Abstract
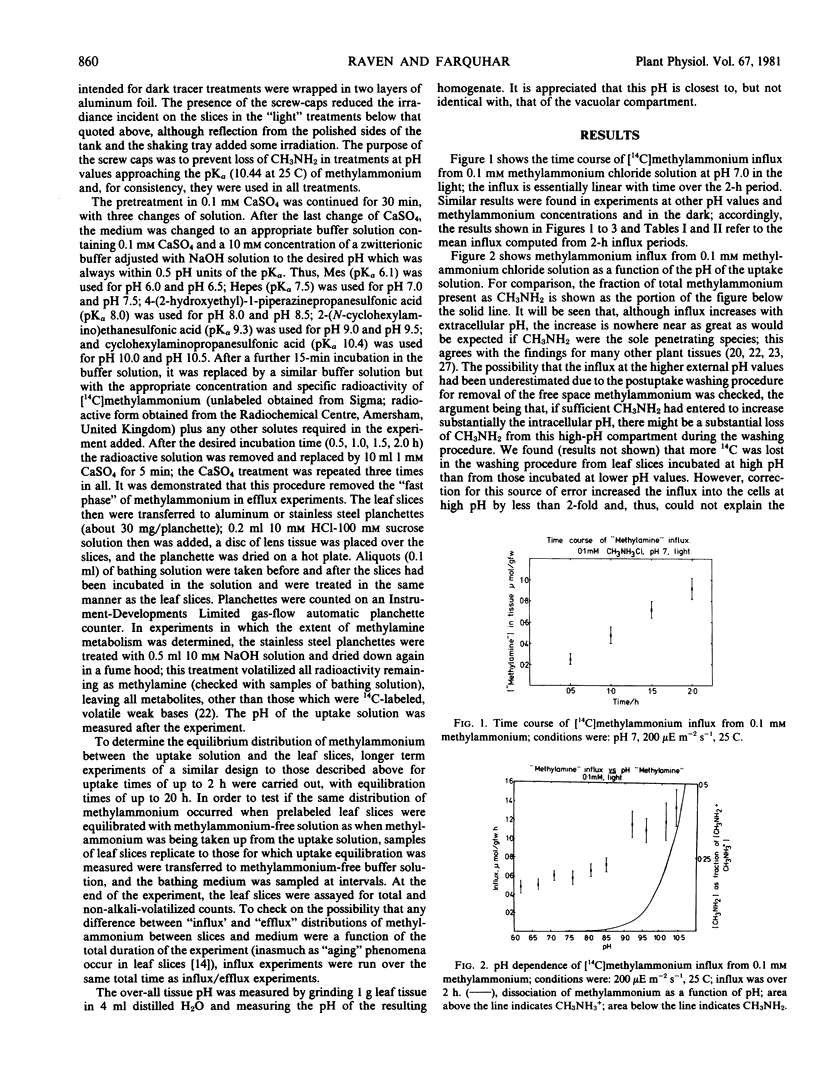
Methylammonium (as a nonmetabolized analog of ammonium) transport was studied in leaf slices of Phaseolus vulgaris L. var. `Hawkesbury Wonder.' The relationship of influx to external pH (6.0-10.5) shows that the influx at low external pH is a larger fraction of that at high external pH than would be expected from the pKα of methylammonium and the assumption that only CH3NH2 is entering the cells. The relationship between methylammonium influx and external methylammonium concentration shows some evidence of saturation; this is a function of the transport system rather than of the (limited) methylammonium metabolism in the cells. The “equilibrium” concentration ratio for methylammonium between leaf slices and bathing medium is far higher than can be explained by the transport of CH3NH2 alone and the pH of the compartments involved. These three lines of evidence strongly suggest that there is an influx of CH3NH3+, possibly by a uniporter driven by the electrical potential of the cytoplasm with respect to the medium, as has been shown for other plant cells. Competitive inhibition of methylammonium influx by ammonium suggests that there is also an ammonium transport system. The significance of this for the recycling of N within the plant and for exchange of gaseous NH3 between leaves and the atmosphere is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Farquhar G. D., Firth P. M., Wetselaar R., Weir B. On the Gaseous Exchange of Ammonia between Leaves and the Environment: Determination of the Ammonia Compensation Point. Plant Physiol. 1980 Oct;66(4):710–714. doi: 10.1104/pp.66.4.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht J., Walter A. Transport of auxin (indoleacetic acid) through lipid bilayer membranes. J Membr Biol. 1980 Aug 21;56(1):65–72. doi: 10.1007/BF01869353. [DOI] [PubMed] [Google Scholar]
- Higinbotham N., Etherton B., Foster R. J. Effect of External K, NH(4), Na, Ca, Mg, and H Ions on the Cell Transmembrane Electropotential of Avena Coleoptile. Plant Physiol. 1964 Mar;39(2):196–203. doi: 10.1104/pp.39.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby B. Light sensitivity of na, rb, and k absorption by different tissues of bean leaves. Plant Physiol. 1975 Jun;55(6):978–981. doi: 10.1104/pp.55.6.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstreth D. J., Nobel P. S. Salinity effects on leaf anatomy: consequences for photosynthesis. Plant Physiol. 1979 Apr;63(4):700–703. doi: 10.1104/pp.63.4.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. S., Layzell D. B., Atkins C. A. Economy of Carbon and Nitrogen in a Nodulated and Nonnodulated (NO(3)-grown) Legume. Plant Physiol. 1979 Dec;64(6):1083–1088. doi: 10.1104/pp.64.6.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven J. A. Nutrient transport in microalgae. Adv Microb Physiol. 1980;21:47–226. doi: 10.1016/s0065-2911(08)60356-2. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The driving force for proton(s) metabolites cotransport in bacterial cells. FEBS Lett. 1976 Jul 15;66(2):159–163. doi: 10.1016/0014-5793(76)80493-0. [DOI] [PubMed] [Google Scholar]


