both aestivating and diapausing individuals compared with the spring and fall active phases (Vinogradova and Pantyuchov, 1995), indicating that the physiological mechanisms between the two types of dormancy may be similar. For males, the metabolic rate during aestivation was not as low as that during diapause, but both were lower than normal active levels (Vinogradova and Pantyuchov, 1995). Insects may undergo aestivation at different developmental stages, which may also impact what physiological changes occur. For example, during pupal aestivation in the tiger moth C. pudica, the metabolic rate decreased to 5-15% of its normal level, and morphological development is halted (Kostal et al., 1998). In contrast, two species of tropical butterfly in the genus Euploea undergo adult aestivation in the cool dry season, characterized by an increased fat body and a lack of egg production (Canzano et al., 2006). For both of these species, the reduction in metabolic rate during this state was estimated at about 28% (Canzano et al., 2006), a less dramatic decline than the range reported above for winter-diapausing insects.
Although metabolic rate was measured in An. coluzzii during the dry season, it is unknown if these mosquitoes were actually in aestivation because they were found indoors and most had recently blood-fed (Huestis et al., 2012). However, some factors which affect metabolic rate have been previously studied in other anophelines and may allow us to make additional predictions about scenarios during dry-season aestivation. For example, metabolic rate of An. quadrimaculatus adults varied with photoperiod: metabolism was higher under long days than under short days, but for mosquitoes collected in the fall, the reverse was found (Lanciani and Anderson, 1993). Furthermore, although this study was presumably conducted using a non-aestivating Floridian population (see above), the short-day photoperiod was always associated with increased longevity, regardless of collection time (Lanciani and Anderson, 1993). For wet-season anophelines in the Sahel, metabolic rate was significantly affected by body size, female gonotrophic status, flight activity, and temperature (Huestis et al., 2011). Huestis et al. (2012) measured seasonal variation in metabolic rate of An. coluzzii and compared a Sahelian population with a riparian population; we found significant seasonal variation at the Sahelian site but not at the riparian site after adjusting for the effect of temperature (Huestis et al., 2012). Surprisingly, metabolic rate at the Sahelian site was the highest in the late dry season, even after accounting for temperature and other factors (Huestis et al., 2012), indicating that these mosquitoes which are hypothesized to be undergoing aestivation do not have a reduced metabolic rate (Table 1). However, because the mosquitoes used in this experiment were collected inside houses and most were freshly blood-fed, they may represent mosquitoes which have temporarily suspended or totally broken aestivation, and thus may not display the same physiological characteristics as mosquitoes which are in shelters. Previous studies have shown that starvation does not decrease metabolic rate in flies (Djawdan et al., 1997) or in crickets (Sinclair et al., 2011) once the effects of fuel-use have been accounted for (since the amount of CO2 produced varies whether the insect is burning lipids or carbohydrates). However, another study showed that starvation slightly decreased an insect's metabolic rate but then resulted in an extreme increase once feeding did occur (Bennett et al., 1999). Additionally, one recent study on diapausing plant bugs (Lygus hesperus) did not find any difference in metabolic rate between winter-diapausing and non-diapausing individuals, because brief periods of activity and feeding occurred (Brent et al., 2013). In conclusion, we predict that mosquitoes hidden in cool, underground shelters may exhibit a reduced metabolic rate (Clarke and Fraser, 2004) while in a dormant, energy-conserving state, but that those mosquitoes which are seeking bloodmeals aboveground no longer show this reduction. It is also unknown if they revert to a reduced metabolism once they presumably return to these shelters after feeding (Huestis et al., 2012); however, pulses of increased metabolic activity followed by a return to lower levels are known to occur in insect winter diapause (Hahn and Denlinger, 2011). Clearly, if mosquitoes could be found within their as-yet-unknown dry-season shelters, it would open up a new avenue of investigation regarding their physiology.
4.2 Nutritional reserves during diapause
To survive a period of inactivity without feeding, insects accumulate nutritional reserves in preparation for diapause (Denlinger, 2002; Hahn and Denlinger, 2011) and reduce their metabolic rate to conserve these acquired reserves as described above. In insect winter diapause, triacylglycerides are the most common form of lipid storage, although insects also continue to use other lipids, carbohydrates, and amino acids (Hahn and Denlinger, 2011). For example, adult Cx. pipiens mosquitoes accumulate lipids prior to winter diapause, and it was found that any remaining lipid resources not used during diapause can be used for egg-production by females after breaking diapause (Zhou and Miesfeld, 2009).
Only a few studies on insect aestivation have measured nutritional reserves accumulated before and fuel usage during aestivation. For example, pupae of the cotton bollworm Helicoverpa amigera undergo summer aestivation and increase their energy storage of lipids and glycogen prior to aestivation (Liu et al., 2006). Similarly, the tiger moth C. pudica also undergoes aestivation in the pupal stage and was found to increase triacylglyceride levels and have altered ratios of saturated to unsaturated lipids (Kostal and Simek, 1998). Adults of the beetle Stenotartus rotundus increase their glycerol and glucose content during dry-season diapause (Pullin and Wolda, 1993). Prior to aestivation, another coleopteran, the weevil H. postica, increases its fat and protein levels while reducing water content, and is hypothesized to use fat as its primary fuel source during aestivation, due to its linear decrease during the 3-month period (Tombes, 1964). In summer-aestivating Cs. inornata, female mosquitoes increased their lipid content nearly fourfold prior to aestivation; lipid levels returned to normal after the 3-month aestivation period, indicating usage of this fuel during this time (Barnard and Mulla, 1978). Similar results were obtained by rearing female Cs. inornata under short- and long-day photoperiods (Barnard and Mulla, 1977). Although nutritional reserves have not yet been explicitly measured in An. coluzzii during the dry season, this work is currently underway and we hypothesize that increased lipid reserves may be found, given the 70% reduction in egg-laying by blood-fed females during the dry season (see above; Yaro et al., 2012). Furthermore, a study of non-aestivating female anophelines showed that, during flight trials, blood-fed females had lower carbohydrate usage than sugar-fed females, indicating that blood-fed females may be able to use some of the nutrients from the bloodmeal for their own activity (Kaufmann and Briegel, 2004). However, as with metabolic rate above, it will be difficult to draw conclusions about the nutritional physiology of aestivating An. coluzzii in the Sahel without being able to identify their shelters and measuring mosquitoes in their dormant state.
4.3 Activity level during diapause
Coupled with increased nutritional reserves and a decreased metabolic rate to make these reserves last longer is a decrease in the activity level of the insect (flight, foraging, and/or reproduction). While this is given for overwintering under low temperature, during aestivation of the tropical beetle S. rotundus, the size of the flight muscles was greatly reduced and flight activity ceased (Pullin and Wolda, 1993). Similarly, during adult winter diapause of the mosquito Cx. pipiens, flight muscle size (measured as amount of beta-tubulin) decreased while flight activity was much reduced (Kim and Denlinger, 2009). For insects which are not actively foraging during dormancy, reducing the amount of energy required to maintain large flight muscles is very beneficial (Denlinger, 1986). In our study comparing anophelines at a Sahelian site with those from a riparian site, we found significantly reduced flight activity prior to and during the dry season in the Sahelian population but no significant seasonal variation in flight activity in the riparian population after accounting for temperature variation (Huestis et al., 2012; Table 1). This study analyzed sound recordings from field-caught mosquitoes placed in individual chambers, generated over a 2-hour timeframe in the field (Huestis et al., 2011). Thus, these results indicate that although we found a significant increase in metabolic rate during the late dry season in the Sahel, mosquitoes may still modify their behavior to conserve energy during the dry season.
Based on these patterns, we presume that, while in shelters, An. coluzzii minimizes its activity and reduces its resting metabolic rate to conserve resources. However once its nutritional resources are depleted, it becomes active and forages for sugars and blood sources. This strategy minimizes the number of foraging trips and bloodmeals a single female will take during the dry season, similar to the strategy of the winter-diapausing plant bug L. hesperus, which reduces its feeding rate during diapause to one-fifth of that during normal activity (Brent et al., 2013). This strategy may explain why malaria transmission is very low throughout the dry season, even if the mosquitoes are very old.
5. Stress tolerance during diapause
During the diapause period, organisms are often inactive (and sometimes incapable of movement), and must cope in situ with the harsh environmental conditions that occur; therefore, increased stress resistance is another hallmark of diapause (Box 1). Desiccation, temperature extremes, and starvation represent stress factors that insects often withstand during inhospitable seasons, especially while in dormancy (Denlinger, 1986; Denlinger and Armbruster, 2014; Masaki, 1980; Tauber et al., 1986).
5.1 Desiccation tolerance
Desiccation tolerance has long been considered a key adaptation of insects living in arid environments, whether they undergo dormancy or not (Benoit et al., 2010a; Chown and Nicolson, 2004; Kostal et al., 1998; Tauber et al., 1986). However, during diapause, water sources may be scarce, the air is dry, and often the insect's mobility is limited; thus desiccation resistance is essential. Higher body water-content, lower rate of water loss, and lower threshold for critical body water-content at death are all components of desiccation tolerance (Chown, 2002; Gibbs et al., 2003). The physiological mechanisms most commonly studied reduce the rate of water loss by i) reducing the amount of time spiracles are open to minimize water vapor loss during gas exchanges, “discontinuous gas exchange” (Gibbs and Johnson, 2004; Lighton, 1996), or ii) increased waterproofing via a higher amount of wax layer in the epicuticle and/or changes in the cuticular hydrocarbon composition (Benoit and Denlinger, 2007; Gibbs et al., 1997). Reduced surface-to-volume ratio associated with increased body size also increases desiccation resistance (Hadley, 1994).
Desiccation resistance mediated by an increased cuticular hydrocarbon layer is considered key to egg diapause in Ae. albopictus (Lounibos et al., 2011; Urbanski et al., 2012). During winter diapause, female Cx. pipiens suppress water loss by doubling the total amount of cuticular hydrocarbons in the epicuticle, lowering metabolic rate (reduces gas exchange), and by increased body size (Benoit and Denlinger, 2007). Whether they drink water or sugar-feed during diapause under natural conditions is unclear. Dehydration stress uses up lipids and glycogen (Benoit et al., 2010b), suggesting that desiccation resistance, nutritional reserves, and starvation resistance may be selected together in populations where extreme winters select for diapause. Unless provided with a sugar source during diapause, depleted nutritional reserves due to desiccation stress reduced female reproductive success after winter diapause (Benoit et al., 2010b)
Rather than comparing their desiccation tolerance under aestivating vs. “normal” conditions, previous studies compared species, populations, and genotypes (within a population) inhabiting dry vs. humid environments. Early studies found clines in frequencies of the inversions 2La and 2Rb to be strongly correlated with aridity on spatial and seasonal scales (Bayoh et al., 2001; Coluzzi et al., 1985, 1979; Toure et al., 1994). These inversions are found in markedly different frequencies between species of the complex (2La is fixed in An. arabiensis and is especially variable between populations of An. coluzzii; Bayoh et al., 2001; Coluzzi et al., 1979, 1985; Toure et al., 1994). It has been hypothesized that the 2La and 2Rb inversions confer higher desiccation tolerance and possibly higher temperature tolerance to their carriers (Bayoh et al., 2001; Coluzzi et al., 1979, 1985; Toure et al., 1994). Using laboratory colonies established from Kenya, female An. arabiensis exhibited a higher desiccation resistance than that of An. gambiae s.s. (Gray and Bradley, 2005). Notably, desiccation resistance of teneral mosquitoes (<24 hrs after adult emergence) of both species was higher than that of 4- and 8-day-old mosquitoes. Additional evidence suggested that the higher water content of An. arabiensis enhances its desiccation resistance and may also explain the advantage of teneral mosquitoes (Gray and Bradley, 2005; Table 1). As in other parts of East Africa, An. arabiensis predominates during the drier season in accordance with this observation. It is also not clear if the larger body size of An. arabiensis (Huestis et al., 2011; Lehmann and Diabate, 2008) has contributed to its higher survival. Comparison of larval tolerance to desiccation between colonies of these species revealed lower tolerance of An. arabiensis from Zimbabwe compared to that of An. gambiae from the Gambia (G3 colony), and that the latter was very similar to the tolerance of An. arabiensis from Sudan (Benedict et al., 2010). However, since the mean difference in survival measured only a few minutes, its importance to existence in dry environments remains unclear. Using F1s of field-collected females from Mali, An. coluzzii exhibited higher desiccation tolerance than An. gambiae s.s. (22.2 vs. 17.6; ∼22%), although variation in body size or age were not considered (Lee et al., 2009; Table 1). Notably, the variation between females and males within a species was larger than differences between species (22.4 vs. 16.8; ∼27%), and the effect of 2La inversion was not significant, although a small sample size reduced the power of this test (Lee et al., 2009).
Within-species variation in desiccation resistance between lines of mosquitoes selected as homozygotes for either the 2La inversion or the standard karyotype showed that the former were more resistant early in life than the latter at 1 and 4 days post-eclosion (14.7 vs. 11.6 hr and 14.7 vs. 12.6 hr, respectively; Gray et al., 2009; Table 1). At 8 days of age, the difference was reversed (13.3 vs. 13.9 hr) or disappeared because it was not statistically significant. Higher water content contributed to higher resistance of 2La homozygotes at day 4 and slower water loss of this karyotype contributed to the resistance of the teneral mosquitoes. In a subsequent study, using 1-day-old mosquitoes representing different karyotypes within An. gambiae s.s. (previously the S molecular form), 2La-homozygotes exhibited higher desiccation tolerance than the standard-homozygotes (612 vs. 537 min or 13%), although the heterozygotes exhibited apparently lower resistance than both (529 min; Fouet et al., 2012; Table 1). Notably, body size of the 2La-homozygotes was larger than that those carrying the standard karyotype, but the heterozygotes were apparently the largest (Fouet et al., 2012). The difference was only found in teneral mosquitoes and no difference was detected after 1 day posteclosion (Fouet et al., 2012).
Environmental factors such as larval nutrition and adult access to water significantly affected survival of An. coluzzii females under desiccation stress (Aboagye-Antwi and Tripet, 2010). In addition to the effect of higher body-water content (prior to desiccation stress), higher glycogen reserves (reflecting access to better larval food) also increased desiccation resistance without affecting body size. In populations of Anopheles stephensi from dry vs. humid environments, smaller spiracle size relative to body size was proposed as a morphological mechanism conferring desiccation tolerance (Nagpal et al., 2003), although desiccation tolerance was not directly assessed.
During the dry season, female An. coluzzii from Sahelian populations exhibited higher desiccation tolerance than that during the wet season (14.6 hr vs. 10.1-12.1, P<0.001), consistent with aestivation (Dao et al., unpublished; Table 1). However, whether a difference of ∼25% (20-35%) in aestivation tolerance explains the long survival of these mosquitoes throughout the long dry season remains unclear.
5.2 Tolerance to temperature extremes
Fewer studies have measured tolerance to temperature extremes in mosquitoes. Using laboratory colonies, An. arabiensis exhibited a higher tolerance to high temperatures than An. gambiae, surviving 112 vs. 67 min at 40°C (Kirby and Lindsay, 2004; Table 1). The effect of larger body size of An. arabiensis was not evaluated. A colony of An. gambiae was used to compare homozygotes of the 2La inversion vs. the standard arrangement in tolerance to high temperature of 4th instar larvae and pupae (Rocca et al., 2009). No difference was found among larvae, but, after acclimation, homozygotes of the 2La inversion exhibited significantly higher tolerance than carriers of the standard arrangement (Table 1). No difference was detected in between pupae regardless of acclimation (Rocca et al., 2009).
In summary, multiple studies have compared desiccation tolerance between species and populations of An. gambiae s.l. selected to represent the extreme opposites in this trait, but the differences measured were modest (<40% although statistically significant, and often restricted to narrow age group), whilst the overlap between these populations was large. The difference “required” to explain aridity tolerance has not been defined, yet we question whether the available estimates (above) would approach this value.
6. Molecular underpinnings of diapause
The molecular regulation of diapause has been previously reviewed thoroughly by Denlinger (2002), MacRae (2010), and Storey and Storey (2012), and was recently summarized in mosquitoes by Denlinger and Armbruster (2014). Additionally, metabolomic and ecdysteroid variation in An. gambiae s.l. under desiccating conditions was recently reviewed (Mamai et al., 2014). Here we will describe common gene-expression changes during winter diapause and focus mainly on those few studies examining molecular changes during aestivation.
6.1 Gene expression during winter diapause
Gene-expression changes associated with winter diapause reveal genes and processes which many organisms have in common, leading to the notion of a “genetic toolkit” for diapause (Poelchau et al., 2013a). For example, in winter-diapausing Drosophila melanogaster, regulation of the insulin pathway shifts so that nutrients are depleted slowly and juvenile hormone increases so that ovarian development ceases (Emerson et al., 2009). Furthermore, genes associated with photoperiod often have widespread downstream effects, as shown in the bean bug Riptortus pedestris, where RNAi knockdown of two photoperiod genes affected both cuticle deposition and ovarian development, important traits for winter diapause of this species (Ikeno et al., 2010). Additionally, defensive proteins such as antifungal and antibacterial peptides are often up-regulated, such as the gene drosomycin in Drosophila triauraria (Daibo et al., 2001).
The Asian tiger mosquito, Aedes albopictus, which undergoes diapause in the pharate first instar stage (i.e., within the egg), has been used as a model for egg diapause. Expression of a gene involved in the cell-cycle arrest process and another gene associated with sugar metabolism were both found to increase in the pre-diapause stage (Poelchau et al., 2013a). These same genes have been identified in other taxa and fit into the “diapause toolkit” concept (above). In a related study, it was found that several genes associated with carbohydrate metabolism and lipid metabolism were over-expressed in the early stages of diapause, while expression of amino-acid metabolism genes were generally reduced (Poelchau et al., 2013b), indicating that, in the early phase of diapause, the dormant embryos are engaged in utilization of stored resources. Later in diapause, however, only lipid metabolism genes were found to be significantly differentially expressed (Poelchau et al., 2013b). Surprisingly, this study did not find evidence for up-regulation of heat-shock proteins throughout diapause (Poelchau et al., 2013b), in contrast to many other studies of insect diapause (Denlinger, 2002).
The expression of structural proteins in the northern house mosquito, Cx. pipiens, such as actin, was shown to be up-regulated in early diapause and further increased after exposure to freezing temperatures (Kim et al., 2006). Actin is presumed to strengthen the cytoskeleton and protect against cellular damage due to freezing temperatures (Kim et al., 2006). The expression level of another structural protein, beta-tubulin, was also studied in midgut and flight muscles of diapausing female Cx. pipiens (Kim and Denlinger, 2009). A significant decrease in microtubule abundance in the flight muscles of diapausing females was found, corresponding to the reduction in flight activity due to low temperature and diapause; in contrast, there was no significant change in the midgut muscles (Kim and Denlinger, 2009). Expression levels of genes associated with feeding and reproduction have also been studied, as it has been shown that female mosquitoes increase sugar intake and decrease bloodfeeding prior to entering diapause (Robich and Denlinger, 2005). In accordance with this shift in meal intake, the enzymes needed to digest a bloodmeal (trypsin and a chymotrypsin-like protease) were down-regulated in the pre-diapause period, while a gene associated with increasing the size of the fat reserve (fatty acid synthase) was up-regulated (Robich and Denlinger, 2005). This preparation happens before diapause begins, consistent with the observation that female Cx. pipiens usually will not take a bloodmeal before or during diapause (and even if they do so, will often expel the blood; see Robich and Denlinger, 2005 and references therein); however, it is possible that they could continue sugar-feeding if the opportunity was available. As the end of diapause nears, the trypsin and chymotrypsin-like expression gradually increases, in preparation for breaking diapause and beginning the gonotrophic cycle (Robich and Denlinger, 2005). Lastly, insulin-signaling genes have been found to be instrumental in initiating the diapause cascade in Cx. pipiens (Sim and Denlinger, 2008), and insulin-like peptides are crucial for stopping ovarian development during overwintering (Sim and Denlinger, 2009). However, ovarian development can be rescued by applying juvenile hormone (Sim and Denlinger, 2009; Spielman, 1974). These studies on adult-diapausing Cx. pipiens can predict some of the gene-expression differences that could be utilized by aestivating An. gambiae s.l. during the Sahelian dry season (see below).
6.2 Gene expression during aestivation
In a review covering gene expression during aestivation in toads, snails, and nematodes, several key classes of genes were found across these taxa, including nutrient-regulation, growth signaling, dehydration responses, and antioxidant defenses (Storey and Storey, 2012). Similarly, a review of gene-expression changes during dormancy across taxa including arthropods, fungi, and mammals revealed that small heat-shock proteins (namely the Hsp70 and Hsp90 families) are up-regulated in different forms of dormancy, including aestivation (Denlinger, 2002), and likely act as protective chaperone proteins. However, few studies have examined changes in gene expression during insect aestivation. For example, pupae of the onion maggot, Delia antiqua, can enter both summer and winter diapause, and suppressive subtractive hybridization identified genes related to stress response (heat-shock proteins), antimicrobial defense, metabolism, and food storage that were expressed significantly more in summer diapause than in inter diapause (Hao et al., 2012). The authors of that study hypothesized that i) heat-shock proteins are needed more in the summer than in winter, due to the extreme heat, and ii) more defense proteins are needed because there are more pathogens active in the soil in summer than in winter, given increased moisture and warmth in the summer. Similarly, a recent study of protein expression in the aestivating snail Pomacea canaliculata also revealed an upregulation of immune-response proteins, antioxidative proteins, and a suite of proteins involved lipid fuel-usage (Sun et al., 2013). Lastly, prior to migration, monarch butterflies (Danaus plexippus) enter a state of reproductive diapause/arrest and increased longevity, and changes in expression of genes associated with circadian rhythm and feeding, along with juvenile hormone, were found in these butterflies, relative to reproductively active summer butterflies (Zhu et al., 2008).
Although no studies to date have measured gene expression during mosquito aestivation, two studies have examined gene-expression changes due to relevant phenotypes: aging and desiccation stress. Using the laboratory G3 colony of An. gambiae (a mix of M and S molecular forms), age-related changes in gene expression, separate from gonotrophic-cycle effects, were measured across ages up to 28 days (Wang et al., 2010). Genes which increased in expression throughout the aging process included two detoxification genes (cytochrome P450 and glutathione S-transferases) and Hsp70, a key stress-response gene (Wang et al., 2010); importantly, these classes of genes have been found in previous studies on aestivation, as mentioned above, and may implicate these genes as part of the “aestivation toolkit.” In a similar study using the G3 colony, changes in gene expression due to desiccation stress were measured, and overall, more genes showed decreased expression during desiccation than those which increased (Wang et al., 2011). Genes which were down-regulated during desiccation stress included chitin metabolism genes, oxidative-stress response genes, and binding function genes, while those which were up-regulated during desiccation stress included amino-acid biosynthesis and Hsp40 (Wang et al., 2011).
Combining these studies on An. gambiae with previous studies during aestivation of other insects, we hypothesize that changes in gene expression will likely occur in key classes of genes. Specifically, similar to previous studies, we predict increased levels of lipid metabolism genes, detoxification and immune-related proteins, genes related to building cuticular hydrocarbons and thickening the cuticle, and heat-shock proteins. Simultaneously, we predict decreased levels of vitellogenesis-related genes and other genes associated with reproduction. Conversely, we predict that expression of some other classes, including flight- and feeding-related genes, will not change dramatically, as mosquitoes are found (albeit in low numbers) throughout the dry season. How gene expression varies during the dry season between active and inactive phases (Adamou et al., 2011; Huestis et al., 2012; Lehmann et al., 2014), as well as throughout the progression of the dry season, might be unique for this strategy and thus render predictions based on studies of overwintering diapause less powerful.
7. Discussion and Conclusions
Here, we have focused on the physiology of mosquitoes during the dry season and mainly on those mechanisms that would potentially allow their persistence in environments without surface water across vast areas for at least three months (Adamou et al., 2011; Lehmann et al., 2010; Omer and Cloudsley-Thompson, 1968, 1970; Simard et al., 2000). Insects, including mosquitoes, employ different forms of dormancy to survive harsh seasons (Box 1). Until recently, aestivation in African anophelines has been discounted by most entomologists, although winter diapause in temperate malaria vectors has been well-documented (Jetten and Takken, 1994), and a few studies provided evidence consistent with it in Sudan (Omer and Cloudsley-Thompson, 1968, 1970). In part, this stemmed from studies conducted in areas that experience a “mild dry season,” during which some larval sites remain available within 5-10 km radius (Charlwood et al., 2000; Jawara et al., 2008; Koenraadt et al., 2003; Minakawa et al., 2001; Ramsdale and Fontaine, 1970a, b; Sogoba et al., 2007). Although less abundant, constantly available larval sites during the dry season probably act as a strong selection force against aestivation. Future studies on this topic must recognize this key criterion in the selection of field sites and source populations of mosquitoes for future laboratory studies on aestivation.
The “big picture” of the physiological mechanisms that allow mosquitoes to cope with seasonal extremes is well understood with respect to winter diapause (e.g., Cx. pipiens, Cx. tarsalis, Wy. smithii, and Ae. albopictus), but is poorly understood with respect to aestivation (e.g., Cs. inornata) and even less so with respect to African anophelines in seasonally dry habitats such as the Sahel (Lehmann et al., 2010). Little attention has been paid to low-transmission periods in the study of disease vectors in sub-Saharan Africa, when it is often difficult to collect enough mosquitoes for experiments, and except for one study (Omer and Cloudsley-Thompson, 1968), induction of aestivation under laboratory conditions have failed. Here, we appraised the current knowledge on the dry-season physiology of African anophelines in reference to characteristics of the ‘diapause syndrome,’ which includes aestivation, seeking to address the question, “Is aestivation the strategy used by An. gambiae s.l.?”
A dramatic extension of lifespan during the dry season was reported for two members of the complex: An. arabiensis (Omer and Cloudsley-Thompson, 1968) and An. coluzzii (Lehmann et al., 2010), adding to an earlier observation of an unknown member(s) of An. gambiae s.l. that survived for over three months after collection from the vicinity of Bobo Dioulasso, Burkina Faso (Holstein, 1954). The physiological processes that facilitate such a dramatic change may well be aestivation or quiescence. Consistent with the dormancy framework, female An. arabiensis were reproductively suppressed in a state of gonotrophic dissociation (Omer and Cloudsley-Thompson, 1970); similarly a ∼70% reduction in reproduction (Yaro et al., 2012) and an 80% reduction in flight activity (Huestis et al., 2012) was measured in An. coluzzii during the dry season as opposed to the wet season. On the other hand, metabolic rate was actually highest during the late dry season (Huestis et al., 2012). Although elevated metabolic rate flies in the face of traditional expectations for dormancy (but see Brent et al., 2013), it is important to qualify studies that were conducted on host-seeking mosquitoes (most of them bloodfed) that were collected indoors, which may not represent the physiological state of the same mosquitoes if found in shelters. These mosquitoes may have interrupted their dormancy to replenish nutritional reserves and therefore may suspend at least certain aspects of their dormancy during that time (Huestis et al., 2012).
Consistent with aestivation rather than with quiescence, the switch from reproduction to long-term survival was not a direct result of the absence of surface water for oviposition (Artis et al., 2014; Dieter et al., 2012), indicating that a token environmental stimulus is involved. Accordingly, An. coluzzii has been observed to nearly disappear from villages approximately one month before the larval sites dry up, presumably in anticipation of the coming dry season, as would be expected according to aestivation and contrary to the quiescence hypothesis (Adamou et al., 2011; Huestis et al., 2012; Lehmann et al., 2010; Yaro et al., 2012). Likewise, induction of diapause by short photoperiod produced only a modest extension of An. coluzzii lifespan in either a field insectary or the laboratory and a similarly small increase in the total cuticular hydrocarbons (Table 1). These results revealed a response to photoperiod in the expected direction, but its limited intensity indicates that the insectary conditions were unsuitable to attain full dormancy, possibly because of inadequate conditions to maintain dormant adults. Accordingly, more field studies (64%, n=11) have revealed evidence consistent with aestivation than laboratory studies (33%, n=6; Table 1).
A number of studies (some unpublished, Table 1) measured a slight increase in desiccation tolerance of An. coluzzii, either in comparison with An. gambiae s.s. (Lee et al., 2009), or in mosquitoes homozygous for the 2La inversion compared with homozygotes to the standard karyotype (Fouet et al., 2012; Gray et al., 2009). However, most of these studies were not designed to test whether mosquitoes were dormant, and they measured minimal differences (although statistically significant) during the wet season (Lee et al., 2009) or between lines that were not induced to undergo dormancy; thus our inferences from their results are limited. Nonetheless, they have demonstrated that the tolerance to desiccation of An. coluzzii during the wet season cannot explain, by itself, this species persistence throughout the dry season in the face of the virtual disappearance of the An. gambiae s.s.
Recognizing the diversity in the manifestation of diapause (aka “diapause syndrome”) between and within species and populations (above), it is difficult to interpret the physiological changes over the season (Table 1 and above) outside of the dormancy spectrum. The specific requirements for induction and latency implied by aestivation (Box 1) are not evident based on classical photoperiod experiments (Table 1). However, the early disappearance of An. coluzzii a month before larval sites dried up (above), the modest extension of lifespan in response to shortened photoperiod (Lehmann et al., 2010, 2014; Table 1), and the suppression of reproduction in the presence of water for oviposition (Yaro et al., 2012; Table 1), as well as the failure to utilize artificial oviposition sites during the dry season by anophelines but not culicines (Lehmann et al., 2010), all agree with an induction and latency. We therefore propose that the existing findings support a form of aestivation as the basis of the strategy of An. coluzzii in the West African Sahel and An. arabiensis in the East African Sahel and possibly in Senegal (Lemasson et al., 1997; Simard et al., 2000). This form of aestivation differs from the hibernal diapause typified by Cx. pipiens and other temperate mosquito species that undergo winter diapause as adults, as explained below.
The winter in temperate latitudes implies freezing temperatures, limited insect activity (flight), a lack of sugar sources (flowers and plant juices), and desiccating conditions; combined, these factors result in greatly reduced mobility or complete immobility of the insect and hence total reliance on physiological solutions to cope with the harsh environment. For Sahelian anophelines, on the other hand, the primary restrictive force of the dry season is the absence of surface waters for larval development (Figure 2). Diel fluctuations in temperature (Figure 1) and the corresponding changes in relative humidity likely confine flight to certain parts of the night (Figure 1), but they promote foraging for blood and available sugar sources and even male swarming (above; Figure 2). We hypothesize that behavioral changes in selecting suitable microhabitats in shelters and suitable periods of activity and rest may have a large role in complementing physiological changes, rather than relying on them completely, as is the case for winter diapause. A greater reliance on behavioral elements probably requires a different set of physiological changes (Figure 2), as may be reflected by the bouts of elevated metabolism (Huestis et al., 2012) and local movement (Lehmann et al., 2014) in the late dry season. Specialized behaviors such as digging into ground and/or cocoon formation exhibited by caterpillars and beetles (Tauber et al., 1986) and the switch into sugar feeding and accumulating lipid reserves in mosquitoes (Robich and Denlinger, 2005) are of paramount importance to overwintering diapause, although they are expressed in the diapause-initiation phase rather than the diapause-maintenance phase, which we presume would be the case for African anophelines. Clearly this hypothesis requires empirical evidence, which presents attractive challenges for future research. Finding the shelters used by mosquitoes during the dry season would allow us to measure the metabolic rate, activity patterns, and blood-feeding responses of mosquitoes in this “active dormant state.” More research is needed to dissect the environmental cues which induce aestivation, the conditions required to maintain it, and those that signal its end. Lastly, gene-expression studies on the molecular basis of the physiological changes which occur during aestivation will benefit from starting with those essential genes noted by studies on hibernal diapause and aestivation in other organisms (e.g., heat-shock, storage-utilization, and reproduction-related proteins, see above).
Figure 2.
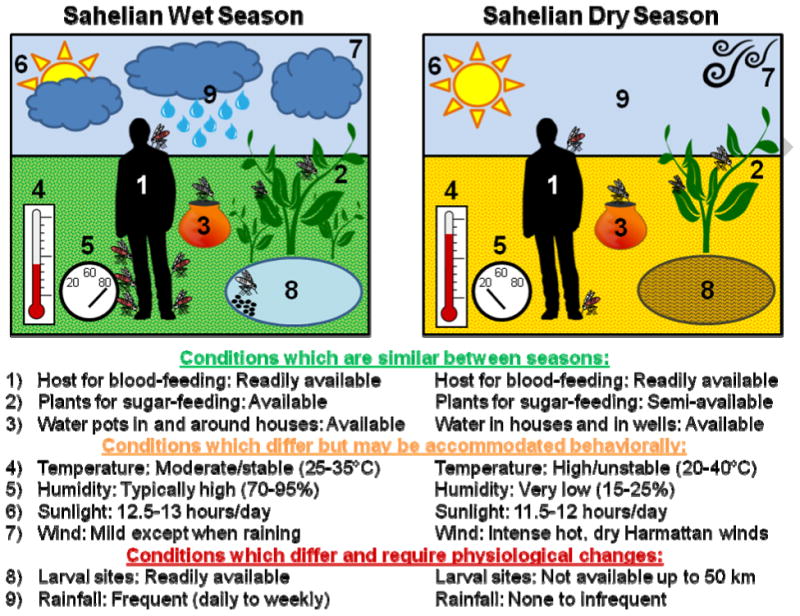
Conditions experienced by mosquitoes during the Sahelian wet season as compared with the Sahelian dry season. Conditions 1-3 are very similar between the wet and dry seasons. Conditions 4-7 are different between the seasons, but presumably mosquitoes could accommodate them behaviorally by seeking cool, humid shelters, only flying at night, etc. Conditions 8 and 9 are the critical differences between the seasons and are why aestivation is required.
A comprehensive picture of the strategy used by mosquitoes to persist throughout the dry season could yield novel vector control strategies and make positive impacts on malaria eradication in the future. Specifically, if the shelters used by these mosquitoes could be identified, predicted, and subjected to vector control, aestivating mosquitoes could be targeted during the dry season, markedly reducing the overall disease burden during the transmission season. Likewise, if the genes underlying this unique physiological state are known, they may be targeted to prevent mosquitoes from completing their aestivation. Thus, the continued studies on the physiological and behavioral strategies used by aestivating anophelines are ongoing and remain a frontier in medical entomology.
Highlights for.
Malaria vectors use unknown physiological adaptations to survive arid habitats.
We review the physiology of dormancy and aridity-tolerance, emphasizing mosquitoes.
Sahelian anophelines may aestivate, with short bouts of activity to gain resources.
This form of aestivation extends survival by depressing movement and reproduction.
Further studies on aestivation physiology could yield novel vector control methods.
Acknowledgments
We thank Dia Elnaiem, Peter Armbruster, Phil Lounibos, and 1 anonymous reviewer for their comments on earlier versions of this manuscript. Research conducted by the authors mentioned throughout was supported by the Division of Intramural Research, NIAID, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboagye-Antwi F, Tripet F. Effects of larval growth condition and water availability on desiccation resistance and its physiological basis in adult Anopheles gambiae sensu stricto. Malar J. 2010;9:225. doi: 10.1186/1475-2875-9-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamou A, Dao A, Timbine S, Kassogue Y, Yaro AS, Diallo M, Traore SF, Huestis DL, Lehmann T. The contribution of aestivating mosquitoes to the persistence of Anopheles gambiae in the Sahel. Malar J. 2011;10:151. doi: 10.1186/1475-2875-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrewartha HG. Diapause in relation to the ecology of insects. Biological Reviews of the Cambridge Philosophical Society. 1952;27:50–107. [Google Scholar]
- Barnard DR, Mulla MS. Effects of photoperiod and temperature on blood feeding, oogenesis, and fat body development in the mosquito, Culiseta inornata. Journal of Insect Physiology. 1977;23:1261–1266. doi: 10.1016/0022-1910(77)90068-3. [DOI] [PubMed] [Google Scholar]
- Barnard DR, Mulla MS. The ecology of Culiseta inornata in the Colorado Desert of California: seasonal abundance, gonotrophic status, and oviparity of adult mosquitoes. Annals of the Entomological Society of America. 1978;71:397–401. [Google Scholar]
- Bates M. The natural history of mosquitoes. MacMillan; New York: 1949. [Google Scholar]
- Bayoh MN, Thomas CJ, Lindsay SW. Mapping distributions of chromosomal forms of Anopheles gambiae in West Africa using climate data. Med Vet Entomol. 2001;15:267–274. doi: 10.1046/j.0269-283x.2001.00298.x. [DOI] [PubMed] [Google Scholar]
- Beach R. Required day number and timely induction of diapause in geographic strains of mosquito Aedes atropalpus. J Insect Physiol. 1978;24:449–455. [Google Scholar]
- Beier JC, Copeland RS, Oyaro C, Masinya A, Odago WO, Odour S, Koech DK, Roberts CR. Anopheles gambiae complex egg stage survival in dry soil from larval development sites in western. Kenya J Am Mosq Control Assoc. 1990;6:105–109. [PubMed] [Google Scholar]
- Bennett VA, Kukal O, Lee RE. Metabolic opportunists: Feeding and temperature influence the rate and pattern of respiration in the high arctic woollybear caterpillar Gynaephora groenlandica (Lymantriidae) J Exp Biol. 1999;202:47–53. doi: 10.1242/jeb.202.1.47. [DOI] [PubMed] [Google Scholar]
- Benoit JB, Denlinger DL. Suppression of water loss during adult diapause in the northern house mosquito, Culex pipiens. J Exp Biol. 2007;210:217–226. doi: 10.1242/jeb.02630. [DOI] [PubMed] [Google Scholar]
- Benoit JB, Lopez-Martinez G, Phillips ZP, Patrick KR, Denlinger DL. Heat shock proteins contribute to mosquito dehydration tolerance. J Insect Physiol. 2010a;56:151–156. doi: 10.1016/j.jinsphys.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit JB, Patrick KR, Desai K, Hardesty JJ, Krause TB, Denlinger DL. Repeated bouts of dehydration deplete nutrient reserves and reduce egg production in the mosquito Culex pipiens. J Exp Biol. 2010b;213:2763–2769. doi: 10.1242/jeb.044883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw WE, Lounibos LP. Evolution of dormancy and its photoperiodic control in pitcher-plant mosquitoes. Evolution. 1977;31:546–567. doi: 10.1111/j.1558-5646.1977.tb01044.x. [DOI] [PubMed] [Google Scholar]
- Brakefield PM, Pijpe J, Zwaan BJ. Developmental plasticity and acclimation both contribute to adaptive responses to alternating seasons of plenty and of stress in Bicyclus butterflies. J Biosci. 2007;32:465–475. doi: 10.1007/s12038-007-0046-8. [DOI] [PubMed] [Google Scholar]
- Brent CS, Klok CJ, Naranjo SE. Effect of diapause status and gender on activity, metabolism, and starvation resistance in the plant bug Lygus hesperus. Entomol Exp Appl. 2013;148:152–160. [Google Scholar]
- Buth JL, Brust RA, Ellis RA. Development time, oviposition activity and onset of diapausein Culex tarsalis, Culex restuans, and Culiseta inornata in southern Manitoba. Journal of the American Mosquito Control Association. 1990;6:55–63. [PubMed] [Google Scholar]
- Canzano AA, Krockenberger AA, Jones RE, Seymour JE. Rates of metabolism in diapausing and reproductively active tropical butterflies, Euploea core and Euploea sylvester (Lepidoptera : Nymphalidae) Physiol Entomol. 2006;31:184–189. [Google Scholar]
- Chambers RJ. Maternal experience of crowding and duration of aestivation in the sycamore aphid. Oikos. 1982;39:100–102. [Google Scholar]
- Charlwood JD, Cuamba N, Tomas EV, Briet OJ. Living on the edge: a longitudinal study of Anopheles funestus in an isolated area of Mozambique. Malar J. 2013;12:208. doi: 10.1186/1475-2875-12-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlwood JD, Vij R, Billingsley PF. Dry season refugia of malaria-transmitting mosquitoes in a dry savannah zone of east. Africa Am J Trop Med Hyg. 2000;62:726–732. doi: 10.4269/ajtmh.2000.62.726. [DOI] [PubMed] [Google Scholar]
- Chown SL. Respiratory water loss in insects. Comparative Biochemistry and Physiology A- Molecular and Integrative Physiology. 2002;133:791–804. doi: 10.1016/s1095-6433(02)00200-3. [DOI] [PubMed] [Google Scholar]
- Chown SL, Nicolson SW. Insect Physiological Ecology: Mechanisms and Patterns. Oxford University Press; Oxford; 2004. [Google Scholar]
- Clarke A, Fraser KPP. Why does metabolism scale with temperature? Funct Ecol. 2004;18:243–251. [Google Scholar]
- Clements AN. The Physiology of Mosquitoes. Pergamon Press; Oxford: 1963. [Google Scholar]
- Clements AN. The Biology of Mosquitoes. Chapman & Halll; London: 1992. [Google Scholar]
- Coetzee M, Hunt RH, Wilkerson R, Della Torre A, Coulibaly MB, Besansky NJ. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa. 2013;3619:246–274. [PubMed] [Google Scholar]
- Coluzzi M. Morphological Divergences in the Anopheles gambiae complex. Riv Malariol. 1964;43:197–232. [PubMed] [Google Scholar]
- Coluzzi M, Petrarca V, Di Deco MA. Chromosomal inversion intergradation and incipient speciation in Anopheles gambiae. Bollettino di Zoologia. 1985;52:45–63. [Google Scholar]
- Coluzzi M, Sabatini A, Petrarca V, Di Deco MA. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg. 1979;73:483–497. doi: 10.1016/0035-9203(79)90036-1. [DOI] [PubMed] [Google Scholar]
- Daibo S, Kimura MT, Goto SG. Upregulation of genes belonging to the drosomycin family in diapausing adults of Drosophila triauraria. Gene. 2001;278:177–184. doi: 10.1016/s0378-1119(01)00713-2. [DOI] [PubMed] [Google Scholar]
- Danilevskii AS. Photoperiodism and Seasonal Development of Insects. Oliver and Boyd; Edinburgh and London: 1965. [Google Scholar]
- Davidson G. Anonopheles gambiae, a complex of species. Bull World Health Organ. 1964;31:625–634. [PMC free article] [PubMed] [Google Scholar]
- Denlinger DL. Dormancy in tropical insects. Annual Review of Entomology. 1986;31:239–264. doi: 10.1146/annurev.en.31.010186.001323. [DOI] [PubMed] [Google Scholar]
- Denlinger DL. Regulation of diapause. Annual Review of Entomology. 2002;47:93–122. doi: 10.1146/annurev.ento.47.091201.145137. [DOI] [PubMed] [Google Scholar]
- Denlinger DL, Armbruster PA. Mosquito diapause. Annu Rev Entomol. 2014;59:73–3. doi: 10.1146/annurev-ento-011613-162023. [DOI] [PubMed] [Google Scholar]
- Depner KR, Harwood RF. Photoperiodic responses of 2 latitudinally diverse groups of Anopheles freeborni (Diptera - Culicidae) Ann Entomol Soc Am. 1966;59:7–11. [Google Scholar]
- Dieter KL, Huestis DL, Lehmann T. The effects of oviposition-site deprivation on Anopheles gambiae reproduction. Parasites Vectors. 2012;5:235. doi: 10.1186/1756-3305-5-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle H. Migration: The Biology of Life on the Move. Oxford University Press; New York: 1996. [Google Scholar]
- Dixon AFG. The effect of population density and nutritive status of the host on the summer reproductive activity of the sycamore aphid, Drepanosiphum platanoides (Schr.) J Anim Ecol. 1966;35:105–112. [Google Scholar]
- Dixon AFG, Wellings PW, Carter C, Nichols JFA. The role of food quality and competition in shaping the seasonal cycle in the reproductive activity of the sycamore aphid. Oecologia. 1993;95:89–92. doi: 10.1007/BF00649511. [DOI] [PubMed] [Google Scholar]
- Djawdan M, Rose MR, Bradley TJ. Does selection for stress resistance lower metabolic rate? Ecology. 1997;78:828–837. [Google Scholar]
- Donnelly MJ, Simard F, Lehmann T. Evolutionary studies of malaria vectors. Trends Parasitol. 2002;18:75–80. doi: 10.1016/s1471-4922(01)02198-5. [DOI] [PubMed] [Google Scholar]
- Douglas AE. Reproductive diapause and the bacterial symbiosis in the sycamore aphid Drepanosiphum platanoidis (Schr.) Ecological Entomology. 2000;25:256–261. [Google Scholar]
- Eldridge BF. Current Topics in Vector Research. Springer; New York: 1987. Diapause and related phenomena in Culex mosquitoes: their relation to arbovirus disease ecology; pp. 1–28. [Google Scholar]
- Emerson KJ, Bradshaw WE, Holzapfel CM. Complications of complexity: integrating environmental, genetic and hormonal control of insect diapause. Trends Genet. 2009;25:217–225. doi: 10.1016/j.tig.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Focks DA, Linda SB, Craig GB, Hawley WH, Pumpuni CB. Aedes albopictus (Diptera: Culicidae): A statistical model of the role of temperature, photoperiod, and geography in the induction of egg diapause. J Med Entomol. 1994;31:276–286. doi: 10.1093/jmedent/31.2.278. [DOI] [PubMed] [Google Scholar]
- Fouet C, Gray E, Besansky NJ, Costantini C. Adaptation to aridity in the malaria mosquito Anopheles gambiae: chromosomal inversion, polymorphism, and body size influence resistance to desiccation. PLoS One. 2012;7:e34841. doi: 10.1371/journal.pone.0034841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs AG, Chippindale AK, Rose MR. Physiological mechanisms of evolved desiccation resistance in Drosophila melanogaster. J Exp Biol. 1997;200:1821–1832. doi: 10.1242/jeb.200.12.1821. [DOI] [PubMed] [Google Scholar]
- Gibbs AG, Fukuzato F, Matzkin LM. Evolution of water conservation mechanisms in Drosophila. J Exp Biol. 2003;206:1183–1192. doi: 10.1242/jeb.00233. [DOI] [PubMed] [Google Scholar]
- Gibbs AG, Johnson RA. The role of discontinuous gas exchange in insects: the chthonic hypothesis does not hold water. J Exp Biol. 2004;207:3477–3482. doi: 10.1242/jeb.01168. [DOI] [PubMed] [Google Scholar]
- Gillies MT, De Meillon B. The Anophelinae of Africa south of the Sahara. 2. South African Institute for Medical Research; Johannesburg, South Africa: 1968. [Google Scholar]
- Gray EM, Bradley TJ. Physiology of desiccation resistance in Anopheles gambiae and Anopheles arabiensis. Am J Trop Med Hyg. 2005;73:553–559. [PubMed] [Google Scholar]
- Gray EM, Rocca KA, Costantini C, Besansky NJ. Inversion 2La is associated with enhanced desiccation resistance in Anopheles gambiae. Malaria Journal. 2009;8:215. doi: 10.1186/1475-2875-8-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guppy M. The biochemistry of metabolic depression: a history of perceptions. Comp Biochem Physiol B-Biochem Mol Biol. 2004;139:435–442. doi: 10.1016/j.cbpc.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Guppy M, Withers P. Metabolic depression in animals: physiological perspectives and biochemical generalizations. Biol Rev Cambridge Philosophic Soc. 1999;74:1–40. doi: 10.1017/s0006323198005258. [DOI] [PubMed] [Google Scholar]
- Hadley NF. Ventilatory patterns and respiratory transpiration in adult terrestrial insects. Physiol Zool. 1994;67:175–189. [Google Scholar]
- Hahn DA, Denlinger DL. Energetics of insect diapause. In: Berenbaum MR, Carde RT, Robinson GE, editors. Annual Review of Entomology. Vol. 56. 2011. pp. 103–121. Annual Reviews, Palo Alto. [DOI] [PubMed] [Google Scholar]
- Hairston NG, Kearns CM. The interaction of photoperiod and temperature in diapause timing – a copepod example. Biol Bull. 1995;189:42–48. doi: 10.2307/1542200. [DOI] [PubMed] [Google Scholar]
- Hao YJ, Li WS, He ZB, Si FL, Ishikawa Y, Chen B. Differential gene expression between summer and winter diapause pupae of the onion maggot Delia antiqua, detected by suppressive subtractive hybridization. J Insect Physiol. 2012;58:1444–1449. doi: 10.1016/j.jinsphys.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Harwood RF, Halfhill E. The effect of photoperiod on fat body and ovarian development of Culex tarsalis (Diptera: Culicidae) Ann Entomol Soc Am. 1964;57:596–600. [Google Scholar]
- Held C, Spieth HR. First evidence of pupal summer diapause in Pieris brassicae L.: the evolution of local adaptedness. J Insect Physiol. 1999;45:587–598. doi: 10.1016/s0022-1910(99)00042-6. [DOI] [PubMed] [Google Scholar]
- Holstein MH. Biology of Anopheles gambiae Research in French West Africa. World Health Organization; Geneva: 1954. [Google Scholar]
- Hudson JE. Induction of diapause in female mosquitoes, Culiseta inornata, by a decrease in daylength. Journal of Insect Physiology. 1977;23:1377–1382. [Google Scholar]
- Huestis DL, Yaro AS, Traore AI, Adamou A, Kassogue Y, Diallo M, Timbine S, Dao A, Lehmann T. Variation in metabolic rate of Anopheles gambiae and A. arabiensis in a Sahelian village. J Exp Biol. 2011;214:2345–2353. doi: 10.1242/jeb.054668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis DL, Yaro AS, Traore AI, Dieter KL, Nwagbara JI, Bowie AC, Adamou A, Kassogue Y, Diallo M, Timbine S, Dao A, Lehmann T. Seasonal variation in metabolic rate, flight activity and body size of Anopheles gambiae in the Sahel. J Exp Biol. 2012;215:2013–2021. doi: 10.1242/jeb.069468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeno T, Tanaka SI, Numata H, Goto SG. Photoperiodic diapause under the control of circadian clock genes in an insect. BMC Biol. 2010;8 doi: 10.1186/1741-7007-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenson TGT, Ameneshewa B. Prehibernation diet and reproductive condition of female Anopheles messeae in Sweden. Med Vet Entomol. 1991;5:243–252. doi: 10.1111/j.1365-2915.1991.tb00547.x. [DOI] [PubMed] [Google Scholar]
- Jawara M, Pinder M, Drakeley CJ, Nwakanma DC, Jallow E, Bogh C, Lindsay SW, Conway DJ. Dry season ecology of Anopheles gambiae complex mosquitoes in The Gambia. Malar J. 2008;7:156. doi: 10.1186/1475-2875-7-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten TH, Takken W. Anophelism without malaria in Europe: a review of the ecology and distribution of the genus Anopheles in Europe. Wageningen Agricultural University Papers 94-5. 1994:1–69. [Google Scholar]
- Kaufmann C, Briegel H. Flight performance of the malaria vectors Anopheles gambiae and Anopheles atroparvus. J Vector Ecol. 2004;29:140–153. [PubMed] [Google Scholar]
- Keilin D. The problem of anabiosis or latent life: history and current concept. Proc R Soc Lond B Biol Sci. 1959;150:149–191. doi: 10.1098/rspb.1959.0013. [DOI] [PubMed] [Google Scholar]
- Kim M, Denlinger DL. Decrease in expression of beta-tubulin and microtubule abundance in flight muscles during diapause in adults of Culex pipiens. Insect Mol Biol. 2009;18:295–302. doi: 10.1111/j.1365-2583.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- Kim M, Robich RM, Rinehart JP, Denlinger DL. Upregulation of two actin genes and redistribution of actin during diapause and cold stress in the northern house mosquito, Culex pipiens. J Insect Physiol. 2006;52:1226–1233. doi: 10.1016/j.jinsphys.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby MJ, Lindsay SW. Responses of adult mosquitoes of two sibling species, Anopheles arabiensis and A. gambiae s.s. (Diptera : Culicidae), to high temperatures. Bulletin of Entomological Research. 2004;94:441–448. doi: 10.1079/ber2004316. [DOI] [PubMed] [Google Scholar]
- Koenraadt CJ, Paaijmans KP, Githeko AK, Knols BG, Takken W. Egg hatching, larval movement and larval survival of the malaria vector Anopheles gambiae in desiccating habitats. Malar J. 2003;2:20. doi: 10.1186/1475-2875-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostal V. Eco-physiological phases of insect diapause. Journal of Insect Physiology. 2006;52:113–127. doi: 10.1016/j.jinsphys.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Kostal V, Hodek I. Photoperiodism and control of summer diapause in the Mediterranean tiger moth Cymbalophora pudica. J Insect Physiol. 1997;43:767–777. doi: 10.1016/s0022-1910(97)00032-2. [DOI] [PubMed] [Google Scholar]
- Kostal V, Simek P. Changes in fatty acid composition of phospholipids and triacylglycerols after cold-acclimation of an aestivating insect prepupa. J Comp Physiol B-Biochem Syst Environ Physiol. 1998;168:453–460. [Google Scholar]
- Kostal V, Sula J, Simek P. Physiology of drought tolerance and cold hardiness of the Mediterranean tiger moth Cymbalophora pudica during summer diapause. J Insect Physiol. 1998;44:165–173. doi: 10.1016/s0022-1910(97)00047-4. [DOI] [PubMed] [Google Scholar]
- Lanciani CA. Photoperiod and longevity in Anopheles crucians. Journal of the American Mosquito Control Association. 1993;9:308–312. [PubMed] [Google Scholar]
- Lanciani CA, Anderson JF. Effect of photoperiod on longevity and metabolic rate in Anopheles quadrimaculatus. Journal of the American Mosquito Control Association. 1993;9:158–163. [PubMed] [Google Scholar]
- Lee Y, Meneses CR, Fofana A, Lanzaro GC. Desiccation resistance among subpopulations of Anopheles gambiae s.s. From Selinkenyi, Mali. Journal of Medical Entomology. 2009;46:316–320. doi: 10.1603/033.046.0216. [DOI] [PubMed] [Google Scholar]
- Lehmann T, Dao A, Yaro AS, Adamou A, Kassogue Y, Diallo M, Sekou T, Coscaron-Arias C. Aestivation of the African malaria mosquito, Anopheles gambiae, in the Sahel. American Journal of Tropical Medicine and Hygiene. 2010;83:601–606. doi: 10.4269/ajtmh.2010.09-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann T, Dao A, Yaro AS, Diallo M, Timbine S, Huestis DL, Adamou A, Kassogue Y, Traore AI. Seasonal variation in spatial distributions of Anopheles gambiae in a Sahelian v or g e, evidence for aestivation. Journal of Medical Entomology. 2014;51:27–38. doi: 10.1603/me13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann T, Diabate A. The molecular forms of Anopheles gambiae: A phenotypic perspective. Infect Genet Evol. 2008;8:737–746. doi: 10.1016/j.meegid.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasson JJ, Fontenille D, Lochouarn L, Dia I, Simard F, Ba K, Diop A, Diatta M, Molez JF. Comparison of behavior and vector efficiency of Anopheles gambiae and An. Arabiensis (Diptera:Culicidae) in Barkedji, a Sahelian area of Senegal. J Med Entomol. 1997;34:396–403. doi: 10.1093/jmedent/34.4.396. [DOI] [PubMed] [Google Scholar]
- Lighton JRB. Discontinuous gas exchange in insects. Annual Review of Entomology. 1996;41:309–324. doi: 10.1146/annurev.en.41.010196.001521. [DOI] [PubMed] [Google Scholar]
- Liu ZD, Gong PY, Wu KJ, Sun JH, Li DM. A true summer diapause induced by high temperatures in the cotton bollworm, Helicoverpa armigera (Lepidoptera : Noctuidae) J Insect Physiol. 2006;52:1012–1020. doi: 10.1016/j.jinsphys.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Escher RL, Lourenco-de-Oliveria R. Asymmetric evolution of photoperiodic diapause in temperate and tropical invasive populations of Aedes albopictus (Diptera : Culicidae) Ann Entomol Soc Am. 2003;96:512–518. [Google Scholar]
- Lounibos LP, Escher RL, Nishimura N. Retention and adaptiveness of photoperiodic egg diapause in Florida populations of invasive Aedes albopictus. Journal of the American Mosquito Control Association. 2011;27:433–436. doi: 10.2987/11-6164.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luker LA, Hatle JD, Juliano SA. Reproductive responses to photoperiod by a south Florida population of the grasshopper Romalea microptera (Orthoptera : Romaleidae) Environ Entomol. 2002;31:702–707. [Google Scholar]
- MacRae TH. Gene expression, metabolic regulation and stress tolerance during diapause. Cell Mol Life Sci. 2010;67:2405–2424. doi: 10.1007/s00018-010-0311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamai W, Mouline K, Blais C, Larvor V, Dabire KR, Ouedraogo GA, Simard F, Renault D. Metabolomic and ecdysteroid variations in Anopheles gambiae s.l. mosquitoes exposed to the stressful conditions of the dry season in Burkina Faso, West Africa. Physiological and Biochemical Zoology. 2014;87:486–497. doi: 10.1086/675697. [DOI] [PubMed] [Google Scholar]
- Masaki S. Summer diapause. Annual Review of Entomology. 1980;25:1–25. [Google Scholar]
- Minakawa N, Githure JI, Beier JC, Yan GY. Anopheline mosquito survival strategies during the dry period in western Kenya. Journal of Medical Entomology. 2001;38:388–392. doi: 10.1603/0022-2585-38.3.388. [DOI] [PubMed] [Google Scholar]
- Nagpal BN, Srivastava A, Kalra NL, Subbarao SK. Spiracular indices in Anopheles stephensi: A taxonomic tool to identify ecological variants. Journal of Medical Entomology. 2003;40:747–749. doi: 10.1603/0022-2585-40.6.747. [DOI] [PubMed] [Google Scholar]
- Omer SM, Cloudsley-Thompson JL. Dry season biology of Anopheles gambiae Giles in the Sudan. Nature. 1968;217:879–880. [Google Scholar]
- Omer SM, Cloudsley-Thompson JL. Survival of female Anopheles gambiae Giles through a 9- month dry season in Sudan. Bull World Health Organ. 1970;42:319–330. [PMC free article] [PubMed] [Google Scholar]
- Poelchau MF, Reynolds JA, Elsik CG, Denlinger DL, Armbruster PA. Deep sequencing reveals complex mechanisms of diapause preparation in the invasive mosquito, Aedes albopictus. Proc R Soc B-Biol Sci. 2013a;280 doi: 10.1098/rspb.2013.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelchau MF, Reynolds JA, Elsik CG, Denlinger DL, Armbruster PA. RNA-Seq reveals early distinctions and late convergence of gene expression between diapause and quiescence in the Asian tiger mosquito, Aedes albopictus. J Exp Biol. 2013b;216:4082–4090. doi: 10.1242/jeb.089508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullin AS, Wolda H. Glycerol and glucose accumulation during diapause in a tropical beetle. Physiol Entomol. 1993;18:75–78. [Google Scholar]
- Ragland GJ, Fuller J, Feder JL, Hahn DA. Biphasic metabolic rate trajectory of pupal diapause termination and post-diapause development in a tephritid fly. J Insect Physiol. 2009;55:344–350. doi: 10.1016/j.jinsphys.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Ramsdale CD, Fontaine RE. Ecological investigations of Anopheles gambiae and Anopheles funestus II. Dry season studies with colony-reared A. gambiae species B, Kaduna Nigeria. 1970a:1–8. WHO/VBC/70.249. [Google Scholar]
- Ramsdale CD, Fontaine RE. Ecological investigations of Anopheles gambiae and Anopheles funestus I. Dry season studies in villages near Kaduna Nigeria. 1970b WHO/VBC/70.248. [Google Scholar]
- Rao VV. On gonotrophic discordance among certain Indian Anopheles. Indian Journal of Malariology. 1947;1:43–50. [Google Scholar]
- Reisen WK, Meyer RP, Milby MM. Studies on the seasonality of Culiseta inornata in Kern County, California. J Am Mosq Control Assoc. 1989;5:183–195. [PubMed] [Google Scholar]
- Reisen WK, Thiemann T, Barker CM, Lu HL, Carroll B, Fang Y, Lothrop HD. Effects of warm winter temperature on the abundance and gonotrophic activity of Culex (Diptera: Culicidae) in California. Journal of Medical Entomology. 2010;47:230–237. doi: 10.1603/me09207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robich RM, Denlinger DL. Diapause in the mosquito Culex pipiens evokes a metabolic switch from blood feeding to sugar gluttony. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15912–15917. doi: 10.1073/pnas.0507958102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca KAC, Gray EM, Costantini C, Besansky NJ. 2La chromosomal inversion enhances thermal tolerance of Anopheles gambiae larvae. Malar J. 2009;8:147. doi: 10.1186/1475-2875-8-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C, Denlinger DL. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6777–6781. doi: 10.1073/pnas.0802067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C, Denlinger DL. A shut-down in expression of an insulin-like peptide, ILP-1, halts ovarian maturation during the overwintering diapause of the mosquito Culex pipiens. Insect Mol Biol. 2009;18:325–332. doi: 10.1111/j.1365-2583.2009.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard F, Lehmann T, Lemasson JJ, Diatta M, Fontenille D. Persistence of Anopheles arabiensis during the sever dry season conditions in Senegal: an indirect approach using microsatellite loci. Insect Mol Biol. 2000;9:467–479. doi: 10.1046/j.1365-2583.2000.00210.x. [DOI] [PubMed] [Google Scholar]
- Sinclair BJ, Bretman A, Tregenza T, Tomkins Jl, Hosken DJ. Metabolic rate does not decrease with starvation in Gryllus bimaculatus when changing fuel use is taken into account. Physiol Entomol. 2011;36:84–89. [Google Scholar]
- Sogoba N, Doumbia S, Vounatsou P, Baber I, Keita M, Maiga M, Traore SF, Toure A, Dolo G, Smith T, Ribeiro JMC. Monitoring of larval habitats and mosquito densities in the Sudan savanna of Mali: Implications for malaria vector control. American Journal of Tropical Medicine and Hygiene. 2007;77:82–88. [PubMed] [Google Scholar]
- Spielman A. Effect of synthetic juvenile hormone on ovarian diapause of Culex pipiens mosquitoes. Journal of Medical Entomology. 1974;11:223–225. doi: 10.1093/jmedent/11.2.223. [DOI] [PubMed] [Google Scholar]
- Spielman A. Structure and seasonality of nearctic Culex pipiens populations. In: White DJ, Morse DL, editors. West Nile Virus: Detection, Surveillance, and Control. New York Acad Sciences; New York: 2001. pp. 220–234. [DOI] [PubMed] [Google Scholar]
- Spielman A, Wong J. Environmental control of ovarian diapause in Culex pipiens (Diptera: Culicidae) Ann Entomol Soc Am. 1973;66:905–907. [Google Scholar]
- Storey KB, Storey JM. Metabolic rate depression and biochemical adaptation in anaerobiosis, hibernation, and estivation. Q Rev Biol. 1990;65:145–174. doi: 10.1086/416717. [DOI] [PubMed] [Google Scholar]
- Storey KB, Storey JM. Metabolic rate depression in animals: transcriptional and translational controls. Biol Rev. 2004;79:207–233. doi: 10.1017/s1464793103006195. [DOI] [PubMed] [Google Scholar]
- Storey KB, Storey JM. Aestivation: signaling and hypometabolism. J Exp Biol. 2012;215:1425–1433. doi: 10.1242/jeb.054403. [DOI] [PubMed] [Google Scholar]
- Sun J, Mu HW, Zhang HM, Chandramouli KH, Qian PY, Wong CKC, Qiu JW. Understanding the regulation of estivation in a freshwater snail through iTRAQ-based comparative proteomics. J Proteome Res. 2013;12:5271–5280. doi: 10.1021/pr400570a. [DOI] [PubMed] [Google Scholar]
- Swellengrebel NH. La dissociation des fonctions sexuelles de nutritives (dissociation gonotrophique) d‘Anopheles maculipennis comme cause du paludisme dans les Pays-Bas et ses rapports ave ‘l’infection domiciliare. Annales de l'Institut Pasteur. 1929;43:1370–1389. [Google Scholar]
- Tauber MJ, Tauber CA, Masaki S. Seaonal Adaptations of Insects. Oxford University Press; New York: 1986. [Google Scholar]
- Tauber MJ, Tauber CA, Nyrop JP, Villani MG. Moisture, a vital but neglected factor in the seasonal ecology of insects: Hypotheses and tests of mechanisms. Environ Entomol. 1998;27:523–530. [Google Scholar]
- Tombes AS. Respiratory and compositional study on the aestivating insect, Hypera postica (Gyll) (Curculionidae) J Insect Physiol. 1964;10:997–1003. [Google Scholar]
- Toure YT, Petrarca V, Traore SF, Coulibaly A, Maiga HM, Sankare O, Sow M, Di Deco MA, Coluzzi M. Ecological genetic studies in the chromosomal form Mopti of Anopheles gambiae s.str. in Mali, West Africa. Genetica. 1994;94:213–223. doi: 10.1007/BF01443435. [DOI] [PubMed] [Google Scholar]
- Tsuda Y, Kim KS. Sudden autumnal appearance of adult Culex tritaeniorhynchus (Diptera : Culicidae) at a park in urban Tokyo: First field evidence for prediapause migration. Journal of Medical Entomology. 2008;45:610–616. doi: 10.1603/0022-2585(2008)45[610:saaoac]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Urbanski J, Mogi M, O'Donnell D, DeCotiis M, Toma T, Armbruster P. Rapid adaptive evolution of photoperiodic response during invasion and range expansion across a climatic gradient. American Naturalist. 2012;179:490–500. doi: 10.1086/664709. [DOI] [PubMed] [Google Scholar]
- Vinogradova An experimental investigation of the ecological factors inducing imaginal diapause in blood sucking mosquitoes. Entomological Review. 1960;39:210–219. [Google Scholar]
- Vinogradova EB, De Statsio B, Gilbert JJ. Diapause in aquatic insects, with emphasis on mosquitoes. In: Alekseev VR, editor. Diapause in Aquatic Invertebrates. Springer; 2007. pp. 83–113. [Google Scholar]
- Vinogradova EB, Pantyuchov GA. Adult diapause and its physiological characteristic in the ragweed leaf beetle, Zygogramma suturalis F (Coleoptera, Chrysomelidae), introduced to Russia from north America. Folia Biol-Krakow. 1995;43:137–141. [Google Scholar]
- Wachira SW, Ndung'u M, Njagi PGN, Hassanali A. Comparative responses of ovipositing Anopheles gambiae and Culex quinquefasciatus females to the presence of Culex egg rafts and larvae. Med Vet Entomol. 2010;24:369–374. doi: 10.1111/j.1365-2915.2010.00913.x. [DOI] [PubMed] [Google Scholar]
- Wagoner KM, Lehmann T, Huestis DL, Cech NB, Ehrmann BM, Wasserberg G. Identification of morphologic and chemical markers of dry- and wet-season conditions in female Anopheles gambiae mosquitoes. Parasites and Vectors. 2014 doi: 10.1186/1756-3305-7-294. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MH, Marinotti O, James AA, Walker E, Githure J, Yan GY. Genome-wide patterns of gene expression during aging in the African malaria vector Anopheles gambiae. PLoS ONE. 2010;5:e13359. doi: 10.1371/journal.pone.0013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MH, Marinotti O, Vardo-Zalik A, Boparai R, Yan GY. Genome-wide transcriptional analysis of genes associated with acute desiccation stress in Anopheles gambiae. PLoS ONE. 2011;6:e26011. doi: 10.1371/journal.pone.0026011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washino RK. Physiological condition of overwintering female Anopheles freeborni in California (Diptera-Culicidae) Ann Entomol Soc Am. 1970;63:210. doi: 10.1093/aesa/63.1.210. [DOI] [PubMed] [Google Scholar]
- Washino RK. The physiological ecology of gonotrophic dissociation and related phenomena in mosquitoes. Journal of Medical Entomology. 1977;13:381–388. doi: 10.1093/jmedent/13.4-5.381. [DOI] [PubMed] [Google Scholar]
- Washino RK, Bailey SF. Overwintering of Anopheles punctipennis (Diptera: Culicidae) in California. Journal of Medical Entomology. 1970;7:95. doi: 10.1093/jmedent/7.1.95. [DOI] [PubMed] [Google Scholar]
- WHO. World Malaria Report 2013. World Health Organization; Geneva, Switzerland: 2013. [Google Scholar]
- Yaro AS, Traore AI, Huestis DL, Adamou A, Timbine S, Kassogue Y, Diallo M, Dao A, Traore SF, Lehmann T. Dry season reproductive depression of Anopheles gambiae in the Sahel. J Insect Physiol. 2012;58:1050–1059. doi: 10.1016/j.jinsphys.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou GL, Miesfeld RL. Energy metabolism during diapause in Culex pipiens mosquitoes. J Insect Physiol. 2009;55:40–46. doi: 10.1016/j.jinsphys.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu HS, Casselman A, Reppert SM. Chasing migration genes: A brain expressed sequence tag resource for summer and migratory monarch butterflies (Danaus plexippus) PLoS ONE. 2008;3:e1345. doi: 10.1371/journal.pone.0001345. [DOI] [PMC free article] [PubMed] [Google Scholar]


