Abstract
α4β7 integrin expressing CD4+ T cells preferentially traffic to gut-associated lymphoid tissues (GALT) and play a key role in HIV/SIV pathogenesis. The administration of an anti-α4β7 monoclonal antibody during acute infection protects macaques from transmission following repeated low-dose intra-vaginal challenges with SIVmac251. In treated animals that became infected the GALT was significantly protected and CD4+ T–cell numbers were maintained. Thus, targeting α4β7 reduces mucosal transmission of SIV in macaques.
Keywords: SIV, α4β7 monoclonal antibody, GI pathology, Repeated Low Dose challenge model, and rhesus macaques, mucosal transmission
INTRODUCTION
CD4+ T-cells residing in gut-associated lymphoid tissues (GALT) are a predominant target of HIV/SIV during the acute phase of infection, and their depletion has been implicated in HIV/SIV disease1–3. CD4+/CCR5+ T-cells that traffic to GALT typically express α4β7, an integrin that functions as a gut-homing receptor4. α4β7 mediates gut-homing by binding to MAdCAM, a ligand expressed on venules that service GALT5. Of note, some strains of HIV/SIV interact with α4β76–8. These findings suggest that the α4β7 subset of CD4+ T-cells may play a key role in transmission, and that targeting them could disrupt early steps in infection, and possibly interfere with disease progression.
To explore that possibility we employed a recombinant rhesus anti-α4β7 mAb (α4β7-mAb)9. Previously, in a high-dose I.V./intra-rectal challenge study, α4β7-mAb treatment mediated a significant decrease in gut tissue pro-viral DNA and a 1–2 log reduction in mean peak viral load. Strikingly, while ten of twelve controls died of AIDS within two years, all ten treated animals remained healthy with CD4+ T-cell counts >500/μl five years post-infection10,11.
In this study, we evaluated the efficacy of α4β7-mAb therapy in preventing transmission. We employed a NHP model based on repeated low-dose intravaginal challenges, which more faithfully mimics HIV transmission. We found that I.V. administration of α4β7-mAb reduced surface exposure of α4β7 on CD4+ T cells in the cervico-vaginal canal, and either prevented or delayed infection. When prevention failed, both viral DNA loads in GALT, and the rate of peripheral CD4+ T-cell depletion were markedly reduced. We conclude that α4β7 antagonists might be useful for prophylaxis or treatment of HIV infection.
RESULTS
The protocol utilized for the entire study is illustrated (Supplementary Fig. 1). Baseline studies designed to optimize the collection and analyses of cytobrush and gut tissue samples were conducted in 24 uninfected female macaques (Phase I). Results are summarized in Supplementary Fig. 2a,b. Macaques were then divided into two groups (n=8), each of which was administered two sequential I.V. injections, at 3-week intervals, of either rhesus recombinant α4β7-mAb or a recombinant isotype matched control, at 50 mg/kg, as previously described10. The monitoring of plasma and CVLα4β7-mAb concentrations10 confirmed that this regimen maintained plasma levels at or above 25 μg/ml and detectable levels in the CVL fluid (Supplementary Fig. 3 a,b). Peripheral blood, rectal biopsies, and cytobrush samples were collected weekly and analyzed to determine the efficiency and kinetics of α4β7 masking. α4β7-mAb treatment inhibited α4β7-mAb binding in all three compartments (Supplementary Fig. 2c) but did not significantly alter the proportion of T-cells expressing the α4 integrin subunit, (Supplementary Fig. 2d), indicating that treatment did not eliminate α4β7+ cells, but rather masked the relevant epitope.
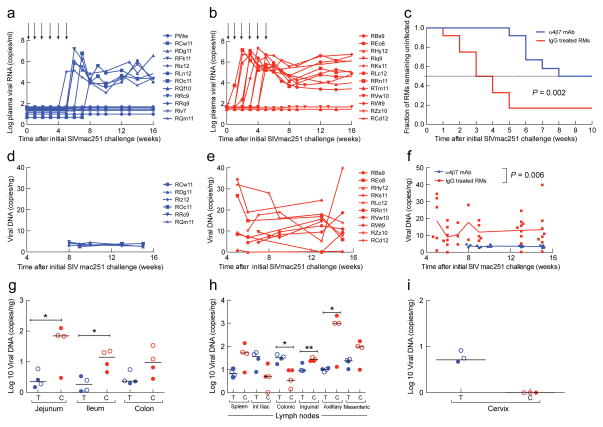
To determine if masking α4β7 affects susceptibility to infection, the 24 female macaques underwent baseline sampling of the frequencies of CD4+α4β7+T cells and Tregs in blood, gut tissues and cervico-vaginal cells for even grouping (Supplementary Fig. 4) and were then divided into two age/reproductive history matched and genetically equivalent groups (n=12 each) (Supplementary Tables 1,2). Each group was administered five injections of either α4β7-mAb or the control mAb, at three-week intervals. Three days after the first injection, animals were challenged intravaginally with a low dose of SIVmac251. Under the predetermined protocol, animals were re-challenged every week until 10 of 12 control animals became infected. Plasma viral RNA loads were then compared (Fig. 1a,b). By week five, 10 of 12 control animals became viremic and challenges were discontinued. In contrast, only one of 12 treated animals became viremic by week five. An additional five animals developed viremia by week eight, while six of 12 remained uninfected. All uninfected animals were subsequently shown not to be intrinsically resistant to infection (Supplementary Fig. 5 a,b).
Fig. 1. Kinetics of plasma viral load and tissue and organ specific pro-viral DNA loads.
Plasma viral loads from two groups of macaques (n=12 for each group) that received 50 mg/kg of either aα4β7-mAb (a) or a rhesus IgG (b) I.V. at 3 week intervals following the initiation of six, once-weekly, low-dose intra-vaginal challenges with SIVmac251 (arrows). Kaplan-Meier curves based on plasma viral RNA (n=12 each group; log-rank test; p=0.002) (c).Pro-viral DNA load (# of copies/ng DNA) in rectal biopsies from α4β7-mAb (d) or IgG control macaques (e) (n=6 for α4β7-mAb, n=10 for IgG control).Median values of data in d and e (random mixed effects (RME) model; p=0.006) (f). Two infected macaques from each group (α4β7-mAb in blue (T), control IgG in red (C)) were sacrificed at 2 weeks p.i. (filled circles) and two sacrificed at 16–18 weeks p.i. (open circles). Pro-viral DNA load (# copies/ng DNA) in jejunal, ileum and colon tissue samples from both groups (n=4; Mann-Whitney U-test; P<0.01) (g), spleen, and internal iliac, colonic, inguinal, axillary, mesenteric lymph-nodes (n=4; Mann-Whitney U-test; P<0.01) from both groups (h). Pro-viral DNA load (# copies/ng DNA) from cervical tissue samples from 3 macaques from both groups (i). Macaque I.D.’s in panels a, b, d and e are specified.
Kaplan-Meier analysis (Fig. 1c) verified that α4β7-mAb treatment significantly increased the number of challenges required for infection (p=0.002, log-rank test). The hazard ratio was 4.3, with 95% confidence limits of 1.5 to 12.2, and p=0.007, using the proportional-hazards regression model. In all, 71 challenges were needed to infect six of 12 treated macaques, while only 44 challenges sufficed to infect 10 of 12 controls, implying a 2.7-fold decreased infection risk with each challenge in the α4β7-mAb-treated group (p<0.05 by Fisher’s exact test).
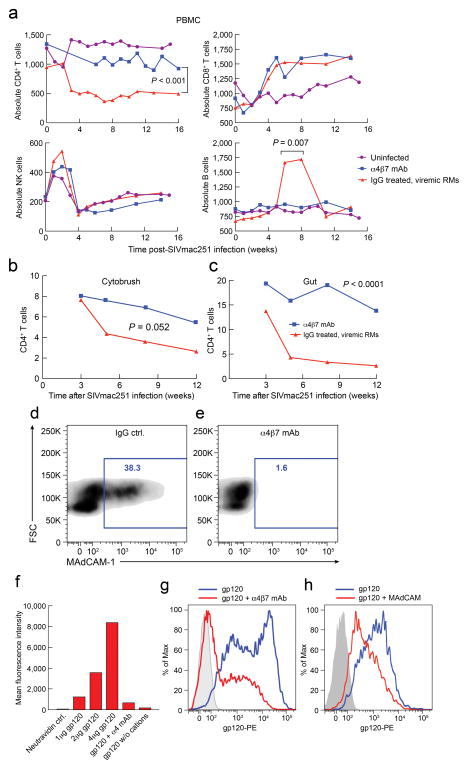
While we found no statistical difference between plasma viral loads in control vs. treated groups, (Fig. 1a,b), proviral DNA in colorectal tissues differed significantly (Fig. 1d,e). GIT biopsies from six treated/infected animals harbored, on average, 3.5 copies/ng DNA, while biopsies from 10 infected controls exhibited a median 12.8 copies/ng DNA (p= 0.006) (Fig. 1f). That disparity was confirmed in a survey of tissues collected from four viremic animals from each group postmortem. Proviral DNA was 5 to 25 fold more abundant in DNA sampled from jejunum, ileum, or colon of control vs. treated animals (Fig. 1g), indicating that α4β7-mAb mediated durable reductions in GALT infection. In contrast, proviral loads in spleen, and various other lymph nodes revealed no consistent disparity between the 2 groups (Fig. 1h). Strikingly, although proviral DNA was undetectable in most other solid organs, it was detected in ecto/endocervical tissues from three of three treated/infected animals, but not in ecto/endocervical tissues of three control/infected animals, sampled post-mortem (Fig. 1i). Thus, for animals that became infected despite treatment, α4β7-mAb was associated with a persistence of infected cells at the portal of infection, and a substantial decrease in SIV-infected cells within GALT. α4β7-mAb-treated animals that became infected maintained higher CD4+ T-cells counts in blood (p<0.001) (Fig. 2a), cytobrush specimens (p=0.0052) (Fig. 2b), and gut tissues (p<0.0001) (Fig. 2c) but had no effect on other cell lineages, confirming that α4β7-mAb treatment did not markedly alter the frequencies of major immune cell types in cervicovaginal tissues.
Fig. 2. Frequency of lymphocyte subsets from infected macaque PBMCs and inhibition of MAdCAM or SIVmac251 gp120 by α4β7-mAb.
Changes in the absolute numbers of CD4+ T cells (top left), CD8+ T cells (top right), NK cells (bottom left) and B cells (bottom right) in PBMC from infected α4β7-mAb (blue) or IgG treated (red) and uninfected (purple) macaques (n=6 for α4β7-mAb infected, n=10 for IgG infected, n=8 for uninfected); median values are given (RME model) (a).Frequencies of CD4+CD45+ T cells in cytobrush samples (n=6 for α4β7-mAb, n=9 for IgG; RME model; p=0.052) (b), and gut biopsy(c) (n=6 for α4β7 mAb and n=9 for IgG; RME model; p<0.0001).Flow-cytometric analysis of CD4+α4β7+ T cells stained with PE labeled MAdCAM-Ig in the presence of IgG (d) or α4β7-mAb (e) in the presence of MgCl2. MAdCAM-Ig-reactive cells are included within the blue box and the percent reactive cells are indicated. Binding of neutravidin-PE alone (lane 1), increasing amounts of PE labeled SIVMac251 gp120 (lanes 2–4), 2μg PE labeled SIVMac251 gp120 in the presence of an unlabeled α4 mAb (lane 5), and 2μg PE labeled SIVMac251 gp120 in the absence of divalent cations (lane 6) as controls, to CD4+α4β7+ T cells (f). Flow-cytometric profile of CD4+α4β7+ T cells stained with neutravidin PE alone (grey) or PE labeled SIVmac251 gp120 in the absence (blue) or presence (red) of unlabeled α4β7-mAb (g). CD4+α4β7+ T cells stained with neutravidin-PE alone (grey) or labeled SIVmac251 gp120 in the absence (blue) or presence (red) of unlabeled MAdCAM-Ig (h). Experiments shown in panels f, g and h were performed in presence of an anti-CD4 mAb.
The protection we observed in α4β7-mAb treated animals raised the question of an underlying mechanism(s). Two of the ways in which α4β7-mAb treatment may have reduced interfered with transmission involve its capacity to a) inhibit α4β7 binding to MAdCAM and/or b) interfere with any potential interactions between α4β7 and SIVmac251 env. To this end we found that α4β7-mAb inhibits binding of α4β7 to both MAdCAM and a gp120 derived from SIVmac251 (Fig. 2d–g). In addition we determined that MAdCAM and SIVmac251 gp120 compete for binding to α4β7 (Fig. 2h). Thus, the α4β7-mAb possesses the capacity, in at least two ways, to interfere with intravaginal transmission of SIVmac251.
DISCUSSION
The present study demonstrates that I.V. administration of α4β7-mAb shortly before and for several weeks after multiple low-dose intravaginal SIV challenges in rhesus macaques significantly decreased the likelihood of viral transmission. Furthermore, in those treated animals that did become infected, GALT viral loads were significantly reduced. Macaques receiving α4β7-mAb prophylactically were, on average, 63% less likely to become infected following any single intravaginal challenge than were controls. The profound destruction of gut CD4+ T cells that typically occurs in acute SIV/HIV infection was prevented in treated but infected animals, which may ameliorate the underlying causes of AIDS1. Our earlier studies10,11 of α4β7-mAb employed high dosages of SIVmac239 that were designed to infect all animals with a single inoculation. Under those conditions, α4β7-mAb markedly impeded disease progression and mortality. The present study was designed to understand the role of α4β7+ T-cells in sexual transmission by using repeated, low-dose, intravaginal challenges. Targeting α4β7+ T-cells not only impeded intravaginal transmission but strikingly, reduced proviral DNA loads in GALT long after treatment was terminated, despite sustained viremia.
Treatment with α4β7-mAb did not greatly alter the numbers of CD4+ T-cells within the cervicovaginal compartment, consistent with the absence of MAdCAM in the FGT under normal conditions (MAdCAM is induced in the FGT by STDs known to increase the susceptibility to HIV12). However, α4β7-mAb masked >99.9% of the α4β7 heterodimers on cells in cervicovaginal compartments (Supplementary Fig. 2c). Masking α4β7 in the FGT might prevent transmission by suppressing the spread of a nascent infection into the largest depot of vulnerable target T-cells in the body, by interfering with the physical interaction of virus with α4β7+/CD4+ cells, or by disrupting cell-cell interactions necessary for efficient viral transmission. In any case, these results strongly suggest that SIVmac251 utilizes α4β7+/CD4+ cells at some key early point in transmission. This conclusion is further supported by the delayed infection observed in a number of treated animals (Fig. 1a). Of note, one of these animals did not become viremic until three weeks after challenges were discontinued. In addition, we detected proviral DNA in cervical tissues of the α4β7-mAb infected/treated macaques but not in controls in necropsies performed early or at 16–18 weeks p.i. (Fig. 1i). Our findings are in accord with numerous studies that implicateα4β7 integrin in promoting mucosal transmission of HIV/SIV13–16. The substantial decrease in the frequency of mucosal infection that we observed in α4β7-mAb-treated animals strongly implicates α4β7 as a key determinant of SIV transmission. Unambiguous evidence of lower tissue proviral loads and sparing of vital T-cell subsets, even after treatment was discontinued, underscores the potential utility of α4β7-directed intervention in SIV/HIV disease. Recent reports from clinical trials of novel drugs targeting α4β7 indicate their safety and efficacy in treating gastrointestinal inflammatory disorders17–20 and raise the possibility that such therapies might prove efficacious in preventing and ameliorating HIV disease.
ONLINE METHODS
Animals
Adult female rhesus macaques (Macaca mulatta) of Indian origin were used for the studies reported herein. The animals were born and housed at the Yerkes National Primate Research Center (YNPRC) of Emory University (Atlanta, GA) and were maintained according to the guidelines of the Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council and the Department of Health and Human Service guideline titled Guide for the Care and Use of Laboratory Animals. These animals were fed monkey diet (Purina) supplemented daily with fresh fruit or vegetables and water ad libitum. The studies reported herein were performed under IACUC protocol #2001725 “Gut homing cells in SIV infection” which was reviewed and approved by the Emory University IACUC and the biosafety review Committee. The YNPRC has been fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International since 1985.
Genetic Typing
PBMCs from each of the rhesus macaques were subjected to MHC class I typing according to protocols previously published 21,22. In addition, each of these macaques was also typed for TRIM-5α, FcR and KIR polymorphisms using our standard laboratory protocols 21,23,24. Results of the genotyping are provided in Supplementary Table 1 a, b & c.
Study Design
The overall objective of this study was to evaluate the efficacy of the α4β7-mAb in influencing the infection susceptibility and kinetics of viremia and factors that impact disease in female rhesus macaques using a low-dose-repeated-challenge model (LDRC). Even though several studies have been conducted using this model 25,26, there is still a paucity of data on the immunological composition of the female reproductive tract in NHP. The studies reported herein were therefore performed in 3 phases each with a distinct objective (see Supplementary Fig. 1). Phase I was designed to optimize the procedure to be utilized for obtaining cytobrush specimens from the macaques and to determine the number and phenotypes of the cells from such cytobrush specimens in order to lay the foundation for studies under Phase II and III. Phase II was designed to determine whether the recombinant α4β7-mAb administered at the doses previously utilized27 reaches the vaginal tissues and to determine its pharmacokinetics. Phase III was the final study designed to assess the efficacy of the α4β7-mAb to influence SIV infection using the LDRC model. Sample sizes for the Phase III study were calculated using parameters obtained from our virus titration studies which utilized 6 female rhesus macaques, and was based upon statistical methods as outlined elsewhere28,29. For 80% power, a 2-sided alpha<0.05, and assuming an infection probability of 0.2 among control animals (e.g. 4 infections per 20 exposures), an effect size of 75%, and a susceptibility of 90–100%, it was determined that a total of 24 adult female rhesus macaques were required, 12 to receive rhesus IgG (controls) and another 12 to receive the anti-α4β7 mAb. The virus stock of SIVmac251 was obtained courtesy of Dr. Nancy Miller (NIAID, NIH). Data from the initial intra-vaginal titration studies suggested that we utilize 1 ml of a 1/20 dilution of the stock virus (5000 TCID50 containing 36 ng of p24). Following the collection of 3 pre- infusion baseline samples from each of the 24 macaques, the 12 control macaques were administered 50 mg/kg of rhesus IgG and the other 12 were administered 50 mg/kg of the α4β7-mAb intravenously every 3 weeks (based on previous PK studies aimed at maintaining a trough plasma level of >5 μg/ml, data not shown). None of the animals were treated with Depo-provera but were monitored for levels of progesterone and estradiol (Biomarkers Core Lab, YNPRC) in order to monitor cycling. Each of the 24 was then challenged 3 days later intra-vaginally with a 1/20 dilution of the stock virus in 1.0 ml of RPMI once weekly according to previously established procedures. Since infection by either the intra-vaginal or intra-rectal route usually reveals that approximately 10% of animals are refractory to infection30–32, it was reasoned that all 24 macaques would be exposed weekly until a majority of the control macaques (10/12) became infected. Infection was defined as plasma positive viremia at 2 consecutive weeks. Macaques were bled weekly for 6 weeks, biweekly until 12 weeks and monthly thereafter for a series of other studies as outlined below, including plasma viral load determinations. The green arrows in Supplementary Figure 1 show weekly bleeds because the 24 macaques were divided into 2 groups and for practical reasons bled on alternative weeks. None of the macaques were biopsied or subjected to cervical brush sampling until they were confirmed as infected in order to avoid any trauma that might promote infection. Shortly after infection, cytobrush specimens and colo-rectal biopsy sampling was performed bi-weekly for 6 weeks and then monthly thereafter. While colo-rectal biopsy specimen collection included the procurement of 6–8 pinch biopsies using a standard bioptome as previously described27, the cytobrush specimen collection procedure had to be optimized (see below). Cervico-vaginal lavage (CVL) fluids from the macaques were collected as previously described33 and aliquots kept at −80°C until used for analyses.
Cytobrush specimen collection
The cytobrush procedure utilized was similar to that utilized for humans34 modified and performed in consultation with Dr. Rupert Kaul (University of Toronto, Toronto, Canada). Briefly, a 3 ml syringe was cut transversally and the rubber plunger inserted within the barrel and the outside of the barrel coated with olive oil and introduced into the vaginal canal. The syringe plunger was then pulled out and the syringe barrel advanced gently against the cervix. A cytobrush+ (Medskin Medical, Cooper Surgical, Berlin, Germany) was then passed through the barrel of the syringe, introduced into the cervix with a rotating motion to collect cells. The cytobrush was then removed and placed in a 15 ml conical tube containing 2 ml of RPMI-1640 media containing DNAse and 10 U/ml of sodium heparin and immediately placed on ice. The cytobrush was then placed successively in 3 tubes containing 3, 5, and 5 ml of media and each tube vortexed for 30 seconds each and the cytobrush then rinsed with 2 ml of media to remove any remaining cells and the content from all 4 tubes pooled (15 ml) and centrifuged at 150xg. The cell pellet was re-suspended in 100 μl of media and an aliquot used to determine cell count and the cells adjusted to 10,000 cells per 100 μl and subjected to flow-cytometric analyses as outlined below. Initial study of 64 such cytobrush samples from uninfected female macaques led to 41 that yielded >10,000 cells/sample (64%) that were sufficient in number to provide an initial profile of the phenotype of cells present in the cytobrush samples. The frequencies of CD3+, CD4+, CD8+ and NKG2a+ cells in the cytobrush samples were similar to those from the GIT biopsies of the corresponding animals (Supplementary Figures 2a & b).
Viral loads
Plasma viral loads were monitored in samples every week for 4 weeks, every other week for 8 weeks and monthly thereafter, using bDNA quantitation (Siemens Inc., Berkeley, CA) on aliquots of EDTA-plasma by Siemens Inc. and expressed as number of copies/ml of plasma. Macaques were only considered infected when their plasma viral loads showed > 1000 vRNA copies/ml on 2 successive weeks, although the threshold for the assay being utilized was 50 vRNA copies/ml of plasma. Cellular pro-viral DNA loads were performed on aliquots of mononuclear cells isolated from colo-rectal biopsy tissue sample as described elsewhere27. Gastro-intestinal tissue (GIT) pro-viral DNA loads were expressed as number of copies/ng DNA with a sensitivity of detection of 1 copy/ng DNA27. Plasma samples from all the Macaques that were classified as negative (below level of detection) were subjected to ultra-sensitive PCR analysis (detection limit 1 copy/ml) by Dr. M. Piatak (NCI, NIH, Frederick, MD) for confirmation. All samples recorded as negative were confirmed to be negative by his lab. In addition, a select number of CVL fluids collected from the macaques prior to the study (controls) and post confirmation of SIV infection were also subjected to RT-PCR analysis for the detection of virus. The CVL fluid (0.5 ml) was centrifuged at 450xg and the resulting cell pellet utilized for RNA extraction, and the subjected to RT-PCR using the same protocol as utilized for the plasma samples. The purpose was primarily to determine if virus could be detected in such samples.
Polychromatic Flow Cytometric Analyses
The frequencies of subsets of mononuclear cells in blood samples and cells isolated from pools of GI tissue biopsies and cytobrush specimens was determined using panels of monoclonal antibody reagents with a focus on analyses of α4β7 expression and blocking as described elsewhere27,35. In brief, the PBMCs were isolated from heparinized blood, and the mononuclear cells were isolated from pools of colo-rectal biopsies by techniques outlined elsewhere27, and cells obtained from cytobrush samples as outlined above. Absolute values of each cell lineage in the blood were calculated from CBCs that were performed on an aliquot of each blood sample. A battery of rhesus macaque reactive commercially purchased monoclonal antibody reagents conjugated with a variety of fluorochromes were used for polychromatic flow cytometric analysis of lymphoid cells utilizing a LSR-II flow cytometer (B-D Immunocytometry Division, Mountain View, CA). Appropriate panels of the monoclonal antibodies were used to identify subsets of T cells, B cells and NK cells as described elsewhere35. Phenotypically NK cells were defined as cells that were CD3-, CD8+, NKG2a+, which includes the 4 major subsets of NK cells. In the case of the cytobrush specimens, the antibody panel included Live/Dead; PE-Cy7 CD45; Alexa700 CD3; PerCP-Cy5.5 CD4; Pac Blue CD8; APC-Cy7 CD20; FITC CCR5; PE- NKG2a; APC α4β7; and PE-Cy5 α4. Following gating on live cells, the cells were gated on CD45+ cells (on average consisting of <10% of the cellular elements) and a minimum of 10,000 events analyzed per cytobrush specimen. Samples showing >2–3% CD20+ B cells were excluded from analysis (reasoned to be contaminated with blood). Cells that stained positively for the anti-α4 integrin but failed to stain with anti-α4β7 was used as a measure of the blocking of α4β7 expressing cells by the infused α4β7-mAb as described previously27.
Plasma and CVL levels of the α4β7-mAb and anti-idiotypic antibodies
Aliquots of the plasma samples and the CVL fluid were analyzed for levels of α4β7-mAb as described elsewhere27. Aliquots of the same plasma samples were also analyzed for levels of anti-idiotypic antibodies using standard ELISA.
In vitro and in vivo susceptibility to SIV infection
PBMCs from the appropriate macaques to be tested were cultured for 2 days in vitro in media (RPMI 1640 medium supplemented with antibiotics, l-glutamine, 10% fetal calf serum) in the presence of anti-CD3/CD28 conjugated immunobeads + rHu-IL-2 (20 U/ml). The cells were then washed and cultured at 2 × 106/ml in either media (control) or inoculated with SIVmac251, and the supernatant fluids collected at varying time intervals up to d14 and assayed for levels of p27, utilizing a standard p27 ELISA. Two of the macaques originally from the control IgG group (RIq9 and RTm11) and 2 originally from the anti-α4β7-mAb group (RRq9 and RVi7) that were resistant to IVAG infection were challenged intra-rectally with 1 ml of the undiluted SIVmac251 stock virus. However, RIq9 and RTm11 were switched in this study to receive α4β7-mAb and RRq9 and RVi7 switched to receive rhesus IgG according to the same dose and schedule as outlined under Supplementary Fig. 1 to neutralize any potential bias from the previous administration of the antibodies in the studies reported under Fig. 1a and b. Plasma samples from each of these 4 macaques were subsequently monitored for SIV using the same assay. Macaques were considered infected if the plasma from the monkey was positive (> 10,000 viral copies/ml) at 2 consecutive weeks.
Necropsy studies
In efforts to determine what differences, if any, could be detected in in vivo organ or tissue localization of SIV, 4 animals from the control group and 4 from the group that received the α4β7-mAb confirmed as SIV positive were subjected to necropsy (2 animals from each of the 2 groups were sacrificed at 2 weeks post infection and data displayed in Fig. 1 as filled circles and 2 from each of the 2 groups sacrificed at 16–18 weeks post infection displayed as open circles in Fig. 1) with 42 different tissues sampled. It is to be noted that we were only able to obtain cervical tissues from 3 of the 4 animals from each group due to technical error. Flash frozen tissue samples were utilized to extract DNA and the DNA quantitated and subjected to pro-viral DNA analysis and the data recorded as number of viral copies/ng DNA.
α4β7-mAb inhibition of MAdCAM and SIVmac251 gp120
Specific inhibition of MAdCAM binding to α4β7 by the α4β7-mAb was carried out as follows. CD4+ T cells were cultured in 1 uM retinoic acid (RA) to induce increased expression of α4β7. Aliquots of 300,000 cells were stained with biotin/neutravidin-PE labeled MAdCAM-Ig (0.5 μg) in the presence of a 5X molar excess of either an irrelevant unlabeled IgG control mAb, α4β7-mAb or the anti-α4 mAb clone 2B4 (R&D Systems), using a standard flow-cytometric staining protocol, with the inclusion of 1mM MgCl2/100μM CaCl2 in the stain and rinse buffers. A recombinant gp120 corresponding to the sequence derived from SIVmac251 (accession number JQ086004) was constructed, expressed, purified and biotin labeled as described in detail in the supplementary methods of reference36. A total of 300,000 RA cultured CD4+ T cells were stained with 1–4 μg of biotin labeled gp120 in the presence of I mM MnCl2/100μM CaCl2. The α4-mAb 2B4 was used to inhibit gp120 binding to α4β7, and cells were stained in the absence of divalent cations as an additional way to inhibit cation-dependent binding of gp120 to α4β736. Inhibition of gp120 binding by the α4β7-mAb to α4β7 was demonstrated by pre-incubation of cells with a 5X molar excess of unlabeled α4β7-mAb, prior to the addition of biotin/neutravidin-PE gp120.
Competition between gp120 and MAdCAM was demonstrated by simultaneously adding biotin/neutravidin-PE labeled gp120 with unlabeled MAdCAM to CD4+ T cells. All data were collected on a BD FACs CANTO (BD Biosciences, San Jose CA).
Statistical Analyses
The Fisher’s exact test (2-sided p-value <0.05) was implemented to assess differences in infection probabilities of α4β7-treated animals relative to IgG control animals. Kaplan-Meier survival curves and the log-rank test were used to plot and compare study group differences in cumulative time (# of exposures) to infection; proportional hazards regression was used to estimate the instantaneous hazard for infection. Means of subject-specific Pearson correlation statistics were computed. Mixed effects regression models were implemented to test for group differences and trends using longitudinal, repeated measurements. Reported p-values are based on two-sided testing and a p-value <0.05 was considered statistically significant. Statistical analyses were performed using Prism® GraphPad Software (version 5, CA) or SAS software, version 9.3 (SAS Institute, Cary, NC, USA).
Supplementary Material
Acknowledgments
This work was supported by a grant from the NIH-NIAID AI-098628-01 (AAA) and OD 51POD1113 to the Yerkes National Primate Research Center. The authors are grateful to Dr. Fawn Connor-Stroud for help with the cytobrush studies and to the veterinary staff and animal caretakers of the Yerkes National Primate Center of Emory University specially Ms. Stephanie Ehnert and her team. The authors also graciously acknowledge the assistance of Dr. M. Piatak (NCI, NIH, Frederick, MD) for performing the ultra sensitive PCR analysis and Dr. Rupert Kaul/Dr. L. R. McKinnon (University of Toronto, Toronto, Canada) for sharing with us his finding on cervical brush analyses in Africa and advising us on how to proceed in adapting their human findings to our nonhuman primates. Recombinant monoclonal antibodies were produced by the Nonhuman Primate Reagent Resource (NIAID, NIH contract # HHSN272200900037C). We apologize to all the authors whose publications we failed to cite due to restrictions in the number of references we could include.
Footnotes
AUTHOR CONTRIBUTIONS
The day-to-day scheduling of the experiments were carried out under the laboratory supervision of A.E.M. and executed by S.B. and B.K. and technically performed by P.D., T.V., and D.L. The experiments described in Fig. 2d–h were performed by F.N. and J.H. The overall planning and direction of the studies was carried out by A.A.A., J.A., C.C., F.V., and P.S. in regularly scheduled consultation with E.N.K., J. M. M. of the CDC, Atlanta, GA and TGP. D.H. provided the statistical planning of the studies and performed the statistical analyses of the data obtained. K.A.R. consulted and provided the large-scale preparation of the recombinant α4β7 monoclonal antibody and the normal rhesus IgG mAbs. M.B. and L.W. performed the MHC typing of the animals and K.R. performed the FcR typing of the animals. AAA and TGP prepared the draft of this manuscript with input from all the authors. A.S.F. provided helpful discussions and review and revision of the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests. The findings in this report are those of the authors and do not necessarily reflect the views of the Centers for Disease Control and Prevention.
References
- 1.Brenchley JM, Douek DC. The mucosal barrier and immune activation in HIV pathogenesis. Curr Opin HIV AIDS. 2008;3:356–361. doi: 10.1097/COH.0b013e3282f9ae9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehandru S, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veazey RS, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 4.Bargatze RF, Jutila MA, Butcher EC. Distinct roles of L-selectin and integrins alpha 4 beta 7 and LFA-1 in lymphocyte homing to Peyer’s patch-HEV in situ: the multistep model confirmed and refined. Immunity. 1995;3:99–108. doi: 10.1016/1074-7613(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 5.Erle DJ, et al. Expression and function of the MAdCAM-1 receptor, integrin alpha 4 beta 7, on human leukocytes. J Immunol. 1994;153:517–528. [PubMed] [Google Scholar]
- 6.Arthos J, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9:301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura GR, Fonseca DP, O’Rourke SM, Vollrath AL, Berman PW. Monoclonal antibodies to the V2 domain of MN-rgp120: fine mapping of epitopes and inhibition of alpha4beta7 binding. PloS one. 2012;7:e39045. doi: 10.1371/journal.pone.0039045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nawaz F, et al. The Genotype of Early-Transmitting HIV gp120s Promotes alpha(4)beta(7) -Reactivity, Revealing alpha(4)beta(7)/CD4 T cells As Key Targets in Mucosal Transmission. PLoS Pathog. 2011;7:e1001301. doi: 10.1371/journal.ppat.1001301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira LE, et al. Preliminary in vivo efficacy studies of a recombinant rhesus anti-alpha(4)beta(7) monoclonal antibody. Cell Immunol. 2009;259:165–176. doi: 10.1016/j.cellimm.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ansari AA, et al. Blocking of alpha4beta7 gut-homing integrin during acute infection leads to decreased plasma and gastrointestinal tissue viral loads in simian immunodeficiency virus-infected rhesus macaques. J Immunol. 2011;186:1044–1059. doi: 10.4049/jimmunol.1003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwa S, et al. Plasmacytoid dendritic cells are recruited to the colorectum and contribute to immune activation during pathogenic SIV infection in rhesus macaques. Blood. 2011;118:2643–2644. doi: 10.1182/blood-2011-02-339515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly KA, Rank RG. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis. Infection and immunity. 1997;65:5198–5208. doi: 10.1128/iai.65.12.5198-5208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cicala C, et al. The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc Natl Acad Sci U S A. 2009;106:20877–20882. doi: 10.1073/pnas.0911796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kader M, et al. Alpha4(+)beta7(hi)CD4(+) memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. 2009;2:439–449. doi: 10.1038/mi.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinelli E, et al. The frequency of alpha4beta7 high memory CD4+ T cells correlates with susceptibility to rectal SIV infection. J Acquir Immune Defic Syndr. 2013;21:21. doi: 10.1097/QAI.0b013e31829f6e1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKinnon LR, et al. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. J Immunol. 2011;187:6032–6042. doi: 10.4049/jimmunol.1101836. [DOI] [PubMed] [Google Scholar]
- 17.Feagan BG, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. The New England journal of medicine. 2013;369:699–710. doi: 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 18.Jovani M, Danese S. Vedolizumab for the treatment of IBD: a selective therapeutic approach targeting pathogenic a4b7 cells. Current drug targets. 2013;14:1433–1443. doi: 10.2174/13894501113146660206. [DOI] [PubMed] [Google Scholar]
- 19.Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. The New England journal of medicine. 2013;369:711–721. doi: 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 20.Danese S, et al. Biological agents for moderately to severely active ulcerative colitis: a systematic review and network meta-analysis. Annals of internal medicine. 2014;160:704–711. doi: 10.7326/M13-2403. [DOI] [PubMed] [Google Scholar]
- 21.Moreland AJ, et al. Characterization of killer immunoglobulin-like receptor genetics and comprehensive genotyping by pyrosequencing in rhesus macaques. BMC genomics. 2011;12:295. doi: 10.1186/1471-2164-12-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Leary CE, et al. Identification of novel MHC class I sequences in pig-tailed macaques by amplicon pyrosequencing and full-length cDNA cloning and sequencing. Immunogenetics. 2009;61:689–701. doi: 10.1007/s00251-009-0397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim SY, et al. TRIM5alpha Modulates Immunodeficiency Virus Control in Rhesus Monkeys. PLoS Pathog. 2010;6:e1000738. doi: 10.1371/journal.ppat.1000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen DC, Scinicariello F, Attanasio R. Characterization and allelic polymorphisms of rhesus macaque (Macaca mulatta) IgG Fc receptor genes. Immunogenetics. 2011;63:351–362. doi: 10.1007/s00251-011-0514-z. [DOI] [PubMed] [Google Scholar]
- 25.Butler K, et al. Susceptibility to repeated, low-dose, rectal SHIVSF162P3 challenge is independent of TRIM5 genotype in rhesus macaques. AIDS research and human retroviruses. 2013;29:1091–1094. doi: 10.1089/aid.2012.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subbarao S, et al. Direct stringency comparison of two macaque models (single-high vs. repeat-low) for mucosal HIV transmission using an identical anti-HIV chemoprophylaxis intervention. Journal of medical primatology. 2007;36:238–243. doi: 10.1111/j.1600-0684.2007.00241.x. [DOI] [PubMed] [Google Scholar]
- 27.Ansari AA, et al. Blocking of alpha4beta7 gut-homing integrin during acute infection leads to decreased plasma and gastrointestinal tissue viral loads in simian immunodeficiency virus-infected rhesus macaques. J Immunol. 2011;186:1044–1059. doi: 10.4049/jimmunol.1003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudgens MG, Gilbert PB. Assessing vaccine effects in repeated low-dose challenge experiments. Biometrics. 2009;65:1223–1232. doi: 10.1111/j.1541-0420.2009.01189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Regoes RR, Longini IM, Feinberg MB, Staprans SI. Preclinical assessment of HIV vaccines and microbicides by repeated low-dose virus challenges. PLoS medicine. 2005;2:e249. doi: 10.1371/journal.pmed.0020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Letvin NL, et al. No evidence for consistent virus-specific immunity in simian immunodeficiency virus-exposed, uninfected rhesus monkeys. Journal of virology. 2007;81:12368–12374. doi: 10.1128/JVI.00822-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Promadej-Lanier N, et al. Resistance to Simian HIV infection is associated with high plasma interleukin-8, RANTES and Eotaxin in a macaque model of repeated virus challenges. J Acquir Immune Defic Syndr. 2010;53:574–581. doi: 10.1097/QAI.0b013e3181d3521f. [DOI] [PubMed] [Google Scholar]
- 32.Veazey RS, Shattock RJ, Klasse PJ, Moore JP. Animal models for microbicide studies. Current HIV research. 2012;10:79–87. doi: 10.2174/157016212799304715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spear G, Rothaeulser K, Fritts L, Gillevet PM, Miller CJ. In captive rhesus macaques, cervicovaginal inflammation is common but not associated with the stable polymicrobial microbiome. PloS one. 2012;7:e52992. doi: 10.1371/journal.pone.0052992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKinnon LR, et al. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. J Immunol. 2011;187:6032–6042. doi: 10.4049/jimmunol.1101836. [DOI] [PubMed] [Google Scholar]
- 35.Pereira LE, et al. Preliminary in vivo efficacy studies of a recombinant rhesus anti-alpha(4)beta(7) monoclonal antibody. Cell Immunol. 2009;259:165–176. doi: 10.1016/j.cellimm.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arthos J, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9:301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.