Abstract
Background
Melanocytic nevi (moles) and freckles are well known biomarkers of melanoma risk, and they are influenced by similar ultraviolet (UV) light exposures and genetic susceptibilities to those that increase melanoma risk. Nevertheless, the selective interactions between UV exposures and nevus and freckling genes remain largely undescribed.
Methods
We conducted a longitudinal study from ages 6 through 10 in 477 Colorado children who had annual information collected for sun exposure, sun protection behaviors, and full body skin exams. MC1R and HERC2/OCA2 rs12913832 were genotyped and linear mixed models were used to identify main and interaction effects.
Results
All measures of sun exposure (chronic, sunburns and waterside vacations) contributed to total nevus counts, and cumulative chronic exposure acted as the major driver of nevus development. Waterside vacations strongly increased total nevus counts in children with rs12913832 blue eye color alleles and facial freckling scores in those with MC1R red hair color variants. Sunburns increased numbers of larger nevi (≥2 mm) in subjects with certain MC1R and rs12913832 genotypes.
Conclusions
Complex interactions between different UV exposure profiles and genotype combinations determine nevus numbers and size, and the degree of facial freckling.
Impact
Our findings emphasize the importance of implementing sun-protective behavior in childhood regardless of genetic make-up; although children with particular genetic variants may benefit from specifically targeted preventive measures to counteract their inherent risk of melanoma. Moreover, we demonstrate, for the first time, that longitudinal studies are a highly powered tool to uncover new gene-environment interactions that increase cancer risk.
Keywords: nevi, freckles, UV, MC1R, OCA2
Introduction
As melanoma incidence rises, interest mounts regarding understanding the interactions between genetic and environmental factors that lead to its development(1). Ultraviolet (UV) rays in sunlight are the principal environmental determinant of melanoma, whereas the number of melanocytic nevi, or moles, is the strongest host risk indicator(2-5). Patterns of UV exposure, such as waterside vacations, sunburns and chronic exposure, during childhood and adolescence, correlate with melanoma incidence and nevi (2, 6). Moreover, most of the known genetic factors that predispose to nevi also confer melanoma risk(7), and UV-induced mutations in oncogenes such as BRAF are found at similar rates in nevi and melanoma(8). Therefore, the shared etiology of nevi and melanoma, and the shared role of UV in their genesis offer a unique opportunity where the study of nevus development in children may shed light on the gene-UV interactions that give rise to melanoma.
Pigmentation phenotypes including fair or red hair, light skin and blue eye color, high nevus counts and dense freckling are known melanoma risk indicators(9-11), and many of the genetic loci responsible for these traits have now been identified and most are associated with melanoma(12). Freckles (or ephelides) are benign, usually small (1-2 mm) pigmented spots that appear on the sun-exposed skin of young fair-skinned or red-haired children(13). Two major pigmentation genes, the Melanocortin-1 receptor (MC1R) and the Oculocutaneous Albinism Type 2 (OCA2) gene contribute to melanoma risk phenotypes (14-16). MC1R, a G protein-coupled receptor, is the major red hair color (RHC) gene (17-19), while the OCA2 locus encodes the P protein, an integral melanosomal protein of uncertain function(20). We and others showed that MC1R variants are associated with fair skin color, freckles, poor tanning, susceptibility to sunburn and reduced nevus counts (16-18, 21). Much of the impact of OCA2 on normal pigmentation may be attributable to a single SNP, rs12913832, which resides in a distal regulatory sequence within the neighboring HERC2 gene(14, 20, 22, 23). rs12913832 modulates the level of OCA2 expression, accounting for >70% of blue vs. brown eye color differences in Caucasians(22, 24). SNPs at this locus are associated with increased freckle and nevus counts, lighter hair and skin, reduced tanning capacity and perhaps increased melanoma risk (16, 25, 26).
This study of a longitudinal cohort of children sought to evaluate the impact of different UV exposure patterns on both nevus and freckle formation in relation to genetic factors. Thus, we present data on differences in nevus counts and freckling scores in children with different sun exposures and different MC1R and OCA2 genotypes.
Materials and Methods
Study Population, Pigmentation and Exposure Variables
A cohort of 1,145 children born in 1998 participated in annual skin exams, and key phenotypic, environmental, and behavioral measures were recorded (27). Of these children, 509 provided DNA samples in 2007-2008 and were included in the analysis. Their parents completed surveys regarding the children's sun-behavioral patterns from mid-June to mid-October over the 2004 – 2008 time periods. The study population was restricted to include white (Hispanic and non-Hispanic) children (n = 477). This study protocol was approved by the Colorado Multiple Institutional Review Board and the Institutional Review Board for Kaiser Permanente of Colorado. Both of these organizations adhere to the Declaration of Helsinki protocols for human subjects research. Parents provided written informed consent and children provided written assent beginning at age 7.
Melanocytic nevus counts, facial freckling density levels, hair, eye, and constitutive skin color were recorded during annual skin exams as previously described (27, 28). UV exposures, and sun-protection practices were assessed at the enrollment interview (2003/2004) and at each annual telephone interview from 2004-2007 (29) using a composite index that included shade seeking and sun-protective clothing, hat and sunscreen use; the higher the composite score, the greater the protection behavior(29). Waterside vacations were considered cumulatively, with numbers taken from birth to the year prior to each skin exam. Cumulative levels of chronic exposure as of each interview year were computed by summing scores on this variable over time starting in 2004. Cumulative total number of sunburns as of each interview year was obtained by summing the number of prior year sunburns across years starting with 2004.
OCA2/HERC2 rs12913832 SNP Genotyping and MC1R sequencing
DNA was collected and extracted from cheek cells using a commercial buccal swab kit (Epicentre Technologies, Madison, WI). The MC1R coding region was sequenced as described (18). Alleles were classified as strong and weak red hair alleles, R and r respectively, and were further grouped into three genotype groups as described (16): [R/R, R/r], [R/+, r/r], [r/+, +/+]. R alleles included D84E, R142H, R151C, R160W, and D294H while r alleles were V60L, V92M and R163Q. We detected additional rare variants including T95M, A139V, I155T, A166V and F196V that we considered as r variants for this analysis. rs12913832 was genotyped using a custom-designed Taqman endpoint genotyping assay shown in Supplemental Methods.
Data Analysis
Preliminary analyses evaluating differences between subjects who did and did not provide DNA were performed using SPSS and SAS/STAT software (Version 9.3 for Windows, SAS Institute, NC, USA). Descriptive statistics (means, standard deviations, proportions) were used to characterize participants in each year and chi-square and one-way analysis of variance tests were used to assess differences in subjects with different MC1R and rs12913832 genotypes. Missing exposure data was imputed as described in Supplemental Methods. SAS PROC MIXED was used to analyze the impact of genotypes, exposures and sun protection behaviors on total body nevus counts and nevi ≥2 mm. SAS PROC GLIMMIX was used to examine interactions among genotypes, exposures and sun protection behaviors that impact facial freckling. Significant interaction effects for each model were plotted using SAS. A detailed analysis plan is included in Supplemental Methods.
Results
Characteristics and Participation of Study Subjects
This study reports data collected annually in 2004–2008 for white children who provided DNA data in either 2007 or 2008 (N=477). Study participants did not differ with regard to race/ethnicity, hair color, eye color, or gender from the other participants in the full study cohort who did not provide DNA (27). Participation was high with 68% of all 477 children completing a skin exam in all five years (age 6-10), 22% had four exams, 6% had three, and 4% had two or one exams (Table S1). 94% completed the survey in all four years, 4% completed three and 2% completed two or fewer surveys.
The number of nevi and freckle density grew in each successive year. The geometric mean total nevus counts increased almost 2.5 fold and the mean freckling score increased 1.75 fold, from age 6 to 10 (Table S2A). Sun exposures also increased: the cumulative number of sunburns, waterside vacations and chronic exposure grew 5-, 1.9- and 3.7-fold, respectively (Table S2B). Average sun protection frequency was approximately 12 on the 4-20 scale, and did not vary markedly each year.
Association of OCA2/HERC2 rs12913832 SNP and MC1R with pigmentation phenotype
rs12913832 alleles (C/T) were found at frequencies of 0.69 and 0.31,respectively (Table S3). As previously reported (16, 22, 30), the C allele was associated with lighter skin, hair, and eye color. The association with eye color was particularly striking where 62.5% of children homozygous for the C allele had blue eyes, and 89.8% of subjects homozygous for the T allele had brown eyes. Thus, the rs12913832 C allele is hereafter referred to as OCA2blue and the T allele as OCA2Brown. OCA2blue was associated with increases in freckling, total body nevus count and nevi ≥2 mm, similarly to previous reports. 62% of children were in the MC1R grouped R/R, R/r category, 25% in R/+, r/r and 13% in r/+, +/+. As expected, MC1R R/R, R/r variants were positively associated with red hair, lighter skin, green eye color and high facial freckle scores, but not total nevus counts or nevi ≥2 mm.
Since it is possible that sun exposure behaviors may be associated with pigmentation characteristics, the potential for significant associations between sun exposure and genotype was examined. Few associations were observed between genotype, sun exposure and sun protection (Table S4, S5); although children in the OCA2blue/blue group experienced more sunburns in both 2004 and 2007, and those with MC1R R/R, R/r variants had more sunburns in 2006 and 2007.
Three major UV exposure variables interact to influence nevus counts in childhood
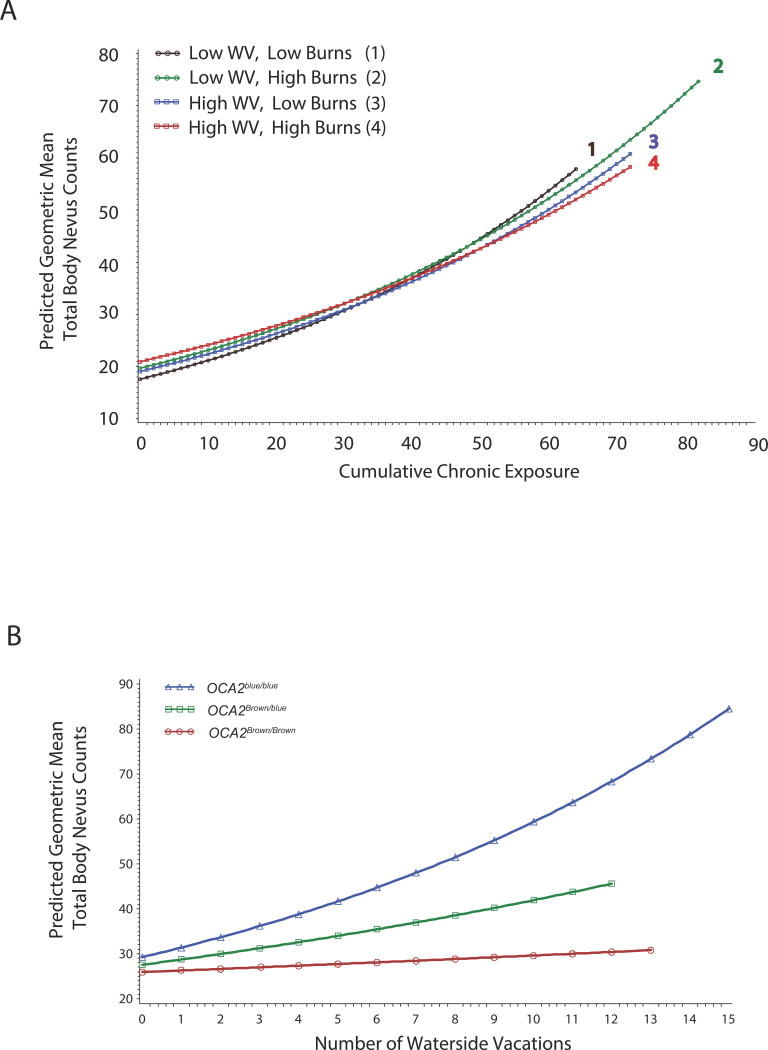
To evaluate associations between exposure patterns such as waterside vacations, sunburns and chronic exposure, and nevus counts among children with MC1R and rs12913832variantswe analyzed the data using linear mixed modeling. A significant 3-way interaction was detected between UV exposures that together were associated with log transformed nevus counts (Table 1). This 3-way interaction showed that the effects of waterside vacations and sunburns on nevus counts are different depending on whether children had low or high chronic exposures. In children with 24 or fewer hours of chronic exposure (mean= 18.25, 3rd quartile = 24), all UV exposures acted cumulatively to increase predicted nevus counts (Figure1A). Thus, among those with little chronic exposure, nevus count increased with each respective unit of exposure. As chronic exposure approached 28 hours, the combined effects of waterside vacations and sunburns started to diminish, and ultimately at higher chronic exposures, their impact appeared to reverse. Thus, the highest predicted rate of nevus accumulation was observed with increasing chronic exposure (>50 hours) in the presence of few sunburns and few waterside vacations (Figure 1A, Group 1). The relationship between chronic exposure and nevus accumulation progressively diminished in individuals who had1) few waterside vacations, many sunburns (Group 2), and 2) many waterside vacations, few sunburns (Group 3), and 3) many waterside vacations and many sunburns (Group 4).
Table 1. Linear mixed model analysis predicting log total nevus counts in children (2005 – 2008).
| Predictor | Estimate | ||||
|---|---|---|---|---|---|
|
| |||||
| b | 95% CI | Antilog (b) | R2 | p | |
|
| |||||
| Intercept | 3.29 | 26.70 | |||
| rs12913832 | 0.12 | 0.04, 0.20 | 1.13 | 0.02 | 0.01 |
| MC1R | |||||
| R/R, R/r | -0.11 | -0.27, 0.05 | 0.90 | 0.006 | 0.19 |
| r/r, R/+ | 0.05 | -0.08, 0.17 | 1.05 | - | 0.47 |
| r/+, +/+ | - | - | - | - | - |
| Gender | |||||
| Female | -0.10 | -0.20, 0.01 | 0.91 | 0.01 | 0.08 |
| Male | - | - | - | - | - |
| Sun Protection Composite | 0.004 | -0.01, 0.02 | 1.00 | 0.003 | 0.51 |
| Water Vacations (#, cum.) | 0.01 | -0.02, 0.05 | 1.01 | 0.0004 | 0.46 |
| Chronic Exposure (cum.) | 0.02 | 0.01, 0.02 | 1.02 | 0.12 | <0.001 |
| Sunburns (#, cum.) | 0.06 | 0.05, 0.08 | 1.07 | 0.04 | <0.001 |
| Water Vacations*rs12913832 | 0.03 | 0.01, 0.05 | 1.03 | 0.004 | 0.01 |
| Water Vacations*Chronic | -0.001 | -0.002, -0.0003 | 0.999 | 0.005 | <0.01 |
| Water Vacations*Sunburns | -0.005 | -0.012, 0.001 | 0.995 | 0.002 | 0.13 |
| Chronic*Sunburns | -0.002 | -0.003, -0.001 | 0.998 | 0.03 | <0.001 |
| Water Vacations*Chronic* Sunburns | 0.0002 | 0.000, 0.0004 | 1.0002 | 0.004 | 0.03 |
#: number, cum: cumulative
Figure 1. Interactions that predict geometric mean total body nevus counts in 6 – 10 year old children as determined by the linear mixed model analysis presented in Table 1.
A) Cumulative hours of chronic exposure interact with cumulative numbers of water vacations and sunburns to predict geometric mean total body nevus counts in the study period. Low and high water vacations and sunburns are used to depict the relationships between these variables in predicting nevus counts. The terms Low and High refer to the 33rd and 66th percentile levels of both cumulative centered sunburns (-1.2 and 0.79) and cumulative centered waterside vacations (-0.88 and 0.12). Exponentiated simple slope estimates and 95% confidence intervals for the effects plotted for each represented exposure group are in Table S6.
B) The OCA2/HERC2 rs12913832 SNP interacts with water vacations to predict geometric mean total body nevus counts. The two-way association between rs12913832 and water vacations is observed in subjects who experience an average level of sunburns (1.88) and chronic exposure (18.25) for the study period. Exponentiated simple slope estimates and 95% confidence intervals for the plotted OCA2 genotypes are in Table S7.
Waterside vacations interact with OCA2blue to elevate childhood nevus counts
An interaction between rs12913832 genotype and cumulative number of waterside vacations was a significant predictor of log transformed nevus counts (Table 1, Figure 1B). At the average level of waterside vacations (2.21), geometric mean nevus counts were increased by 13% with each OCA2blue allele. Each additional waterside vacation increased that effect by another 3%. Thus, waterside vacations had the greatest effect in promoting nevus formation in children who carried OCA2blue/blue (Figure1B). In fact, OCA2blue/blue subjects increased their predicted geometric mean nevus counts around 2.5 fold when waterside vacations increased from themean level (2.21) to the maximum of 12. In contrast, predicted geometric mean nevus counts increased only 1.9 fold in OCA2Brown/blue children and 1.2 fold in OCA2Brown/Brown children who experienced the same increase in waterside vacations.
Genetic interaction of MC1R with OCA2 in sunburned kids influences ≥2 mm nevus counts
Since it is possible that nevi of different sizes develop via different pathways, the same potential Interactions among MC1R and rs12913832 and the three different UV exposure variables were investigated using nevi ≥2 mm as the outcome (Table 2). In this model, a significant three-way interaction of MC1R and rs12913832 genotypes and number of sunburns was observed. Sunburns preferentially accelerated nevus counts ≥2 mm in children who were in the intermediate MC1R r/r, R/+ genotype group in combination with OCA2Brown/Brown (Figure 2A). For this MC1R/rs12913832 combination, sunburns increased geometric mean nevus count 3.6 fold with 8 sunburns versus the overall average of 2 sunburns for the study period. The effect of sunburns on nevus counts among children with intermediate MC1R was progressively reduced as the number of OCA2blue alleles increased (Figure 2B, 2C). Nevi also increased in response to sunburns in the MC1R R/R, R/r genotype group, and there was a tendency for this sunburn responsiveness to increase with each OCA2blue allele, however, this was not statistically significant. The MC1R +/+, r/+ genotype was minimally responsive to sunburns in any combination with rs12913832 genotype.
Table 2. Linear mixed model analysis predicting log total nevus counts ≥2 mm in children (2005 – 2008).
| Predictor | Estimate | ||||
|---|---|---|---|---|---|
|
| |||||
| b | 95% CI | Antilog (b) | R2 | p | |
|
| |||||
| Intercept | 0.70 | 0.41, 0.99 | 2.02 | <0.001 | |
| rs12913832 | 0.19 | 0.07, 0.30 | 1.21 | 0.01 | 0.001 |
| MC1R | |||||
| R/R, R/r | 0.11 | -0.41, 0.63 | 1.12 | 0.01 | 0.67 |
| r/r, R/+ | 0.30 | -0.01, 0.62 | 1.35 | - | 0.06 |
| r/+, +/+ | - | - | - | - | - |
| Gender | |||||
| Female | -0.06 | -0.18, 0.06 | 0.94 | 0.002 | 0.35 |
| Male | - | - | - | - | - |
| Sun Protection Composite | 0.004 | -0.014, 0.02 | 1.00 | 0.0001 | 0.66 |
| Water Vacations (#, cum.) | 0.03 | 0.01, 0.05 | 1.03 | 0.01 | 0.01 |
| Chronic Exposure (cum.) | 0.01 | 0.005, 0.01 | 1.01 | 0.02 | <0.001 |
| Sunburns (#, cum.) | 0.02 | -0.05, 0.09 | 1.02 | 0.005 | 0.54 |
| rs12913832*MC1R | |||||
| R/R, R/r | -0.11 | -0.44, 0.22 | 0.90 | 0.003 | 0.52 |
| r/r, R/r | -0.12 | -0.32, 0.08 | 0.89 | - | 0.25 |
| r/+, +/+ | - | - | - | - | - |
| rs12913832*Sunburns | 0.01 | -0.03, 0.05 | 1.01 | 0.001 | 0.68 |
| MC1R*Sunburns | |||||
| R/R, R/r | 0.04 | -0.11, 0.20 | 1.05 | 0.004 | 0.57 |
| r/r, R/+ | 0.14 | 0.03, 0.24 | 1.15 | - | 0.01 |
| r/+, +/+ | - | - | - | - | - |
| rs12913832*MC1R*Sunburns | |||||
| R/R, R/r | 0.01 | -0.08, 0.10 | 1.01 | 0.005 | 0.81 |
| r/r, R/+ | -0.08 | -0.15, -0.02 | 0.92 | - | 0.01 |
| r/+, +/+ | - | - | - | - | - |
#: number, cum: cumulative
Figure 2. Three-way interaction that predicts geometric mean nevus counts≥2 mm in 6 – 10 year old children as determined by the linear mixed model analysis of longitudinal data presented in Table 2.
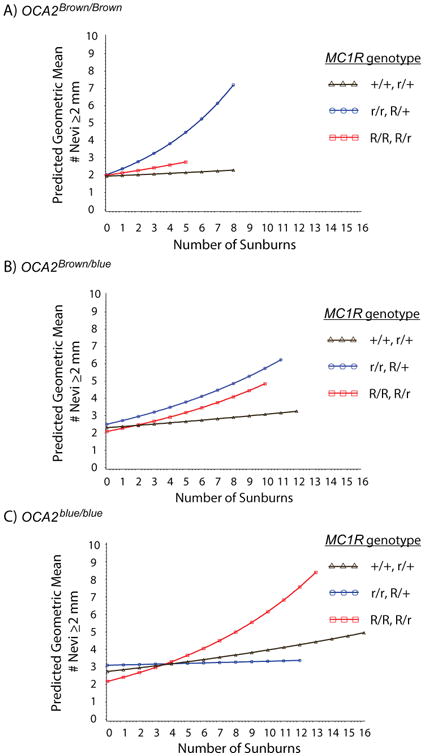
A), B), and C) A three-way interaction with cumulative number of sunburns (x-axis) predicts nevus counts in children from each combination of OCA2Brown and OCA2blue alleles together with each MC1R genotype grouping. Nevus counts were only plotted where actual sunburn data was available within each genotype group. Exponentiated simple slope estimates and 95% confidence intervals for each plotted MC1R and OCA2 genotype group are shown in Table S8.
Waterside vacations enhance facial freckling in children with red hair color alleles
Despite the widespread belief that freckles appear with increasing sun exposure, and the recognition that both freckles and UV exposures are melanoma risk factors, the relationship between these two risk factors has not been previously described. Linear mixed model analysis was used to examine the impact of rs12913832, MC1R variants and UV exposures on facial freckling (Table 3). No interaction was observed between the rs12913832 and any of the UV exposure variables although there was a significant main effect of rs12913832, with eachOCA2blue allele increasing the odds of being in both the high and medium freckle groups relative to the group with no freckles. Of the other UV exposure variables, chronic exposure and sunburns both elevated the odds of having higher freckling levels, and these effects were independent of MC1R and rs12913832 genotypes.
Table 3. Generalized linear mixed model analysis predicting facial freckle level in children (2005 – 2008).
| Predictor | Parameter | ||
|---|---|---|---|
|
| |||
| Odds Ratio | 95% CI | p | |
|
| |||
| Intercept | |||
| high freckling | 0.001 | 0.0001, 0.01 | <0.001 |
| medium freckling | 0.07 | 0.01, 0.41 | 0.003 |
| low freckling | 0.43 | 0.13, 1.39 | 0.16 |
| rs12913832 | |||
| high freckling | 3.40 | 1.50, 7.75 | 0.004 |
| medium freckling | 1.90 | 1.16, 3.09 | 0.01 |
| low freckling | 1.08 | 0.80, 1.44 | 0.62 |
| MC1R | |||
| high freckling | 17.65 | 8.07, 38.60 | <0.001 |
| medium freckling | 4.51 | 2.77, 7.34 | <0.001 |
| low freckling | 1.52 | 1.09, 2.11 | 0.01 |
| Female gender | |||
| high freckling | 1.10 | 0.39, 3.06 | 0.86 |
| medium freckling | 1.22 | 0.64, 2.34 | 0.55 |
| low freckling | 1.42 | 0.95, 2.11 | 0.09 |
| Sun Protection Composite | |||
| high freckling | 1.12 | 0.95, 1.31 | 0.17 |
| medium freckling | 1.00 | 0.64, 2.34 | 0.97 |
| low freckling | 1.00 | 0.95, 2.11 | 1.00 |
| Water Vacations | |||
| high freckling | 0.73 | 0.55, 0.97 | 0.03 |
| medium freckling | 0.99 | 0.84, 1.17 | 0.92 |
| low freckling | 1.02 | 0.92, 1.13 | 0.72 |
| Chronic Exposure | |||
| high freckling | 1.03 | 1.01, 1.06 | 0.01 |
| medium freckling | 1.02 | 1.00, 1.04 | 0.05 |
| low freckling | 1.01 | 1.00, 1.03 | 0.04 |
| Sunburns | |||
| high freckling | 1.38 | 1.17, 1.63 | 0.000 |
| medium freckling | 1.25 | 1.09, 1.43 | 0.001 |
| low freckling | 1.06 | 0.96, 1.18 | 0.23 |
| MC1R*Water Vacations | |||
| high freckling | 1.45 | 1.11, 1.88 | 0.01 |
| medium freckling | 1.04 | 0.86, 1.25 | 0.71 |
| low freckling | 0.92 | 0.79, 1.06 | 0.22 |
reference group = no freckles
A significant interaction between waterside vacations and MC1R was detected. Children who had MC1R R/R, R/r variants were increasingly found in the highest freckle group as they experienced more waterside vacations (Table 3; Figure 3). In fact, for MC1R R/R, R/r children, the predicted odds ratio for being in the high versus no freckle group was negligible (0.1) in the absence of water vacations, and increased to over 7when children experienced 9 waterside vacations (Figure.3). The odds of being classified in the high freckle group were unaffected by waterside vacations for youth in the other two MC1R groups.
Figure 3. An interaction between waterside vacations and MC1R predicts facial freckle scores as determined by the model presented in Table 3.
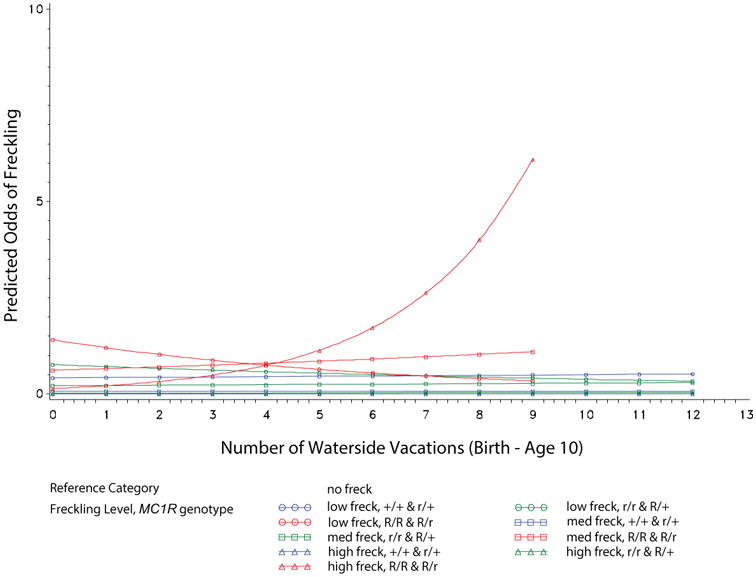
The odds ratios for being in each freckle group (low, moderate, high) versus the no freckles reference group for each MC1R genotype was plotted for each waterside vacation. Exponentiated simple slope estimates and 95% confidence intervals for each plotted freckle density level and MC1R genotype group are shown in Table S9.
Discussion
Much has been written about the power of longitudinal studies to uncovergene-environment interactions(31, 32); however, to our knowledge, our study is the first report of a longitudinal analysis where gene-environment interactions for cancer risk phenotypes are identified. With this in mind, our study uncovered a number of novel findings. We demonstrated that chronic exposure plays a central role in the formation of nevi: children with the highest chronic exposure had the highest nevus counts. Within the low range of chronic exposure, all UV exposures acted cumulatively to increase nevus number. However, at high levels of chronic exposure, the nevus promoting effect of waterside vacations and sunburns was reduced. Secondly, we identified interactions between waterside vacations, sunburns and specific rs12913832 and MC1R genotypes that significantly enhanced total nevus counts, counts of nevi ≥2 mm and the degree of facial freckling. Taken together, our data suggest that to the extent that nevi are a marker for melanoma risk, all children will benefit from sun protection. The results also indicate that a personalized sun protection strategy based on a child's genetic makeup may be of value in counteracting melanoma risk as a complement to current population-wide preventive efforts.
While intense intermittent UV exposure is associated with increased nevus counts and melanoma (2, 33), the role of chronic exposure is less clear(34)(2). We present a new perspective for the role of chronic exposure in nevo genesis and melanoma risk. In children with low levels of chronic exposure, all types of UV exposure contribute to nevus counts in a cumulative manner. This relationship tended to reverse in children who experienced higher levels of chronic exposure, where the nevo genic effect of this exposure tended to be reduced along with that of waterside vacations and sunburns as their respective numbers increased. These observations suggest that high levels of chronic exposure may counteract the effects of intense intermittent UV exposure on melanoma risk(33-35), possibly by inducing an adaptive response of skin to UVR. Alternatively, there may be a threshold of UV that a child experiences, equivalent to around 30 – 40 hrs of cumulative chronic exposure by the age of 10 in our study, at which the effects of all types of exposures are dampened. Previous studies, including our own, have not reported such a strong effect of chronic exposure on nevus counts(29). This may be due to differences between cross-sectional versus longitudinal analytical approaches, failure to examine interactions between exposure types, and our multi-year prospective study design where yearly sun exposure information is collected annually, rather than more distant retrospective assessment of sun exposure behaviors based on locality of past residence or recall. Chronic exposure may be particularly hard to assess retrospectively, and analysis of a single year of chronic exposure data may underestimate the effects of this variable. That is, chronic exposure may act in a cumulative fashion over multiple years, and one year's exposure may not accurately represent this.
The total nevus count model showed that waterside vacations, rather than sunburns or chronic exposures, preferentially interacted with theOCA2blue allele to increase nevus counts. This finding builds upon the known association between OCA2blue, blue eye color and elevated nevus count(22), and is the first indication that these children are susceptible to nevi following UVR. Previously, the methylthio adenosine phosphorylase (MTAP) rs7023329 SNP was shown to interact with sunburns to increase nevus counts in adults(34). We also observed that sunburns acted on the intermediate MC1R genotype group, R/+, r/r, in combination with OCA2Brown alleles to increase counts of nevi >2 mm. These findings extend the previous report of interaction between MC1R and OCA2 to include the effect of UV exposure on nevi (16). This interaction was not observed for total nevi, and altogether, these data may indicate that nevi of different sizes form through different mechanisms in response to different types of UV exposure.
Freckling is generally considered to be genetically determined (36), although a pre-established pattern of freckles may become visible following sun exposure(37, 38). We showed for the first time that the number of waterside vacations strongly interacted with MC1R RHC variants to enhance facial freckling in children. We note that MC1R is the major freckling gene, although variants in ASIP,BNC2, IRF4, OCA2 and TYR also contribute, albeit to a much lesser degree, to formation of this trait(13, 14). We did not identify any interaction between rs12913832 and any sun exposure variable, so freckling by this pathway may be entirely inherent. Further studies are needed to unravel the relative contributions of sun exposure and genes to formation and natural history of freckles.
While our studies indicate that environment-environment and gene-environment interactions are associated with melanoma risk phenotypes in children, the extent to which these findings can be generalized to melanoma itself remains to be determined. The GEM study group previously reported an association between UV exposure and MC1R R variants(39), and we also observed a tendency towards increased nevi ≥2 mm in the OCA2blue/blueMC1R R/R, R/r group. Moreover, MC1R R/+ subjects are known to be at elevated risk for melanoma(40). OCA2 variants, including rs12913832, are likely weakly penetrant for melanoma, as some studies show an association(25, 26) but not others(12, 41). Our data suggest that associations between rs12913832 and melanoma may strengthen as future studies include UV exposure covariates. Our data also support the idea that GWA studies factoring in relevant exposure variables could uncover new cancer risk loci.
In the models for nevi and freckles, no significant effect of sun protection was observed. The sun protection composite score incorporates information on hat and sunscreen use, sun-safe clothing use and shade seeking behavior, and is likely to have an impact on our models by influencing the number of sunburns experienced by any individual(34). There is a moderate yet significant inverse correlation between sun protection composite score and number of sunburns experienced by subjects within the study period (r = -0.07 - -0.13; P = 0.038 – 0.005; n = 446 – 457 from 2005 to 2007; 2004 was not significant). It is possible that new alternatives for intervention, perhaps including genetic information would show greater impact on these longitudinal models.
It will be useful to replicate the findings reported here using additional cases from the cohort being followed in this study. Such replication will not only help to validate the present findings but may reveal more robust associations, given a larger sample size and increased statistical power. Although the size of the current sample was sufficient for the analyses reported here, we did use imputation procedures to fill in the small amount of missing data that existed on our sunburn, waterside vacation, and chronic exposure variables. The imputation procedure clearly allowed us to maximize our analytic power with minimal negative impact our findings.
It will also be important to confirm these findings in other locations where sun exposure patterns may vary from those experienced by children in Colorado. Colorado is characterized by a high altitude (>5,000 foot elevation) and a sunny climate (>300 days of sunshine per year), and a 30% higher ambient UV level compared to sea level. Repeat studies in other locations will determine if differences exist in gene-environment interactions where ambient UV levels are substantially lower or higher, resulting in marked differences in the impact of chronic exposure.
Our understanding of the gene-environment interactions that influence melanoma risk phenotypes, and indeed melanoma itself, is in its infancy. Given that much of the sun exposure associated with later life melanoma risk may be accrued during childhood and adolescence, a clear understanding of the impact of different types of sun exposure is needed to engage the most effective prevention strategies. While some studies suggest that sun protective behaviors may reduce nevus counts in children(42-45), a more efficient and consistent preventive strategy is needed. To this end, our unique longitudinal study of nevus development provides concrete evidence for what has been long suspected- that different patterns of sun exposure interact in different ways with each other and with different genetic factors to influence melanoma risk phenotypes. Our data suggest that all children may benefit from sun protection, and that a personalized sun protection strategy based on a child's genetic makeup may be of additional value in counteracting melanoma risk.
Supplementary Material
Acknowledgments
We thank the health care practitioners and support staff for conducting the skin exams, all of the families of the Colorado Kids Sun Care Program for their time and enthusiasm. We also thank Dr. Jon Weisiger for technical help.
Funding: A. Barón, J. Morelli, N. Box, M. Berwick, R. Dellavalle, S. Mokrohisky and L. Crane are co-Investigators on NIH/NCI 5R01CA74592
N. Box and L. Crane were supported by NIH/NCRR (UL1RR025780).
L. Crane, N. Box and R. Dellavalle were supported by NIH/NCI (P30CA046934).
T. Terzian was supported by grants from NIH/NIAMS (K01AR063203), NIH/NCATS (UL1 TR001082) and a research grant from the Dermatology Foundation.
Footnotes
Conflict of Interest: The authors report no conflicts of interest
References
- 1.Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129:1666–74. doi: 10.1038/jid.2008.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Picconi O, Boyle P, et al. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer. 2005;41:45–60. doi: 10.1016/j.ejca.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Chang YM, Newton-Bishop JA, Bishop DT, Armstrong BK, Bataille V, Bergman W, et al. A pooled analysis of melanocytic nevus phenotype and the risk of cutaneous melanoma at different latitudes. Int J Cancer. 2009;124:420–8. doi: 10.1002/ijc.23869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang YM, Barrett JH, Bishop DT, Armstrong BK, Bataille V, Bergman W, et al. Sun exposure and melanoma risk at different latitudes: a pooled analysis of 5700 cases and 7216 controls. International journal of epidemiology. 2009;38:814–30. doi: 10.1093/ije/dyp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Abeni D, Boyle P, et al. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur J Cancer. 2005;41:28–44. doi: 10.1016/j.ejca.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Carli P, Naldi L, Lovati S, La Vecchia C. The density of melanocytic nevi correlates with constitutional variables and history of sunburns: a prevalence study among Italian school children. Int J Cancer. 2002;101:375–9. doi: 10.1002/ijc.10629. [DOI] [PubMed] [Google Scholar]
- 7.Law MH, Montgomery GW, Brown KM, Martin NG, Mann GJ, Hayward NK, et al. Meta-analysis combining new and existing data sets confirms that the TERT-CLPTM1L locus influences melanoma risk. J Invest Dermatol. 2012;132:485–7. doi: 10.1038/jid.2011.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill VK, Gartner JJ, Samuels Y, Goldstein AM. The genetics of melanoma: recent advances. Annual review of genomics and human genetics. 2013;14:257–79. doi: 10.1146/annurev-genom-091212-153429. [DOI] [PubMed] [Google Scholar]
- 9.Bliss JM, Ford D, Swerdlow AJ, Armstrong BK, Cristofolini M, Elwood JM, et al. Risk of cutaneous melanoma associated with pigmentation characteristics and freckling: systematic overview of 10 case-control studies. The International Melanoma Analysis Group (IMAGE) Int J Cancer. 1995;62:367–76. doi: 10.1002/ijc.2910620402. [DOI] [PubMed] [Google Scholar]
- 10.MacKie RM. Incidence, risk factors and prevention of melanoma. Eur J Cancer. 1998;34(Suppl 3):S3–6. doi: 10.1016/s0959-8049(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 11.Titus-Ernstoff L, Perry AE, Spencer SK, Gibson JJ, Cole BF, Ernstoff MS. Pigmentary characteristics and moles in relation to melanoma risk. Int J Cancer. 2005;116:144–9. doi: 10.1002/ijc.21001. [DOI] [PubMed] [Google Scholar]
- 12.Law MH, Macgregor S, Hayward NK. Melanoma genetics: recent findings take us beyond well-traveled pathways. J Invest Dermatol. 2012;132:1763–74. doi: 10.1038/jid.2012.75. [DOI] [PubMed] [Google Scholar]
- 13.Praetorius C, Sturm RA, Steingrimsson E. Sun-induced freckling: ephelides and solar lentigines. Pigment Cell Melanoma Res. 2014 doi: 10.1111/pcmr.12232. [DOI] [PubMed] [Google Scholar]
- 14.Duffy DL, Montgomery GW, Chen W, Zhao ZZ, Le L, James MR, et al. A three-single-nucleotide polymorphism haplotype in intron 1 of OCA2 explains most human eye-color variation. Am J Hum Genet. 2007;80:241–52. doi: 10.1086/510885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bastiaens M, ter Huurne J, Gruis N, Bergman W, Westendorp R, Vermeer BJ, et al. The melanocortin-1-receptor gene is the major freckle gene. Hum Mol Genet. 2001;10:1701–8. doi: 10.1093/hmg/10.16.1701. [DOI] [PubMed] [Google Scholar]
- 16.Duffy DL, Box NF, Chen W, Palmer JS, Montgomery GW, James MR, et al. Interactive effects of MC1R and OCA2 on melanoma risk phenotypes. Hum Mol Genet. 2004;13:447–61. doi: 10.1093/hmg/ddh043. [DOI] [PubMed] [Google Scholar]
- 17.Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet. 1995;11:328–30. doi: 10.1038/ng1195-328. [DOI] [PubMed] [Google Scholar]
- 18.Box NF, Wyeth JR, O'Gorman LE, Martin NG, Sturm RA. Characterization of melanocyte stimulating hormone receptor variant alleles in twins with red hair. Hum Mol Genet. 1997;6:1891–7. doi: 10.1093/hmg/6.11.1891. [DOI] [PubMed] [Google Scholar]
- 19.Raimondi S, Sera F, Gandini S, Iodice S, Caini S, Maisonneuve P, et al. MC1R variants, melanoma and red hair color phenotype: a meta-analysis. Int J Cancer. 2008;122:2753–60. doi: 10.1002/ijc.23396. [DOI] [PubMed] [Google Scholar]
- 20.Visser M, Kayser M, Grosveld F, Palstra RJ. Genetic variation in regulatory DNA elements: the case of OCA2 transcriptional regulation. Pigment Cell Melanoma Res. 2014;27:169–77. doi: 10.1111/pcmr.12210. [DOI] [PubMed] [Google Scholar]
- 21.Dellavalle RP, Johnson KR, Hester EJ, Deas AM, Mokrohisky S, Morelli JG, et al. Children with red hair have more freckles but fewer melanocytic nevi: results from a cohort study of 280 three-year-olds. Arch Dermatol. 2005;141:1042–3. doi: 10.1001/archderm.141.8.1042. [DOI] [PubMed] [Google Scholar]
- 22.Sturm RA, Duffy DL, Zhao ZZ, Leite FP, Stark MS, Hayward NK, et al. A single SNP in an evolutionary conserved region within intron 86 of the HERC2 gene determines human blue-brown eye color. Am J Hum Genet. 2008;82:424–31. doi: 10.1016/j.ajhg.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Visser M, Kayser M, Palstra RJ. HERC2 rs12913832 modulates human pigmentation by attenuating chromatin-loop formation between a long-range enhancer and the OCA2 promoter. Genome Res. 2012;22:446–55. doi: 10.1101/gr.128652.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eiberg H, Troelsen J, Nielsen M, Mikkelsen A, Mengel-From J, Kjaer KW, et al. Blue eye color in humans may be caused by a perfectly associated founder mutation in a regulatory element located within the HERC2 gene inhibiting OCA2 expression. Hum Genet. 2008;123:177–87. doi: 10.1007/s00439-007-0460-x. [DOI] [PubMed] [Google Scholar]
- 25.Jannot AS, Meziani R, Bertrand G, Gerard B, Descamps V, Archimbaud A, et al. Allele variations in the OCA2 gene (pink-eyed-dilution locus) are associated with genetic susceptibility to melanoma. European journal of human genetics : EJHG. 2005;13:913–20. doi: 10.1038/sj.ejhg.5201415. [DOI] [PubMed] [Google Scholar]
- 26.Amos CI, Wang LE, Lee JE, Gershenwald JE, Chen WV, Fang S, et al. Genome-wide association study identifies novel loci predisposing to cutaneous melanoma. Hum Mol Genet. 2011;20:5012–23. doi: 10.1093/hmg/ddr415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crane LA, Mokrohisky ST, Dellavalle RP, Asdigian NL, Aalborg J, Byers TE, et al. Melanocytic nevus development in Colorado children born in 1998: a longitudinal study. Arch Dermatol. 2009;145:148–56. doi: 10.1001/archdermatol.2008.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aalborg J, Morelli JG, Mokrohisky ST, Asdigian NL, Byers TE, Dellavalle RP, et al. Tanning and increased nevus development in very-light-skinned children without red hair. Arch Dermatol. 2009;145:989–96. doi: 10.1001/archdermatol.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pettijohn KJ, Asdigian NL, Aalborg J, Morelli JG, Mokrohisky ST, Dellavalle RP, et al. Vacations to waterside locations result in nevus development in Colorado children. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:454–63. doi: 10.1158/1055-9965.EPI-08-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han J, Kraft P, Nan H, Guo Q, Chen C, Qureshi A, et al. A genome-wide association study identifies novel alleles associated with hair color and skin pigmentation. PLoS Genet. 2008;4:e1000074. doi: 10.1371/journal.pgen.1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robertson S, Poulton R. Longitudinal studies to detect genexenvironment interactions in common disease--bang for your buck? A commentary on Chaufan's “how much can a large population study on genes, environments, their interactions and common diseases contribute to the health of the American people?” (65:8, 1730-1741(2007)) Soc Sci Med. 2008;67:666–72. doi: 10.1016/j.socscimed.2008.04.010. discussion 75-83. [DOI] [PubMed] [Google Scholar]
- 32.Guttmacher AE, Hirschfeld S, Collins FS. The National Children's Study--a proposed plan. N Engl J Med. 2013;369:1873–5. doi: 10.1056/NEJMp1311150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer. 1997;73:198–203. doi: 10.1002/(sici)1097-0215(19971009)73:2<198::aid-ijc6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 34.Newton-Bishop JA, Chang YM, Iles MM, Taylor JC, Bakker B, Chan M, et al. Melanocytic nevi, nevus genes, and melanoma risk in a large case-control study in the United Kingdom. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:2043–54. doi: 10.1158/1055-9965.EPI-10-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newton-Bishop JA, Chang YM, Elliott F, Chan M, Leake S, Karpavicius B, et al. Relationship between sun exposure and melanoma risk for tumours in different body sites in a large case-control study in a temperate climate. Eur J Cancer. 2011;47:732–41. doi: 10.1016/j.ejca.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bataille V, Snieder H, MacGregor AJ, Sasieni P, Spector TD. Genetics of risk factors for melanoma: an adult twin study of nevi and freckles. J Natl Cancer Inst. 2000;92:457–63. doi: 10.1093/jnci/92.6.457. [DOI] [PubMed] [Google Scholar]
- 37.Wilson PD, Kligman AM. Experimental induction of freckles by ultraviolet-B. Br J Dermatol. 1982;106:401–6. doi: 10.1111/j.1365-2133.1982.tb04531.x. [DOI] [PubMed] [Google Scholar]
- 38.Ezzedine K, Mauger E, Latreille J, Jdid R, Malvy D, Gruber F, et al. Freckles and solar lentigines have different risk factors in Caucasian women. Journal of the European Academy of Dermatology and Venereology : JEADV. 2013;27:e345–56. doi: 10.1111/j.1468-3083.2012.04685.x. [DOI] [PubMed] [Google Scholar]
- 39.Kricker A, Armstrong BK, Goumas C, Kanetsky P, Gallagher RP, Begg CB, et al. MC1R genotype may modify the effect of sun exposure on melanoma risk in the GEM study. Cancer causes & control : CCC. 2010;21:2137–47. doi: 10.1007/s10552-010-9633-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmer JS, Duffy DL, Box NF, Aitken JF, O'Gorman LE, Green AC, et al. Melanocortin-1 receptor polymorphisms and risk of melanoma: is the association explained solely by pigmentation phenotype? Am J Hum Genet. 2000;66:176–86. doi: 10.1086/302711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guedj M, Bourillon A, Combadieres C, Rodero M, Dieude P, Descamps V, et al. Variants of the MATP/SLC45A2 gene are protective for melanoma in the French population. Hum Mutat. 2008;29:1154–60. doi: 10.1002/humu.20823. [DOI] [PubMed] [Google Scholar]
- 42.Smith A, Harrison S, Nowak M, Buettner P, Maclennan R. Changes in the pattern of sun exposure and sun protection in young children from tropical Australia. J Am Acad Dermatol. 2013;68:774–83. doi: 10.1016/j.jaad.2012.10.057. [DOI] [PubMed] [Google Scholar]
- 43.Bauer J, Buttner P, Wiecker TS, Luther H, Garbe C. Effect of sunscreen and clothing on the number of melanocytic nevi in 1,812 German children attending day care. American journal of epidemiology. 2005;161:620–7. doi: 10.1093/aje/kwi086. [DOI] [PubMed] [Google Scholar]
- 44.Autier P, Dore JF, Cattaruzza MS, Renard F, Luther H, Gentiloni-Silverj F, et al. Sunscreen use, wearing clothes, and number of nevi in 6- to 7-year-old European children. European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Natl Cancer Inst. 1998;90:1873–80. doi: 10.1093/jnci/90.24.1873. [DOI] [PubMed] [Google Scholar]
- 45.Harrison S, Buettner PG, MacLennan R, Woosnam J, Hutton L, Nowak M. Sun-Safe clothing helps to prevent the development of pigmented moles-results of a randomized controlled trial in young Australian children. Annals of the Australian College of Tropical Medicine. 2010;11:50. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.