Abstract
Background
Disproportionally low retention of minority populations can adversely affect the generalizability of clinical research trials. We determine the overall retention rates for White and Black participants from the Selenium and Vitamin E Cancer Prevention Trial (SELECT) and explore participant and site characteristics associated with retention failure (study disengagement)for these groups.
Methods
A secondary analysis of 28,118 White (age ≥55), and 4,322 Black (age ≥ 50) SELECT participants used multivariate Cox regression to estimate overall retention rates and to calculate hazard ratios (HR) and 95% confidence intervals (CI).
Results
Blacks had higher age-adjusted risk of disengagement than Whites (HR=1.92; 95% CI,1.77-2.08). Among Black participants, those age 50-54 were at three times the risk of disengagement than those age ≥65 (HR=3.61; 95%CI,2.41-5.41). Blacks age ≥65 had 1.6 times the risk of disengagement than Whites age ≥65 (HR=1.60; 95%CI,1.38-1.87). By six years post-randomization, 84% of Whites and 69% of Blacks remained engaged in the study. Current smoking status was an independent risk factor for study disengagement for both White and Black participants. For both groups, sites whose staffs missed SELECT training sessions or who received SELECT Retention and Adherence grants were associated with increased and decreased disengagement risks, respectively.
Conclusions
SELECT retention was disproportionately lower for Blacks than for Whites.
Impact
The observed difference in retention rates for Blacks and Whites and factors identified by race for study disengagement in SELECT may inform retention efforts for future long-term, cancer prevention trials.
Keywords: clinical trial, retention, minority retention, African Americans, prostate cancer prevention
Introduction
Disproportionally low recruitment and retention of minority populations can adversely affect the generalizability of clinical research trials.This is evident with respect to African American (“Black”) participation in large randomized trials (1-5).Low Black recruitment and retention rates may be particularly consequential in clinical cancer studies, where Blacks bear disproportionately higher disease burdens for breast, prostate, colorectal and lung cancers.Suboptimal recruitment and retention of black participants has been noted in cancer screening (6, 7), prevention (8-10) and treatment trials (2, 11).A wide variety of factors have emerged to explainrecruitment and retention problems among Blacks and have broadly included sociodemographic factors, belief and trust issues, competing priorities, co-morbidity burdens and ineffective research team practices both before and during implementation of trials (1, 3-5, 12).Interventions directed to address some of these factors have been shown to be modestly effective (6, 10, 13, 14).
The incidence of prostate cancer and its mortality rate are higher in Black men than among Whites and other racial-ethnic groups (14, 15).Blacks have 1.6 times the incidence of prostate cancer than Whites and 2.4 times the deaths from prostate cancer than Whites (15). Adequate recruitment and retention of Blacks in prostate cancer trials is critical to determining potential differential responses for treatment benefit or harm.
The Selenium and Vitamin E Cancer Prevention Trial (SELECT)was a large SWOG coordinated randomized trial for the prevention of prostate cancer (16,17).SELECT took special efforts to recruit a representative proportion of minority participants, particularly Blacks, through its selection of study sites, modified eligibility criteria and creation of sub-committees and grants designed to enhance both overall and minority recruitment and retention (18-20). Eligibility criteria were adjusted to permit registration of men with controlled co-morbid conditions, allowing for more eligible Black participants because of the generally higher rates of comorbidities among Blacks (21-23). Additionally, the minimum age for Blacks was lowered from 55 years to 50 years because the risk of prostate cancer among Blacks at age 50 is equivalent to Whites’ risk at age 55 (15).
The goal of the SELECT recruitment plan was to provide multiple strategies, materials and resources to the variety of participating institutions that included academic sites, Community Clinical Oncology Programs (CCOPs), specialty and general hospitals, Veterans Affairs (VA) facilities and health maintenance organizations.The SELECT Recruitment/Retention and Adherence Committee (RAC) and the Minority and Medically Underserved Subcommittee were established prior to the trial with the purpose of monitoring overall and minority recruitment and implementing strategies to increase recruitment and retention of SELECT participants. Specific SELECT recruitment and retention strategies are shown in Table 1; a full discussion of SELECT's minority recruitment strategies is presented elsewhere (18). SELECT recruitment was very successful, surpassing the study's accrual goal 28 months ahead of schedule. Because accrual was more rapid than anticipated, the study needed to move quickly to implement recruitment plans, especially those targeted at Blacks. Overall SELECT minority recruitment was 22%, and Black recruitment was 15%.
Table 1. SELECT Recruitment and Retention Strategies.
| General strategy description | Specific SELECT application of this strategy |
|---|---|
| Recruitment Strategies | |
|
|
|
| Establish study committees charged with monitoring recruitment and suggesting strategies to improve it as needed. | The SELECT Recruitment/Retention and Adherence Committee (RAC) and the Minority and Medically Underserved Subcommittee (MMUS) were established prior to the trial with the purpose of monitoring overall and minority recruitment and implementing strategies to increase recruitment of SELECT participants. |
| Study site selection | The study selected sites that would likely have a high proportion of eligible black participants, including the VA, which has had traditionally high black enrollment in its cooperative studies. (20) |
| Recruitment materials for physicians and lay public | Study branded recruitment materials were designed for promoting the study with physicians using scientific information and for the lay public using ninth grade or lower reading levels whenever possible. |
| Grants for recruitment activities | SELECT offered both Recruitment grants and Minority Recruitment Enhancement grants (19) designed to improve overall and minority recruitment. |
| Minority site training Workshops | Small interactive workshops for selected Principal Investigator and staff that focused on existing and new accrual strategies. (18) |
| Retention Strategies | |
|
| |
| Establish study committees charged with monitoring retention and suggesting strategies to improve it as needed. | The SELECT RAC and MMUS were established prior to the trial with the purpose of monitoring overall and minority retention and implementing strategies to increase retention of SELECT participants. |
| Site staff retention training | Site staffs attended semi-annual Workshops, which provided retention training. They could request mentoring visits to help increase site performance, including issues with retention. |
| Free Study Multivitamin | Participants and their partners were offered a free SELECT multivitamin, made without vitamin E or selenium, so they could continue taking a multivitamin while on SELECT (most commercial formulations contain vitamin E and selenium). |
| Participant newsletter | The SELECT Coordinating Center provided a semi-annual national newsletter which was available in English, French and Spanish. Study sites were encouraged to also provide a local newsletter. |
| Establish an advisory board comprised of study participants | Study sites had access to the SELECT National Participant Advisory Board (NPAB), whose members were available to attend events and provide a participant's perspective. NPAB members also appeared in motivation videos for sites to use. |
| Grants for retention activities | SELECT offered Adherence grants designed to improve retention. |
Despite achieving a notable level of Black recruitment and continuing retention efforts, ongoing monitoring of the SELECT population indicated that Black participants were being lost to follow-up to a greater extent than were Whites.The extent to which this was occurring and what the related factors may have been were not certain.The objectives of this secondary analysis is to determine the overall retention rates for White and Black SELECT participants and to determine the participant and site characteristics associated with retention failure for these groups.
Materials and Methods
SELECT description
The present study is a secondary analysis of data from SELECT (NCT00006392), sponsored by the National Cancer Institution (NCI) and coordinated by SWOG. SELECT was a phase III, double blind, placebo controlled clinical trial to assess the impact of selenium and vitamin E, alone and in combination, on the clinical incidence of prostate cancer. SELECT randomized 35,533 men from 427 study sites in the United States, Puerto Rico and Canada. Minimum age eligibility was 50 years for Black men and 55 years for all others. The primary outcome was prostate cancer incidence, as determined by standard of care at the study sites. Follow-up ranged from 4.2 to 7.3 years, with a median of 5.5 years. Due to lack of efficacy in each of the three intervention arms, as determined at interim data analysis presented to the Data and Safety Monitoring Committee, study supplementation ended in October 2008. Details of the trial design, eligibility criteria and the composite results have been presented previously (16, 17, 24). A later analysis using longer follow-up showed vitamin E was associated with an increased risk of prostate cancer (25).
Defining race
All SELECT participants self-identified race and ethnicity at enrollment using standard NCI guidelines. Participants chose one or more of the following standard NCI racial categories: “White or Caucasian”; “Black or African American”; “Native Hawaiian or Other Pacific Islander”; “Asian”; “American Indian or Alaska Native”; “Unknown”.In this manuscript, we use the terms “Black” and “White” to identify “Black or African American” and “White or Caucasian” groups of study participants, respectively. Hispanic ethnicity (yes or no) was collected on all participants.
Study population
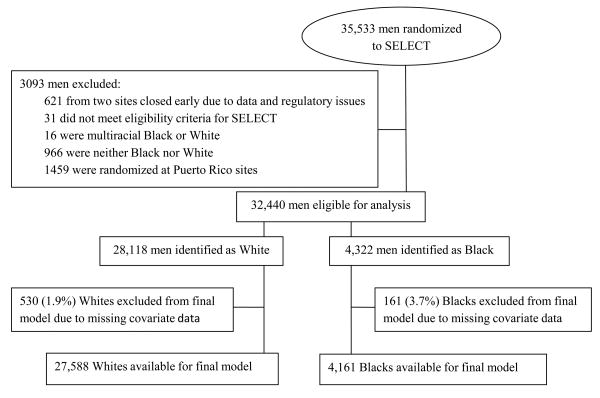
Eligibility criteria for the current analysis are shown in the Cohort Diagram. (Figure 1) Participants were excluded if they were deemed not eligible for SELECT based on SELECT Statistical Center review (n=31), randomized to either of two SELECT sites that were closed early due to data and regulatory issues (n=621), were randomized at Puerto Rican sites (n=1459), were self-identified as multiracial Black or White (n=16), or were self-identified as neither Black nor White (n=966).An additional 691 participants (161 Blacks and 530 Whites) were excluded from the final model due to missing covariate data (see Figure 1).For the current analysis, there were 28,118 White and 4322 Black participants available for the survival analysis, and 27,589 White and 4,255 Black participants available for modeling in the multivariate analysis.
Figure 1. Establishment of study cohort (CONSORT Diagram).
Primary Outcome Variables
SELECT participants were scheduled to have follow-up visits, either in person or by telephone, every six months regardless of supplementation status. Missed visits were documented to record reason for being missed. Participants who adamantly refused follow-up were documented and, with Principal Investigator consent, no longer followed.
We defined time to disengagement as the days to the earlier of either (1) the second consecutive missed visit (in person or via telephone) or (2) refusal of all future contact with study staff. Missing two consecutive visits (a year's worth of visits) did not necessarily mean the participant would no longer be involved on the study or would be lost to follow-up, but was considered a consistent, measurable parameter and a reasonable indicator of retention failure and that a participant's bond/commitment to the study was weak.
Censoring occurred when a participant met his initial study commitment for follow-up, defined as the earliest times of the following: prostate cancer diagnosis; death; or the end of study-wide supplementation. Censoring also occurred at the time of site closure, such as for natural disasters (e.g., Hurricane Katrina) or other issues.
Potential model covariates
Participant covariates considered for inclusion in the models were collected at baseline and include demographics, comorbidities and the six self-reported reasons for participating in SELECT (Table 2).
Table 2. Baseline characteristics of SELECT participants included in the retention analysis, by race.
| White (n=28,118) | Black (n=4,322) | |||
|---|---|---|---|---|
| N | % | N | % | |
| DEMOGRAPHIC | ||||
| Age group (years) a | ||||
| 50-54 | 1374 | 32% | ||
| 55-59 | 9271 | 33% | 1240 | 29% |
| 60-64 | 7776 | 28% | 803 | 19% |
| ≥65 | 11071 | 39% | 905 | 21% |
| Ethnicity | ||||
| Hispanic | 567 | 2% | 40 | 1% |
| Not Hispanic | 27326 | 97% | 4244 | 98% |
| Missing | 225 | 1% | 38 | 1% |
| Family history of prostate cancer | ||||
| Yes | 5333 | 19% | 720 | 17% |
| No | 22755 | 81% | 3567 | 83% |
| Missing | 30 | 0% | 35 | 1% |
| Living arrangements | ||||
| Alone | 3265 | 12% | 923 | 21% |
| With others | 24703 | 88% | 3313 | 77% |
| Missing | 150 | 1% | 86 | 2% |
| Education level | ||||
| Up to high school graduate | 5370 | 19% | 1245 | 29% |
| Vocational/some college | 7304 | 26% | 1556 | 36% |
| College graduate/some graduate school | 8111 | 29% | 846 | 20% |
| Completed graduate school | 7125 | 25% | 586 | 14% |
| Missing | 208 | 1% | 89 | 2% |
| BASELINE COMORBIDITY | ||||
| Smoking status | ||||
| Current | 1671 | 6% | 798 | 18% |
| Former | 14291 | 51% | 1761 | 41% |
| Never | 12072 | 43% | 1699 | 39% |
| Missing | 84 | 0% | 64 | 1% |
| Cardiovascular health historyb | ||||
| Yes | 4560 | 16% | 570 | 13% |
| No | 23549 | 84% | 3749 | 87% |
| Missing | 9 | 0% | 3 | 0% |
| Diabetes | ||||
| Yes | 2341 | 8% | 741 | 17% |
| No | 25768 | 92% | 3578 | 83% |
| Missing | 9 | 0% | 3 | 0% |
| Hypertension | ||||
| Yes | 10045 | 36% | 2318 | 54% |
| No | 18064 | 64% | 2001 | 46% |
| Missing | 9 | 0% | 3 | 0% |
| REASONS FOR PARTICIPATING ON SELECTc | ||||
| “May help others in the future” | ||||
| Yes | 25393 | 90% | 3478 | 80% |
| No | 2693 | 10% | 808 | 19% |
| Missing | 32 | 0% | 36 | 1% |
| “It may help me be healthier” | ||||
| Yes | 20651 | 73% | 3251 | 75% |
| No | 7435 | 26% | 1035 | 24% |
| Missing | 32 | 0% | 36 | 1% |
| “It may prevent prostate cancer” | ||||
| Yes | 22433 | 80% | 3477 | 80% |
| No | 5653 | 20% | 809 | 19% |
| Missing | 32 | 0% | 36 | 1% |
| “My wife or others in my family want me to join” | ||||
| Yes | 4192 | 15% | 614 | 14% |
| No | 23894 | 85% | 3672 | 85% |
| Missing | 32 | 0% | 36 | 1% |
| “It makes me proud to be part of a study” | ||||
| Yes | 10453 | 37% | 1565 | 36% |
| No | 17633 | 63% | 2721 | 63% |
| Missing | 32 | 0% | 36 | 1% |
| “It gives me a chance to see someone about my health” | ||||
| Yes | 7865 | 28% | 1609 | 37% |
| No | 20221 | 72% | 2677 | 62% |
| Missing | 32 | 0% | 36 | 1% |
White participants were eligible for SELECT at age 55 and older; Black participants were eligible at age 50 and older.
A patient has a positive cardiovascular health history if he reported any of the following at baseline: Embolism, thrombosis; TIA; Stroke; CHF; Arrhythmia; Angina; MI; CABG; Angioplasty.
All participants completed a form with the question, “Why have you chosen to be on this study?” Participants may have marked more than one response.
Specific site characteristics were chosen because they either reflected site performance and investment in SELECT or they had the potential to impact accrual and/or retention. SELECT offered four ancillary studies. Staff training workshops were held every six months throughout SELECT; at least one person from each site was expected to attend each workshop. The workshops focused on a variety of topics, including recruitment, retention and adherence. Attendance data are available for the last 10 of 16 workshops. SELECT Retention and Adherence grants (R&A grants) were awarded twice yearly and were primarily intended to support overall site retention and adherence activities. Sites applied for these funds, generally $150 to $1500, to support specific promotional activities or for direct support of participants for items such as transportation or parking. All site factors except for type of site are included in the models as time-varying covariates (Table 3).
Table 3. Characteristics of SELECT sites with participants included in the retention analysis.
| N | % | |
|---|---|---|
| NUMBER OF SITES | 417 | 100% |
| SITE CHARACTERISTICS | ||
| Type of site | ||
| Veterans Affairs | 45 | 11% |
| Community Clinical Oncology Programs | 126 | 30% |
| Other | 246 | 59% |
| Total accrual | ||
| <100 | 334 | 80% |
| 100 - 499 | 72 | 17% |
| ≥500 | 11 | 3% |
| Percent African American accrual at site | ||
| <5% | 197 | 47% |
| 5-19% | 124 | 30% |
| ≥20% | 96 | 23% |
| Site participated in ancillary studiesa | ||
| Yes | 362 | 87% |
| No | 55 | 13% |
| Number of training workshops missed by site staff b | ||
| 0, 1, or 2 | 117 | 28% |
| 3 or 4 | 77 | 18% |
| 5 or more | 223 | 53% |
| Site ever received a SELECT R&A Grantc | ||
| Yes | 127 | 30% |
| No | 290 | 70% |
Site registered at least one participant to a SELECT ancillary study
Staff were required to attend SELECT training workshops, held every 6 months
SELECT Retention and Adherence (R&A) grants were available to all sites via an application process, for study-related activities
Data Analysis
Cox regression was used to model time to disengagement using PROC PHREG in SAS 9.2 (SAS Institute Inc., Cary, North Carolina). Participants were clustered at sites, and participants at the same site will likely be more similar than participants at different sites; the models account for this correlation.Participant and site characteristics are described with frequencies and percentages.Survival curves were produced using the Kaplan-Meier method.Retention for Black versus White participants was compared directly using hazard ratios obtained from a Cox regression model adjusted for age.
Cox regression models were used to obtain hazard ratios for the multivariate analyses. In preparation for the model building, the covariates were checked for collinearity.Univariate analyses checking the smoothed deviance residuals were used to confirm the covariates were in the correct form. The proportionality assumption was assessed using the empirical score process, based on the Martingale residuals. Multivariate models were constructed separately for Blacks and Whites and include factors with significant bivariate relationships. The final models use all factors identified from both racial groups to produce a common covariate model, which allows parameters to be compared between groups.
Results
Descriptive findings
Of 35,533 initially randomized SELECT participants, 28,118 White participants and 4,332 Black participants were available for this analysis; their baseline descriptive characteristics are presented in Table 2.Whites are age 55 and older; 39% are at least age 65. Blacks are age 50 and older; only 21% are at least age 65 and 32% are age 50-54. Blacks generally have higher rates of comorbidities than Whites. Only 6% of Whites are current smokers compared to 18% of Blacks. The most commonly cited reason for participating in SELECT, for all participants, was that it “may help others in the future” (90% for Whites and 80% for Blacks).
Thirty percent (n=126) of SELECT sites are CCOP sites and 11% (n=45) are part of the VA system (Table 3).Twenty percent (n=83) of sites accrued 100 or more participants to SELECT and Black participant accrual was at least 20% at 96 (23%) sites.Only 28% of sites (n=117) missed two or fewer of the semi-annual SELECT training workshops whereas 53% (n=223) missed five or more of these sessions.Thirty percent of sites (n=127) applied for and received at least one SELECT R&A grant.
Retention by Race and Age
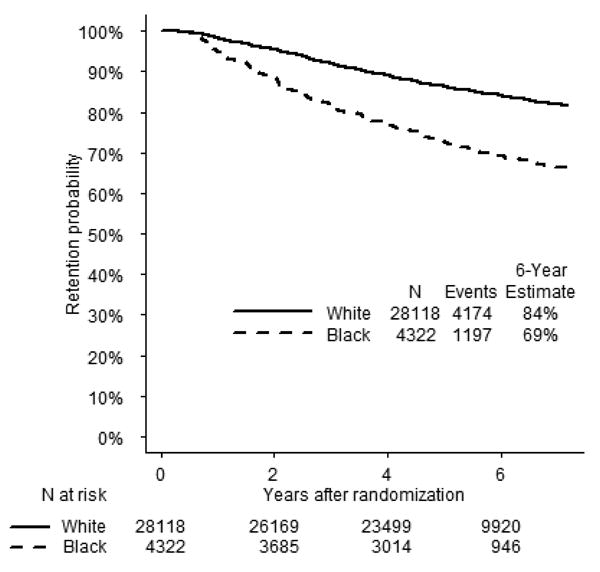
Blacks had higher risk of disengagement than Whites (age-adjusted HR=1.92; 95% CI,1.77-2.08). By six years post-randomization, 84% of Whites were still retained on the study compared to 69% of Blacks (Figure 2). The difference between Black and White participant retention was evident early in the trial, and continued to diverge over its duration.
Figure 2. Time to disengagement from SELECT, stratified by race.
Among Black participants at six years post-randomization, the youngest age group (50-54 years) demonstrated the lowest retention (64%) and the oldest age group (≥65 years) demonstrated the highest retention (76%) (Figure 3).A comparison of these two groups shows more than a three-fold greater risk for disengagement in the younger group (HR=3.61; 95% CI,2.41-5.41).This finding is in contrast to that for White participants, where those aged 55-64 years and ≥65 years had nearly identical retention rates six years post-randomization (HR= 0.97; 95% CI,0.85-1.10).Among men age 55-64 years, Blacks had twice the risk of Whites for disengagement (HR=2.08; 95% CI,1.90-2.28).Among men age ≥65 years, Blacks had 1.6 times the risk of Whites for disengagement (HR=1.60; 95% CI,1.38-1.87).
Figure 3.
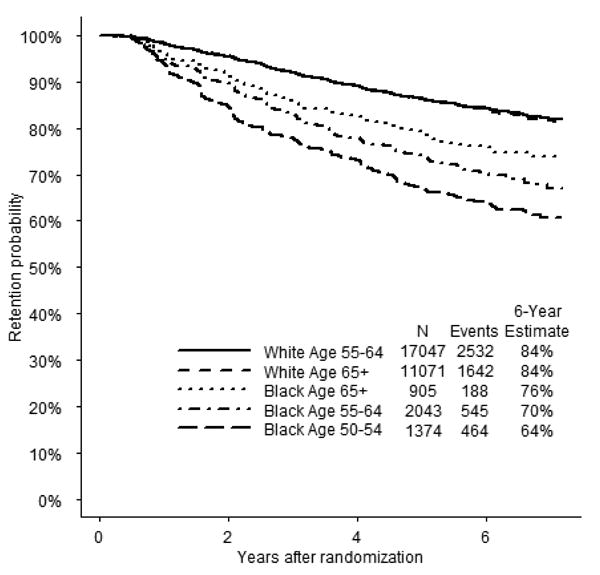
Time to disengagement from SELECT, stratified by race and age. Note: the two curves for the White age groups overlap.
Disengagement risk factors
Several participant characteristics are associated with retention failure (Table 4).The hazard of disengagement is higher among White participants who indicated Hispanic ethnicity, did not have a college degree, were living alone or were current smokers. Among Blacks, the younger participants (age 50-59 years) had higher risk compared to those ≥65 years. Black current smokers also had higher hazards compared to former or never smokers.Different reasons cited for joining SELECT within each racial group associated with retention: “It makes me proud to be part of a study” and “It may help others in the future”for Whites; “My wife or others in my family wanted me to join” for Blacks. Participant characteristics that showed no association with retention are: treatment assignment; cardiovascular health history; diabetes; hypertension; and reasons for joining SELECT (“It may help me be healthier”,“It may prevent prostate cancer”, “It gives me a chance to see someone about my health”).
Table 4. Hazard ratio estimates for factors associated with disengagement by race.
| Model for White participants (n=27,588) |
Model for Black participants (n=4161) |
|||
|---|---|---|---|---|
|
|
|
|||
| Hazard ratio (95% Confidence interval) |
p-value | Hazard ratio (95% Confidence interval |
p-value | |
|
|
|
|||
| DEMOGRAPHICS | ||||
| Age | ||||
| 50-54 years | 1.58 (1.31, 1.89) | <.0001 | ||
| 55-59 years | 1.07 (0.98, 1.16) | 0.1494 | 1.41 (1.15, 1.73) | 0.0012 |
| 60-64 years | 0.94 (0.86, 1.04) | 0.2273 | 1.04 (0.84, 1.28) | 0.7426 |
| 65 years and older | 1.00 (reference) | 1.00 (reference) | ||
| Hispanic ethnicity vs. not | 1.83 (1.34, 2.50) | 0.0001 | 1.37 (0.82, 2.28) | 0.2332 |
| Living alone vs. not | 1.35 (1.20, 1.50) | <.0001 | 1.27 (1.12, 1.45) | 0.0003 |
| College degree vs. not | 0.81 (0.75, 0.88) | <.0001 | 0.88 (0.75, 1.03) | 0.1150 |
| COMORBIDITIES | ||||
| Family history of prostate cancer | 0.91 (0.84, 0.99) | 0.0302 | 0.76 (0.64, 0.90) | 0.0017 |
| Current smoker vs. former or never smoker | 1.51 (1.33, 1.71) | <.0001 | 1.47 (1.27, 1.70) | <.0001 |
| WHY PARTICIPATING IN SELECT | ||||
| "It makes me proud to be part of a study” vs. not | 0.85 (0.79, 0.91) | <.0001 | 1.03 (0.91, 1.16) | 0.6619 |
| “It may help others in the future” vs. not | 0.84 (0.74, 0.94) | 0.0035 | 0.90 (0.78, 1.03) | 0.1276 |
| “My wife or others in my family want me to join” vs. not | 1.00 (0.90, 1.11) | 0.9417 | 0.76 (0.63, 0.92) | 0.0040 |
| SITE CHARACTERISTICS | ||||
| Received an R&A grant vs. not | 0.66 (0.45, 0.97) | 0.0355 | 0.76 (0.57, 1.00) | 0.0532 |
| Number of training meetings missed | ||||
| 5 or more | 1.92 (1.38, 2.67) | 0.0001 | 2.00 (1.20, 3.35) | 0.0079 |
| 3 or 4 | 1.40 (0.94, 2.08) | 0.0954 | 1.74 (1.17, 2.57) | 0.0056 |
| 0, 1 or 2 | 1.00 (reference) | 1.00 (reference) | ||
| Type of site | ||||
| CCOP | 0.67 (0.52, 0.86) | 0.0014 | 0.70 (0.51, 0.96) | 0.0277 |
| VA | 1.99 (1.26, 3.15) | 0.0032 | 0.87 (0.65, 1.16) | 0.3523 |
| Other | 1.00 (reference) | 1.00 (reference) | ||
Covariates are adjusted for all other covariates presented in the table.
Abbreviations: R&A = Retention and Adherence. CCOP=Community Clinical Oncology Program. VA=Veterans Affairs
Site characteristics also are associated with disengagement. The number of missed training workshops by site staffs had a direct relationship with retention failure: the more workshops missed, the greater the risk of disengagement. Both White and Black participants at sites that received R&A grants were at lower risk of disengagement, as were White and Black participants at a CCOP site when compared with all non-CCOP, non-VA sites, including academic centers and community hospitals and clinics.White participants at VA site had twice the risk of disengagement when compared to all non-VA, non-CCOP sites; Black participants at VA sites did not show an increased risk of disengagement. Site factors that showed no association with retention are:total accrual; percent of Black participants; and ancillary study participation.
Discussion
In SELECT, a large randomized, phase III trial testing whether selenium and/or vitamin E prevent prostate cancer (16, 17), Black participants had lower retention rates than Whites, with the age-adjusted hazard for disengagement for Blacks being nearly twice that for Whites (HR=1.92; 95% CI,1.77-2.08).Black retention was lowest in the youngest age group and increased with age, but even among Blacks age 65 and older, the disengagement rate was 1.6 times that of Whites in that age group.Age was not associated with retention among Whites. Concordant with these respective rates of disengagement, at the end of six years 84% of Whites and 69% of Blacks were retained in the study.
Lowering the eligibility age was the most important tool for increasing Black recruitment, but it was also a major factor in Black disengagement. In developing SELECT, lowering the eligibility age for Blacks was thought of as a valid way to preferentially enrich the Black population, since Blacks at age 50 have a similar prostate cancer risk to Whites at age 55. Lowering the eligibility age for Blacks was a successful recruitment tactic (32% of Blacks were age 50-54), so the low retention rate for this group is particularly disappointing.Although no other studies have published comparable definition of retention, overall or by race, overall loss to follow-up rates are usually available. SELECT's loss to follow-up rate (4.6%)(17) is similar to that of other large prevention trials of similar duration, which range from 2.3% (WHI) to 7.7% (PCPT) (26-29).
It is not certain why an age-related association with retention failure occurred in Blacks but not in Whites in SELECT.To our knowledge, the differential effect of race on the association of age and study retention has not been studied in long-term prevention trials outside of SELECT. Other studies in a variety of disease settings (HIV, obesity, cancer, aging, lower urinary tract symptoms, hernias) show an association between younger age and loss to follow-up (8, 30-39), covering all ages, several continents and a variety of follow-up methods, but the relationship between race, age and loss to follow-up seems unexplored.Future studies are needed to investigate and address the issues related to age and study disengagement for Blacks.
For both Blacks and Whites, current smoking emerged as a significant risk factor for disengagement, increasing the risks by about 50% for both groups.Although the prevalence of current smokers was much higher among Blacks than Whites, smoking status did not affect retention differentially by race (Pinteraction=0.74).Smoking has been shown to be a risk factor for poor adherence among Black participants 55 years and older in a cancer screening trial (7) and in longitudinal trials of HIV positive patients (40, 41). Current smoking status may be particularly important in prostate cancer trials, as heavy smoking has been shown to be a risk factor for prostate cancer and more aggressive prostate cancer among Blacks (42).The high prevalence of smoking among Blacks compared to Whites, coupled with lower retention rates, may have disproportionately reduced the opportunity to detect incident prostate cancer among Black smokers, although this sub-population of 798 participants represents only 2.5% of the total SELECT population.Such concerns may warrant special efforts to retain smokers, particularly in cancer prevention trials where smoking is an identified risk factor.It is important to note that the SELECT men included in this analysis had lower current smoking rates by one third to one half of their peers (43).
Our analysis excluded participants from Puerto Rican sites and therefore included only non-Puerto Rican Hispanic participants (2% of Whites,1% of Blacks).Despite only a small number of such participants, Hispanic self-identity among Whites was independently associated with double the risk of disengagement (HR=1.86; 95%CI,1.36-2.53).Prior studies have indicated both lower recruitment and retention of Hispanic participants, due to a number of putative social and behavioral factors (10-12, 14, 40, 44).This issue has not been studied directly in cancer prevention trials; with the increasing prevalence of Hispanics in the United States and with data indicating greater risks for stomach, liver, uterine cervix and gallbladder cancers for Hispanics than for the general population (45), there is a need to do so.
Black participants who joined SELECT because “…my family wanted me to join” had higher retention rates compared to those who did not endorse this reason.A lack of family support may indicate someone is living alone, and our results reflect this.Blacks living alone had lower retention rates when compared to those not living alone (HR=1.27; 95% CI,1.12-1.45).Living alone among Whites is also associated with an increased risk of disengagement (HR=1.35; 95% CI,1.20-1.50). Living alone can be a surrogate for one or more demographic and social factors including marital status, education, employment, income, and substance use disorders, each of which could reasonably affect a person's ability or willingness to remain in a long-term clinical trial (1, 3-5, 12, 23).From our findings however, we cannot conclude that there are substantial racial differences in the influence of these often linked demographic and social factors on study retention in SELECT.
It is unclear why White participants from VA sites had substantially greater risks of retention failure than did participants from CCOP and non-CCOP, non-VA sites, whereas Black participants from VA sites had similar rates to those from non-CCOP, non-VA sites (Table 4). The general success in recruiting Blacks for VA Cooperative Studies Program trials has been previously documented (20), but to our knowledge there has been no data regarding differential rates of Black and White retention in VA studies. Of note, however, Oddone et al. (20) reported that many of the VA trials which targeted diseases more common in Blacks than in Whites (e.g., diabetes, hypertension, renal failure) exceeded their expected enrollment of minority patients.
Our data from site characteristics also point toward the potential advantages of providing ongoing training and additional resources to participating site staffs.Retention rates for both Whites and Blacks were highest when staff at their sites attended more SELECT training sessions. These sessions included specific workshops on recruitment, adherence and retention strategies.It is likely that those sites whose staff had regular attendance generally functioned at a higher level in all aspects of the study, and that higher retention was part of that overall good performance.In this regard, participants at CCOP sites had higher retention rates than participants at other types of SELECT sites. CCOP sites are community hospitals or consortia funded through a stable, 5 year NIH grant (46); other types of SELECT sites received per capita funding for recruitment and follow-up.
SELECT offered R&A grants, which were designed to improve retention and adherence.Although overall retention of Blacks, as shown in this analysis, fell below that of Whites, the data indicate that among both White and Black participants, those at sites receiving R&A grants had respectively higher retention rates than those at sites that did not receive such grants.Because sites had to apply for these grants, this finding probably reflects self-selection on behalf of the sites. We postulate that these sites had higher retention rates because their staffs were more engaged or interested in SELECT and so were motivated to successfully apply for the R&A grants.
We did not include study medication adherence as a covariate in our analysis, but recognize its importance in the conduct of long-term trials.In an additional analysis, we found that disengagement from the study was much higher for participants who demonstrated non-adherence to study drugs, defined for this purpose as the first time a participant had adherence less than 80% for both study supplements. The age- and race-adjusted hazard ratio for retention failure for those who were non-adherent is 15.37 (95% CI,12.36-19.12).In our current analysis, we focused on baseline covariates known at randomization; whereas study medication adherence was measured over the course of the trial, we could not include this as a baseline characteristic in the multivariate analysis.Nevertheless, our data indicates a logical and strong statistical association between medication non-adherence and study disengagement, so that the factors that predict adherence and retention failures may be similar.
There are several limitations to this analysis. This secondary analysis relied on derived outcome criteria, failure to complete two consecutive six-month study visits or frank refusal for further contact.Study staff were instructed to attempt to reestablish contact and reactivate these participants, although successes in these attempts were modest: approximately three-fourths of disengaged participants either were not able to be contacted or provided no additional or timely (within six additional months) data. Data collection for SELECT did not fully meet the needs of this analysis.Determinants of participant socio-economic status (except for education level and living arrangements) were unavailable for the analysis. SELECT did not collect direct information from either participants or staff as to the reasons for disengagement, nor were other potentially relevant site staff characteristics available, such as staff turnover rates and staff race.We have no direct knowledge of study staff practices with respect to enhancing study retention, and conversely, practices that may have inadvertently discouraged participants’ long-term commitments to SELECT.We did not analyze other racial groups due to small numbers. Puerto Rican participants were excluded due to excessive missing racial data.Despite these limitations, we believe that our findings from the large SELECT study population allow for a direct comparison of both the respective rates of study retention and some of the key factors that contributed to study disengagement for White and Black participants.
Our results indicate a disproportionate, age-controlled increase in Black participant disengagement from SELECT.We found no personal or site characteristics that explained this racial difference.Differential retention rates between racial groups are of concern to study generalizability: significantly greater attrition by a particular group means less follow-up and reduces the opportunity for endpoint ascertainment in that group. We did identify factors that associated with increased risks of disengagement across both racial groups, specifically living alone, smoking and Hispanic self-identification – each of which may be critical in planning recruitment and enhancing retention in long-term cancer prevention, clinical trials.We also believe that the site characteristics associated with an increased risk of disengagement are important.SELECT made available special site grants to enhance recruitment and retention, both generally and minority-targeted, and participants at those sites that applied for and received such grants had lower risks of study disengagement.Participants at sites where staffs demonstrated higher rates of participation in SELECT training workshops also had lower risks for disengagement.Although we cannot discern cause and effect from this analysis, the evidence shows study site commitment is positively associated with better study retention.Thus, along with addressing personal factors that might affect long-term study participation, training and support of sites, particularly in the area of overall minority recruitment and retention, should be a key focus in the conduct of long-term clinical cancer trials.
Acknowledgments
This research was supported in part by Public Health Service Cooperative Agreement grant CA37429 (KB Arnold, KB Anderson, CM Tangen, PI: C Blanke) awarded by the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, and in part by the National Center for Complementary and Alternative Medicine (National Institutes of Health). Study agents and packaging were provided by Perrigo Company (Allegan, Michigan), Sabinsa Corporation (Piscataway, New Jersey), Tishcon Corporation (Westbury, New York), and DSM Nutritional Products Inc.The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- CCOP
Community Clinical Oncology Program
- CI
Confidence interval
- HR
Hazard ratio
- NCI
National Cancer Institutes
- R&A
Retention and Adherence
- RAC
Recruitment/Retention and Adherence Committee
- SELECT
Selenium and Vitamin E Cancer Prevention Trial
- VA
Veterans Affairs
Footnotes
Clinical trials identifier: NCT00006392
SELECT (16,17,24) was a phase III, double blind, placebo controlled clinical trial to assess the impact of selenium and vitamin E, alone and in combination, on the clinical incidence of prostate cancer.
The authors have no conflicts of interest to disclose.
References
- 1.Gadegbeku CA, Stillman PK, Huffman MD, Jackson JS, Kusek JW, Jamerson KA. Factors associated with enrollment of African Americans into a clinical trial: results from the African American study of kidney disease and hypertension. Contemporary clinical trials. 2008;29(6):837–42. doi: 10.1016/j.cct.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell EP. Prognostic impact of race and ethnicity in the treatment of colorectal cancer. The Medical clinics of North America. 2005;89(5):1045–57. 54. doi: 10.1016/j.mcna.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Levkoff S, Sanchez H. Lessons learned about minority recruitment and retention from the Centers on Minority Aging and Health Promotion. The Gerontologist. 2003;43(1):18–26. doi: 10.1093/geront/43.1.18. [DOI] [PubMed] [Google Scholar]
- 4.Brown DR, Topcu M. Willingness to participate in clinical treatment research among older African Americans and Whites. The Gerontologist. 2003;43(1):62–72. doi: 10.1093/geront/43.1.62. [DOI] [PubMed] [Google Scholar]
- 5.Gorelick PB, Harris Y, Burnett B, Bonecutter FJ. The recruitment triangle: reasons why African Americans enroll, refuse to enroll, or voluntarily withdraw from a clinical trial. An interim report from the African-American Antiplatelet Stroke Prevention Study (AAASPS) Journal of the National Medical Association. 1998;90(3):141–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Ford ME, Havstad SL, Tilley BC. Recruiting older African American men to a cancer screening trial (the AAMEN Project) The Gerontologist. 2003;43(1):27–35. doi: 10.1093/geront/43.1.27. [DOI] [PubMed] [Google Scholar]
- 7.Ford ME, Havstad SL, Fields ME, Manigo B, McClary B, Lamerato L. Effects of baseline comorbidities on cancer screening trial adherence among older African American men. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17(5):1234–9. doi: 10.1158/1055-9965.EPI-08-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumenthal DS, Sung J, Coates R, Williams J, Liff J. Recruitment and retention of subjects for a longitudinal cancer prevention study in an inner-city black community. Health services research. 1995;30(1 Pt 2):197–205. [PMC free article] [PubMed] [Google Scholar]
- 9.Moinpour CM, Atkinson JO, Thomas SM, Underwood SM, Harvey C, Parzuchowski J, et al. Minority recruitment in the prostate cancer prevention trial. Annals of epidemiology. 2000;10(8 Suppl):S85–91. doi: 10.1016/s1047-2797(00)00185-x. [DOI] [PubMed] [Google Scholar]
- 10.McCaskill-Stevens W, Wilson JW, Cook ED, Edwards CL, Gibson RV, McElwain DL, et al. Clinical trials. 2. Vol. 10. London, England: 2013. National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene trial: advancing the science of recruitment and breast cancer risk assessment in minority communities; pp. 280–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and agebased disparities. Jama. 2004;291(22):2720–6. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 12.Psaty BM, Cheadle A, Koepsell TD, Diehr P, Wickizer T, Curry S, et al. Race- and Ethnicity-specific Characteristics of Participants Lost to Follow-up in a Telephone Cohort. American Journal of Epidemiology. 1994;140(2):161–71. doi: 10.1093/oxfordjournals.aje.a117226. [DOI] [PubMed] [Google Scholar]
- 13.Ford ME, Havstad S, Vernon SW, Davis SD, Kroll D, Lamerato L, et al. Enhancing adherence among older African American men enrolled in a longitudinal cancer screening trial. The Gerontologist. 2006;46(4):545–50. doi: 10.1093/geront/46.4.545. [DOI] [PubMed] [Google Scholar]
- 14.Ofstedal MB, Weir DR. Recruitment and retention of minority participants in the health and retirement study. The Gerontologist. 2011;51(Suppl 1):S8–20. doi: 10.1093/geront/gnq100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA a cancer journal for clinicians. 2005;55(1):10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 16.Lippman SM, Goodman PJ, Klein EA, Parnes HL, Thompson IM, Jr, Kristal AR, et al. Designing the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Journal of the National Cancer Institute. 2005;97(2):94–102. doi: 10.1093/jnci/dji009. [DOI] [PubMed] [Google Scholar]
- 17.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Jama. 2009;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook ED, Moody-Thomas S, Anderson KB, Campbell R, Hamilton SJ, Harrington JM, et al. Clinical trials. 5. Vol. 2. London, England: 2005. Minority recruitment to the Selenium and Vitamin E Cancer Prevention Trial (SELECT) pp. 436–42. [DOI] [PubMed] [Google Scholar]
- 19.Cook ED, Arnold KB, Hermos JA, McCaskill-Stevens W, Moody-Thomas S, Probstfield JL, et al. Clinical trials. 1. Vol. 7. London, England: 2010. Impact of supplemental site grants to increase African American accrual for the Selenium and Vitamin E Cancer Prevention Trial; pp. 90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oddone EZ, Olsen MK, Lindquist JH, Orr M, Horner R, Reda D, et al. Enrollment in clinical trials according to patients race: experience from the VA Cooperative Studies Program (1975-2000) Controlled clinical trials. 2004;25(4):378–87. doi: 10.1016/j.cct.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Svensson CK. Representation of American blacks in clinical trials of new drugs. Jama. 1989;261(2):263–5. [PubMed] [Google Scholar]
- 22.Swanson GM, Ward AJ. Recruiting minorities into clinical trials: toward a participant-friendly system. Journal of the National Cancer Institute. 1995;87(23):1747–59. doi: 10.1093/jnci/87.23.1747. [DOI] [PubMed] [Google Scholar]
- 23.Probstfield JL, Frye RL. Strategies for recruitment and retention of participants in clinical trials. Jama. 2011;306(16):1798–9. doi: 10.1001/jama.2011.1544. [DOI] [PubMed] [Google Scholar]
- 24.Klein EA. SELECT: the selenium and vitamin E cancer prevention trial rationale and design. Prostate Cancer and Prostatis Diseases. 2000;3:145–51. doi: 10.1038/sj.pcan.4500412. [DOI] [PubMed] [Google Scholar]
- 25.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Jama. 2011;306(14):1549–56. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. Jama. 2004;291(14):1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 27.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O'Sullivan MJ, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. The New England journal of medicine. 2006;354(7):684–96. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 28.Hercberg S, Galan P, Preziosi P, et al. The su.vi.max study: A randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Archives of Internal Medicine. 2004;164(21):2335–42. doi: 10.1001/archinte.164.21.2335. [DOI] [PubMed] [Google Scholar]
- 29.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. The New England journal of medicine. 2003;349(3):215–24. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed I, Gugsa ST, Lemma S, Demissie M. Predictors of loss to follow-up before HIV treatment initiation in Northwest Ethiopia: a case control study. BMC public health. 2013;13(1):867. doi: 10.1186/1471-2458-13-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alvarez-Uria G, Naik PK, Pakam R, Midde M. Factors associated with attrition, mortality, and loss to follow up after antiretroviral therapy initiation: data from an HIV cohort study in India. Global health action. 2013;6:21682. doi: 10.3402/gha.v6i0.21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishijima T, Gatanaga H, Komatsu H, Takano M, Ogane M, Ikeda K, et al. Illicit Drug Use Is a Significant Risk Factor for Loss to Follow Up in Patients with HIV-1 Infection at a Large Urban HIV Clinic in Tokyo. PloS one. 2013;8(8):e72310. doi: 10.1371/journal.pone.0072310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rice BD, Delpech VC, Chadborn TR, Elford J. Loss to follow-up among adults attending human immunodeficiency virus services in England, Wales, and Northern Ireland. Sexually transmitted diseases. 2011;38(8):685–90. doi: 10.1097/OLQ.0b013e318214b92e. [DOI] [PubMed] [Google Scholar]
- 34.Merrill RM, Aldana SG. Cardiovascular risk reduction and factors influencing loss to follow-up in the coronary healthimprovement project. Medical science monitor : international medical journal of experimental and clinical research. 2008;14(4):Ph17–25. [PubMed] [Google Scholar]
- 35.Lebouche B, Yazdanpanah Y, Gerard Y, Sissoko D, Ajana F, Alcaraz I, et al. Incidence rate and risk factors for loss to follow-up in a French clinical cohort of HIV-infected patients from January 1985 to January 1998. HIV medicine. 2006;7(3):140–5. doi: 10.1111/j.1468-1293.2006.00357.x. [DOI] [PubMed] [Google Scholar]
- 36.Dalle Grave R, Calugi S, Molinari E, Petroni ML, Bondi M, Compare A, et al. Weight Loss Expectations in Obese Patients and Treatment Attrition: An Observational Multicenter Study. Obesity Research. 2005;13(11):1961–9. doi: 10.1038/oby.2005.241. [DOI] [PubMed] [Google Scholar]
- 37.Zunzunegui MV, Beland F, Gutierrez-Cuadra P. Loss to follow-up in a longitudinal study on aging in Spain. Journal of clinical epidemiology. 2001;54(5):501–10. doi: 10.1016/s0895-4356(00)00325-5. [DOI] [PubMed] [Google Scholar]
- 38.Blanker MH, Prins J, Bosch JL, Schouten BW, Bernsen RM, Groeneveld FP, et al. Loss to follow-up in a longitudinal study on urogenital tract symptoms in Dutch older men. Urologia internationalis. 2005;75(1):30–7. doi: 10.1159/000085924. [DOI] [PubMed] [Google Scholar]
- 39.Lertsithichai P, Pornchai S. Factors influencing loss to follow-up after elective inguinal herniorrhaphy. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2012;95(1):37–41. [PubMed] [Google Scholar]
- 40.Dudley J, Jin S, Hoover D, Metz S, Thackeray R, Chmiel J. The Multicenter AIDS Cohort Study: Retention after 9½ Years. American Journal of Epidemiology. 1995;142(3):323–30. doi: 10.1093/oxfordjournals.aje.a117638. [DOI] [PubMed] [Google Scholar]
- 41.Krishnan S, Wu K, Smurzynski M, Bosch RJ, Benson CA, Collier AC, et al. Incidence rate of and factors associated with loss to follow-up in a longitudinal cohort of antiretroviral-treated HIV-infected persons: an AIDS Clinical Trials Group (ACTG) Longitudinal Linked Randomized Trials (ALLRT) analysis. HIV clinical trials. 2011;12(4):190–200. doi: 10.1310/HCT1204-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy AB, Akereyeni F, Nyame YA, Guy MC, Martin IK, Hollowell CM, et al. Smoking and prostate cancer in a multi-ethnic cohort. The Prostate. 2013;73(14):1518–28. doi: 10.1002/pros.22699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Health, United States, 2005 with Chartbook on Trends in the Health of Americans. Hyattsville, Maryland: National Center for Health Statistics; 2005. [PubMed] [Google Scholar]
- 44.Coatsworth JD, Duncan LG, Pantin H, Szapocznik J. Patterns of retention in a preventive intervention with ethnic minority families. The journal of primary prevention. 2006;27(2):171–93. doi: 10.1007/s10935-005-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. CA: a cancer journal for clinicians. 2012;62(5):283–98. doi: 10.3322/caac.21153. [DOI] [PubMed] [Google Scholar]
- 46.Minasian LM, Carpenter WR, Weiner BJ, Anderson DE, McCaskill-Stevens W, Nelson S, et al. Translating research into evidence-based practice: the National Cancer Institute Community Clinical Oncology Program. Cancer. 2010;116(19):4440–9. doi: 10.1002/cncr.25248. [DOI] [PMC free article] [PubMed] [Google Scholar]